Abstract
Dysfunctional expression of T-bet, a transcription factor that is critical for interferon (IFN)-γ production, has been implicated in the development of asthma. To investigate in detail the mechanisms responsible for exacerbated disease in the absence of T-bet expression, BALB/c wild-type (WT) and T-bet−/− mice were used in a murine model of ovalbumin (OVA)-induced allergic lung inflammation. Following OVA challenge, T-bet−/− mice displayed increased histological inflammation in the lungs as well as greater thickening of the bronchiole linings, increased numbers eosinophils and neutrophils in the lung, and enhanced airway hyperresponsiveness, compared to WT mice. However, the production of Th2 cytokines in T-bet−/− mice did not appear to be significantly greater than in WT mice. Interestingly, a marked increase in the levels of the pro-inflammatory cytokine IL-17 was observed in T-bet−/− mice. Neutralization of pulmonary IL-17 in T-bet−/− mice by anti-IL-17 mAb treatment during OVA challenge resulted in decreased levels of neutrophilic infiltration into the airways and decreased airway inflammation, essentially reversing the development of allergic asthma development. These findings indicate that IL-17 is a key mediator of airway inflammation in the absence of T-bet. The results of this study suggest a possible target for therapeutic intervention of human asthma.
Keywords: Allergy, Cytokines, Lung
Introduction
Allergic asthma, the most common form of asthma, is a chronic inflammatory disorder of the lung in which CD4 T helper cells play a key role. Experimental evidence indicates that there is a predominance of CD4 T helper type 2 (Th2) immune responses during the asthmatic disease process (1). Th2 cells are characterized by the production of IL-4, IL-5 and IL-13 (2), which in response to inhaled allergens, results in the classic phenotypic traits of allergic asthma; specifically, eosinophilic infiltration, bronchial hyperresponsiveness, mucus hypersecretion and allergen-specific IgE production (3, 4).On the other hand, expression of interferon (IFN)-γ has been shown to prevent development of allergen-induced airway inflammation (5, 6). IFN-γ is mainly produced by CD4 Th1 cells and suppresses Th2 responses. Therefore, allergic airway inflammation appears to be regulated by a sensitive balance between an over-active Th2 response and an inhibitory Th1 response.
The T-box transcription factor, T-bet, is crucial for Th1 cell differentiation (7).Expression of T-bet induces IFN-γ production and suppresses Th2-associated transcription factors (8). In agreement with its key regulatory in immune responses, dysfunctional expression of T-bet has been implicated in the development of allergic asthma (9). Indeed, reduced expression of T-bet in pulmonary T cells from asthmatic patients has been observed compared to T cells from non-asthmatic patients (9). In addition, mice that are genetically deficient in T-bet expression have been found to spontaneously exhibit airway hyper-responsiveness (AHR), as well as increased production of pulmonary Th2 cytokines and enhanced eosinophilic infiltration into the lung. These changes mimic both acute and chronic human asthma (9). Recently, the role of T-bet in asthma has been further underscored by reports that polymorphisms of the T-bet gene are associated with allergic asthma and airway hyperresponsiveness (10, 11).Thus, T-bet expression appears to be a critical genetic factor in allergic asthma. However, the precise role of T-bet in the regulation of allergen-induced airway inflammation remains unknown.
Recent evidence has also suggested a role for the proinflammatory cytokine IL-17, in the pathogenesis of airway disease, including allergic asthma. IL-17 is primarily secreted by CD4 Th cells and aids in neutrophil influx into peripheral sites partially through the induction of G-CSF and other chemokines (12). Not only has IL-17 expression been found to be upregulated in the airways of mice and humans following allergen-induced airway inflammation (13–16), but IL-17 has also been found to induce pulmonary inflammation (17). In addition, there appears to be strong evidence that in response to IL-17 production, there is a neutrophilic influx into the lung, which contributes to pulmonary diseases including asthma (18, 19). Similar studies have suggested that IL-17 is crucial for allergen specific bronchial hyperresponsiveness (20).Thus, accumulating evidence indicates that IL-17 has a key role in allergic asthma.
Interestingly, T-bet-deficient cell cultures secrete higher levels of IL-17 than WT cultures in response to T-cell receptor and cytokine stimulation, suggesting that T-bet is a critical negative regulator of Th17 cells (21). In addition, it has been shown that Th17 cells can develop in vitro in the absence of T-bet and STAT1 (22). Indeed, T-bet appears to be an inhibitory factor for IL-17 production and the loss of T-bet suggests a possible default pathway leading to IL-17 production. However, the interplay between the regulatory role of T-bet and the production of IL-17 following allergen-specific provocation remains to be investigated. In this study, we have now employed the established murine model of ovalbumin (OVA)-induced lung inflammation to test the hypothesis that in the absence of T-bet regulation, an enhanced proinflammatory IL-17 response would lead to increased pulmonary inflammation. We further hypothesized that neutralization of IL-17 in T-bet−/− mice would prevent development of asthmatic symptoms.
Materials and Methods
Mice
BALB/c WT mice were obtained from Charles River Laboratory through a contract with the National Cancer Institute. BALB/c T-bet−/− mice were obtained from the Jackson Laboratory. IL-17R−/− mice were provided by Amgen (Seattle, WA) and backcrossed seven generations on to the BALB/c background. All mice were housed in microisolator cages under pathogen-free conditions at Albany Medical College, and the Institutional Animal Care and Use Committee approved all procedures concerning the use of these mice.
Antigen-induced allergic airway inflammation
Allergic lung inflammation was induced as previously described (23). Mice (5–7 weeks of age) were immunized intraperitoneally (i.p.) twice with 10 ug of OVA (Sigma-Aldrich) in 4 mg alum (Reheis) at weekly intervals for two weeks. One week following second immunization, the sensitized mice were lightly anesthetized and challenged intranasally (i.n.) with 100 ug OVA in saline once daily for five days. Controls included naive mice and mice that were OVA sensitized but challenged with only saline. Twenty-four hrs after the final inoculation of OVA, the mice were sacrificed. Bronchoalveolar lavage (BAL) fluids were collected with a microsyringe fitted with a cannula that was gently inserted into the trachea and the lungs were lavaged three times with 1 ml of PBS. The amount of recovered lavage fluid was 750–900 µl per mouse ± 5% variability among groups. The BAL fluids were centrifuged at 300xg for 10 min at 4°C, and the cell free lavage fluids were stored at −80°C until analysis. Numbers of viable cells in BAL were determined by trypan blue exclusion, and the cells were mounted on slides for cytospin analysis by centrifugation at 110xg for 5 min at room temperature. In addition, portions of lung tissues were snap frozen in liquid nitrogen and stored at −80°C for cytokine analysis. Other lung tissue samples were fixed in formalin (Fisher Scientific) for histological analysis.
Histology
After collection of BAL fluids, lung tissues were fixed overnight in 5% buffered formalin, dehydrated and embedded in paraffin blocks (Richard Allan Scientific). The blocks were sectioned (5 µm thickness) and stained with hematoxylin and eosin (H&E) (Fisher Scientific). Slides were blinded and the peribronchial regions, six to eight per mouse, were scored at X200 magnification for inflammatory cellular infiltrate severity around airways as previously described (24) (0, normal; 1, <3 cells diameter thick; 2, 3–10 cells thick; 3, >10 cells thick). The presence of inflammatory cells was assessed using standard light microscopy; inflammation manifested as infiltrates in the perivascular and peribronchiolar regions of the lung.
Inflammatory cells isolated from BAL fluids were evaluated using a commercially available modified Wright-Giemsa stain (HEMA 3; Biochemical Sciences). Cytospins were stained according to the manufacturer’s instructions and examined under oil-immersion light microscopy.
Cytokine levels
Protein levels of IL-4, IL-5, IL-13 and IFN-γ in BAL samples and lung homogenates were determined by cytometric bead array (CBA) analysis using a BD FACSArray. Protein levels of homogenized lung tissues were normalized to total lung tissue protein in order to determine cytokine levels in different groups of mice. The following were used to quantitate other cytokine protein levels: IL-17, DuoSet ELISA (R&D Systems); IL-23, Elisa Ready-Set-Go! (eBioScience); and IL-25, ElisaMax (BioLegend), per the manufacturer’s instructions.
Quantitative Real-Time PCR
Total RNA was prepared from lung homogenates with TriZol reagent (Invitrogen). Complementary DNA (cDNA) was synthesized with superscript reverse transcriptase and oligo(dT) primers (Invitrogen). Gene expression was examined using a Bio-Rad iCycler Optical System and an iQ™ SYBR green real-time PCR kit (Bio-Rad Laboratories). The ΔΔCt method was used to normalize transcript to GAPDH and to calculate expression relative to untreated mice. Threshold cycle values were validated with different concentrations of RNA and the number of fold inductions remained constant. The primers for IL-17A and RORγt were previously described (25).
Measurement of airway responsiveness
Bronchial responsiveness to aerosolized methacholine (Mch) was measured 24 hrs following final OVA challenge as previously described, using anesthetized, tracheostomized, paralyzed mice connected to a computer-controlled small-animal mechanical ventilator (flexiVent; SCIREQ). Used forced oscillation measurements of Airway resistance (R), tissue damping (G) and tissue elastance (H) were obtained in response to increasing doses of methacholine by fitting the constant phase model to the impedance data (26–28).
Flow cytometry
Single-cell suspensions were obtained from the lungs of T-bet+/+ and T-bet−/− mice by collagenase digestion (2.5 mg/ml collagenase D, 0.25 mg/ml DNaseI, and 1 mM MgCl2) (Roche) for 1 hr at 37°C, followed by passage through a nylon mesh. The cells were first incubated with 2.4G2 mAb to mouse FcγRII/RIII for 20 min and then incubated with PE-rat anti-mouse IL-17 mAb (BD Pharmingen) or rabbit anti-mouse IL-23p19 Ab (Abcam) at 4°C for 30 min. Cells incubated with anti-IL-23p19 were washed and incubated with secondary PE-conjugated anti-rabbit IgG (Jackson ImmunoResearch).Abs were titrated before use to determine specificity and optimal staining concentrations. Stained cells were detected with a FACSCanto flow cytometer and analyzed using FACSDiva (BD Biosciences) software. The lymphocyte population was gated based on forward and side scatter and ten thousand events per gate per sample were collected, and the data were reported as the percentage of cells within the lymphocyte gate.
Anti-IL-17 mAb treatment
To neutralize pulmonary IL-17, rat anti-murine IL-17A mAb (Amgen) or control rat IgG (Jackson Immunoresearch Laboratories) was inoculated both i.n. at a dose of 5 µg on days 1 and 2 of OVA challenge phases as well as i.p. at a dose of 50 µg on days 1, 3,and 5 of OVA challenge.
Statistics
Statistical comparisons between mice were performed using the two-tailed Students t test or ANOVA for multiple groups of mice with a Tukey multiple comparison posttest (GraphPad InStat 3). Values of p<0.05 were considered to be statistically significant.
Results
Allergen-induced airway inflammation is enhanced in BALB/c T-bet−/− mice compared to WT mice
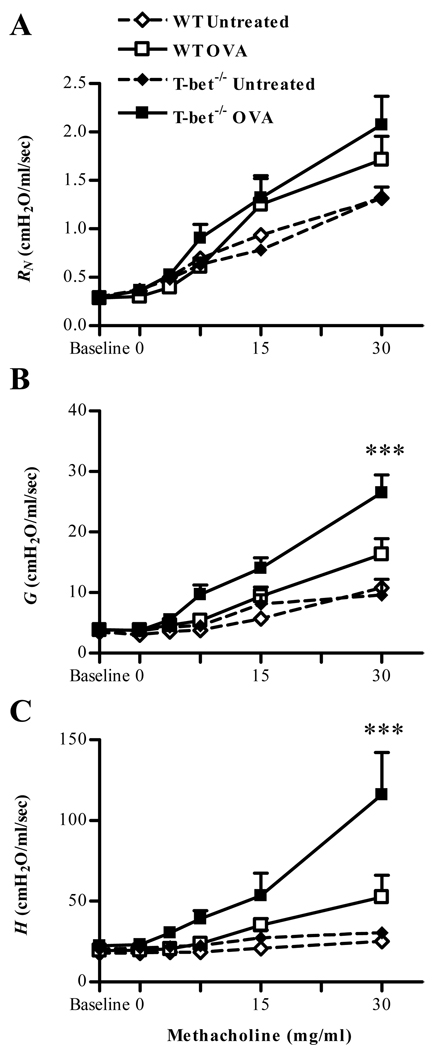
To assess development of antigen-induced allergic airway inflammation in the absence of T-bet regulation, a murine model of allergic lung inflammation was utilized. BALB/c T-bet+/+ and T-bet−/− mice were sensitized by i.p. injection of OVA in alum and then challenged i.n. for five consecutive days with soluble OVA. Following challenge, lung tissues and BAL fluids were obtained for histological and cytokine analyses. Histological examination showed that antigen-induced inflammation was apparent in the peribronchial and perivascular areas of the lung following OVA challenge, and the extent of inflammation was much greater in T-bet−/− mice compared to T-bet+/+ mice (supplementary Fig. 1A). No pulmonary inflammation was seen in either naive orantigen-sensitized and saline only challenged T-bet−/− and T-bet+/+ mice (supplementary Fig. 1A and data not shown). In addition to histological inflammation, it was found that T-bet−/− mice challenged with OVA developed a significantly increased pulmonary tissue resistance (G) and tissue elastance (H), to aerosolized Mch compared to T-bet+/+ mice (Fig. 1B, C). There was no significant effect on airway resistance (RN) in T-bet−/− mice compared to T-bet+/+ mice following OVA challenge. As expected, enhanced airway inflammation correlated with increased infiltration of inflammatory granulocytic cells into the airways. Expression of granulocytes was increased in both T-bet−/− and T-bet+/+ mice following OVA challenge, and levels of both eosinophils and neutrophils were greater in T-bet−/− mice compared to T-bet+/+ animals (supplementary Fig. 1B). Numbers of granulocytes did not differ among naïve animals or animals that were OVA-sensitized and challenged with saline (data not shown). Taken together, the results show that BALB/c T-bet−/− mice exhibit more severe OVA-induced allergic lung inflammation compared to T-bet+/+ counterparts.
FIGURE 1. Ova-induced airway hyperresponsiveness is enhanced in T-bet−/− mice.
5–7-week-old BALB/c T-bet+/+ and T-bet−/− mice were sensitized to OVA and challenged i.n. for 5 consecutive days. After challenge, airway function was assessed with forced oscillations utilizing the constant phase model for lung mechanics. A. Newtonian resistance (RN), B. tissue damping (G) and C. tissue elastance (H) were obtained in response to increasing doses of methacholine in naive and OVA-sensitized and OVA-challenged T-bet+/+ and T-bet−/− mice. The data are shown as means ± SEM; 6 mice/group; ***P<0.001.
Enhanced airway inflammation in the absence of T-bet does not correlate with increased Th2 cytokine production
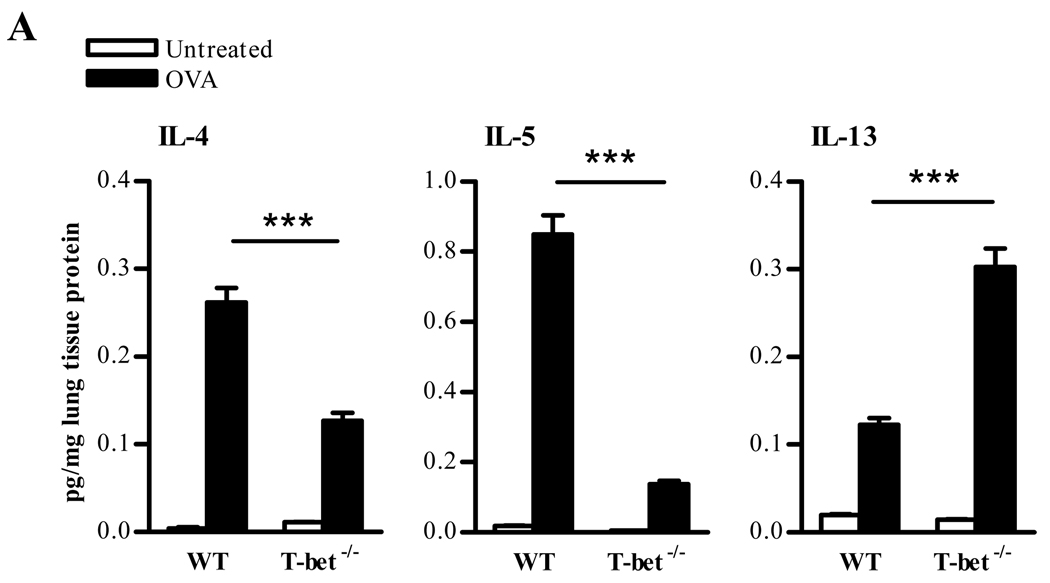
To investigate whether the absence of T-bet regulation leads to a dominant Th2 response following OVA challenge, cytokine expression in lung homogenates was analyzed. As expected, the lungs of T-bet+/+ animals showed increased levels of Th2-like cytokines, namely, IL-4, IL-5 and IL-13, following a five day OVA inhalation challenge (Fig. 2).However, these cytokines were not elevated in T-bet−/− mice. In fact, the cytokine levels appeared to be lower in the lungs T-bet−/− mice compared to T-bet+/+ mice, with the exception of increases in IL-13. Cytokine levels were also measured in BAL fluids and the pattern of cytokine expression remained consistent (data not shown). Intracellular cytokine staining for IL-4 expression confirmed a deficiency of IL-4 expression in T-bet−/− mice (data not shown). Thus, there is a paucity of Th2 cytokine production following specific antigen challenge of T-bet−/− mice, implying that additional factors are responsible for the enhanced antigen-induced airway inflammation observed in these mice.
FIGURE 2. Levels of Th2 cytokines in T-bet−/− compared to T-bet+/+ mice.
Lung cytokine levels following allergic lung inflammation induction. Twenty-four hrs after a 5 day i.n. OVA challenge, lung tissues were collected from T-bet+/+ and T-bet−/− mice. Protein levels were normalized to total lung tissue protein and cytokine levels were measured by murine CBA. The data represent means ± SEM; 8 mice/group; ***P<0.001.
IL-17 production is significantly enhanced in T-bet−/− mice following OVA challenge
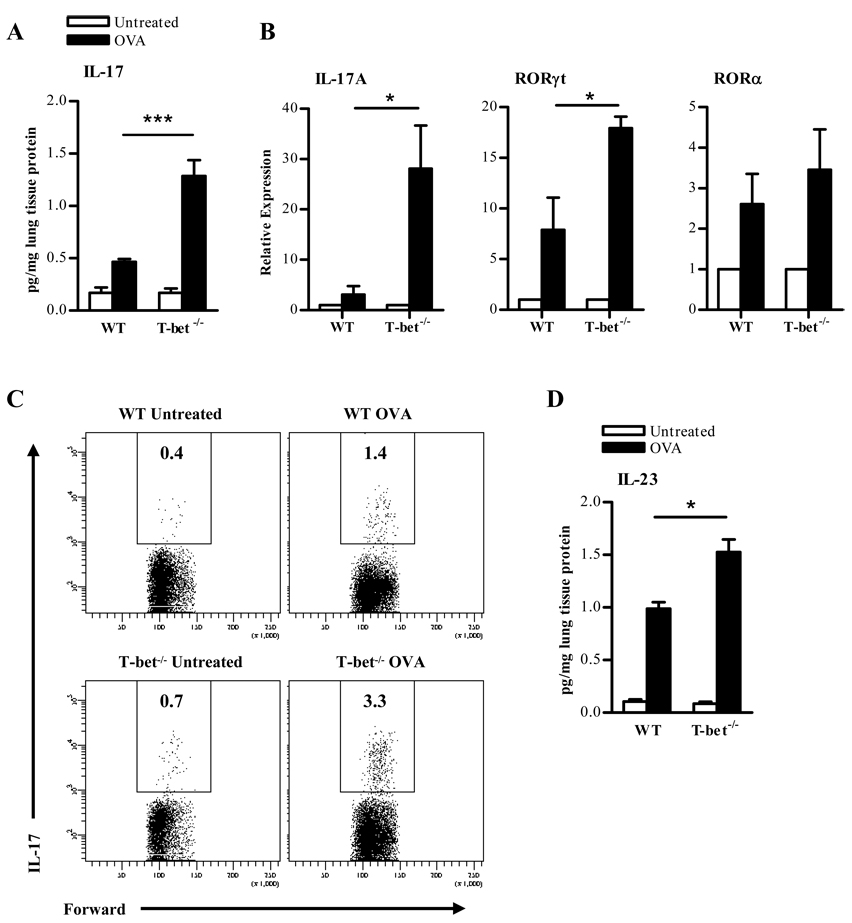
IL-17 has been implicated in airway granulocyte recruitment (29). To determine whether IL-17 was produced during allergic lung inflammation in T-bet−/− mice, lung homogenates from OVA-sensitized and challenged animals were analyzed. T-bet+/+ animals demonstrated an increase in pulmonary IL-17 levels but interestingly, IL-17 levels were even more dramatically increased in T-bet−/− animals following OVA-induced allergic lung inflammation (Fig. 3A). There were no detectable levels of IL-17 in the BAL fluids obtained from these mice. IL-17A transcripts were also significantly increased in T-bet−/− mice following OVA sensitization and challenge (Fig 3B). Similar patterns were observed for RORγt expression, a key regulator of IL-17A transcription (Fig 3B). RORα expression was also measured and there appeared to be no significant difference between T-bet+/+ and T-bet−/− animals (Fig. 3B). Intracellular cytokine staining of lung lymphocytes also demonstrated an increase in IL-17 production in T-bet−/− mice (Fig. 3C). Serum IL-17 levels were low in all mice (data not shown), suggesting that IL-17 expression is locally amplified and particularly enhanced in T-bet deficient lungs following allergen challenge. Many of the IL-17-expressing cells isolated from the lungs were found to be CD4+ (supplementary Fig. 2). In both the naïve and OVA challenged groups, little if any CD8+IL-17+ cells were found (data not shown), suggesting a major role for Th17 cells in response to inhaled allergen. Furthermore, IL-23, a critical cytokine for IL-17 production, was detectable in the lungs of OVA sensitized and challenged mice (Fig. 3D). The levels of IL-23 were increased in T-bet+/+ animals following OVA challenge but were increased to an even greater extent in T-bet−/− animals. In addition, 13.5 ± 1.4 percent of lung lymphocytes isolated from T-bet−/− animals were IL-23+ compared with 10.1 ± 0.7 percent IL-23+ lymphocytes in T-bet+/+ lungs. Also there was a significant increase in IL-23 receptor expression in T-bet−/− lungs (39.2 ± 13.8 relative expression) compared to T-bet+/+ lungs (16.1 ± 2.2 relative expression) following OVA challenge. These data correlate and reinforce the significant role of IL-17 in T-bet−/− animals.
FIGURE 3. IL-17 production is increased in the lungs of T-bet−/− mice following OVA-induced allergic lung inflammation.
A. IL-17 cytokine levels in the lung homogenates of naïve and OVA-sensitized and -challenged T-bet+/+ and T-bet−/− mice. Twenty-four hrs after a 5 day i.n. OVA challenge, lung tissues were collected from T-bet+/+ and T-bet−/− mice. Following normalization to total lung protein, cytokine levels were measured by ELISA. The data represent means ± SEM; 8 mice/group; ***P<0.001. B. IL-17, RORγt and RORα transcripts were detected by quantitative RT-PCR 24 hrs following final OVA challenge. C. Flow cytometric detection of IL-17-producing cells in lung lymphocyte preparations from naïve and OVA-sensitized T-bet+/+ and T-bet−/− mice following a 5 day i.n. OVA challenge. The data represent mean percentages of positively-stained cells within the lymphocyte gate of 6 mice/group. D. Expression of IL-23 in the lungs of sensitized mice upon antigen challenge. Lungs were collected from naïve and OVA-sensitized mice that were challenged with OVA 24 hrs earlier and levels of IL-23 were measured by ELISA. The data represent means ± SEM; 8 mice/group; *P<0.05.
IL-17 receptor signaling is necessary for antigen-induced airway inflammation
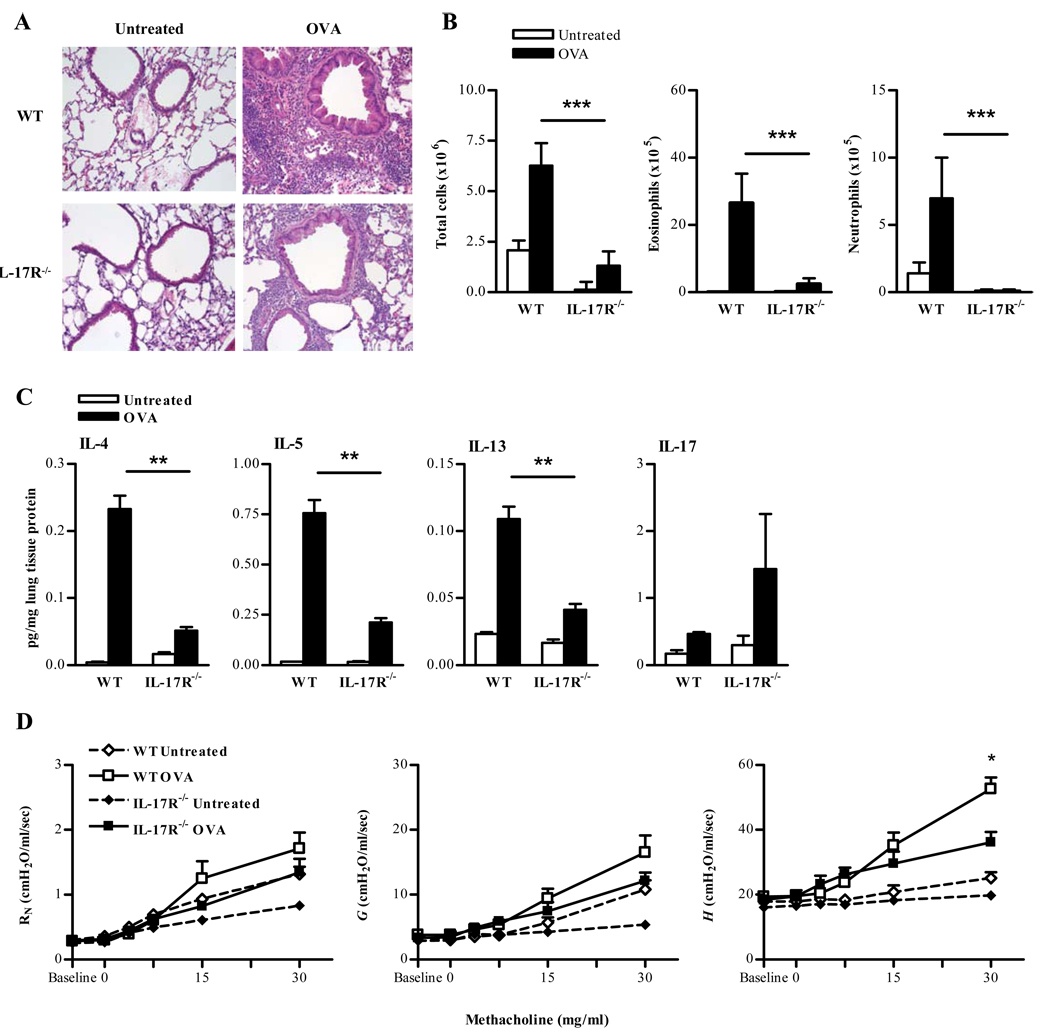
In order to determine the requirement for IL-17 in allergic asthma, IL-17R−/− mice were utilized. Following OVA challenge, granulocytic pulmonary inflammation was decreased in the peribronchial and perivascular regions of IL-17R−/− lungs compared to IL-17R+/+ lungs (Fig. 4A). Similarly, recruitment of eosinophils as well as neutrophils, was decreased in IL-17R−/− mice compared to IL-17R+/+ mice (Fig. 4B). Furthermore, expression of IL-4, IL-5, and IL-13 was reduced in IL-17R−/− versus IL-17R+/+ mice following antigen challenge (Fig. 4C). Levels of IL-17 in IL-17R−/− mice were normal or even somewhat higher than in WT mice. In response to aerosolized Mch, IL-17R−/− mice demonstrated an overall reduction in airway response to Mch compared with WT mice (Fig. 4D). Therefore, signaling through IL-17R is required for the development of antigen-induced allergic asthma.
FIGURE 4. IL-17 receptor signaling is critical for antigen induced airway inflammation.
A. Representative H&E stained lung tissues from 5–7-week-old naïve and OVA challenged BALB/c IL-17R−/− mice (X200; 4/group). B. Numbers of total cells, eosinophils and neutrophils in BAL fluids were evaluated 24 hrs after OVA instillation. BAL cells were stained with Wright’s modified stain and counted according to standard morphological criteria. The data represent means ± SEM; 4 mice/group. C. Cytokine levels in lungs following allergic lung inflammation induction. Twenty-four hrs after a 5 day i.n. OVA challenge, lung tissues were collected from naïve and OVA-challenged IL-17R−/− mice. IL-5, IL-4 and IL-13 cytokine levels were measured by CBA, IL-17 levels were measured by ELISA. The data represent means ± SEM; 4 mice/group. D. Mch dose-response curves 24 hrs following 5 day i.n. OVA challenge. The data are represented as means ± SEM; 6 mice/group *P<0.05.
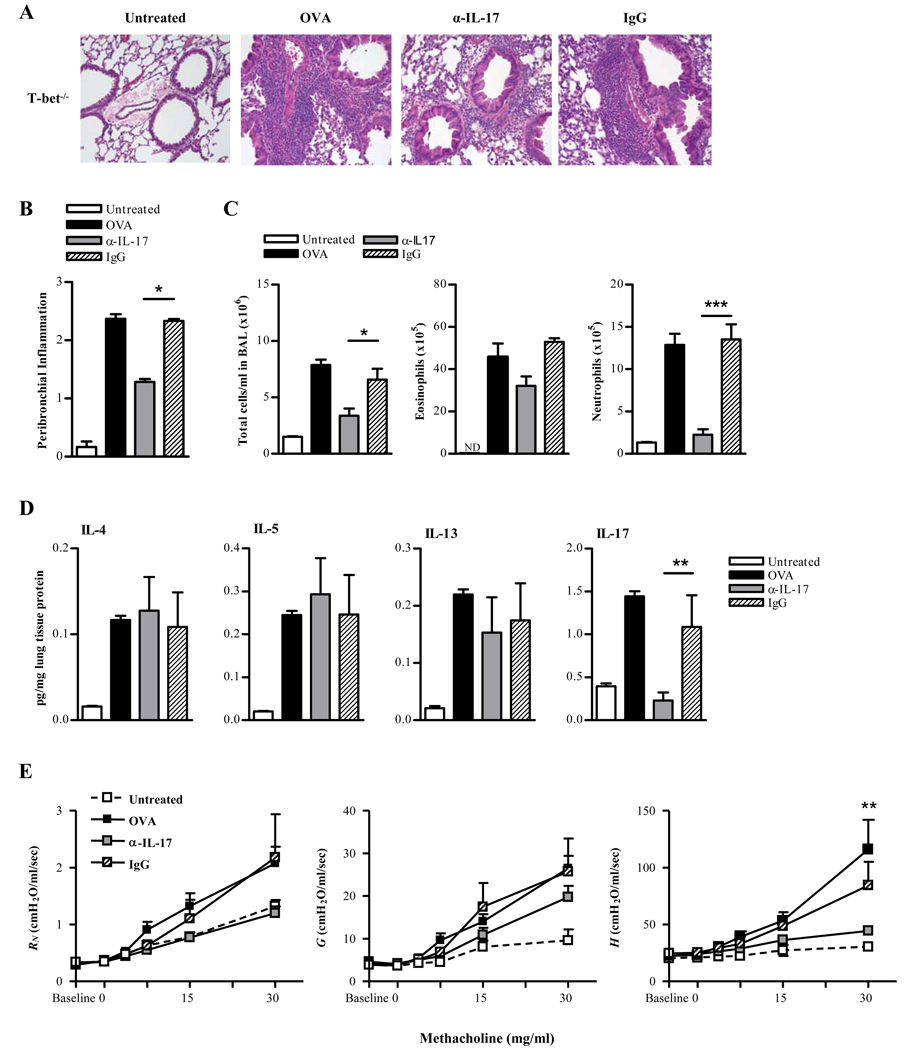
To confirm these findings and to determine whether IL-17 is necessary for mediating airway inflammation in T-bet−/− mice, pulmonary IL-17 activity was neutralized by in vivo rat anti-IL-17 mAb treatment during OVA challenge. Protein levels of IL-17 in T-bet−/− mice were significantly decreased following antibody treatment, demonstrating that the procedure was effective. Anti-IL-17 treatment essentially reversed the perivascular and peribronchial inflammation observed in control rat IgG-treated T-bet−/− mice (Fig. 5A, B). This reduction in inflammation correlated with reduced neutrophilic infiltration (Fig. 5C). However, anti-IL-17 antibody treatment had little effect on the levels of lung Th2 cytokine production (Fig. 5D). It also appears the anti-IL-17 antibody treatment had little effect on the airway resistance and tissue damping, but did have a significant effect on tissue elastance in T-bet−/− mice (Fig. 5E) suggesting a specific role for IL-17-induced inflammation in the peripheral airways.
FIGURE 5. IL-17 mediates allergen-induced airway inflammation in T-bet−/− mice.
A. Representative H&E stained lung sections from naïve and OVA-challenged BALB/c T-bet−/− mice (X200; 4 mice/group. Neutralizing anti-IL-17 mAb (α-IL-17) and isotype control mAb (IgG) were administered on days 1 through 3 of the OVA i.n. challenge.). B. H&E-stained lung sections were scored for levels of peribronchial inflammation. C. Numbers of total cells, eosinophils and neutrophils in BAL fluids were evaluated 24 hrs after OVA challenge. BAL cells were stained with Wright’s modified stain and counted according to standard morphological criteria. The data represent means ± SEM; 8 mice/group. ND, none detected. D. Cytokine levels in T-bet−/− lungs following induction of allergic lung inflammation and treatment with neutralizing anti-IL-17 mAb. Cytokine levels were measured by CBA (IL-5, IL-4, and IL-13) and by ELISA (IL-17). The data represent means ± SEM; 6 mice/group; **P<0.01. E. Mch dose-response curves 24 hrs following 5 day i.n. OVA challenge. The data represent means ± SEM; 6 mice/group; **P<0.01.
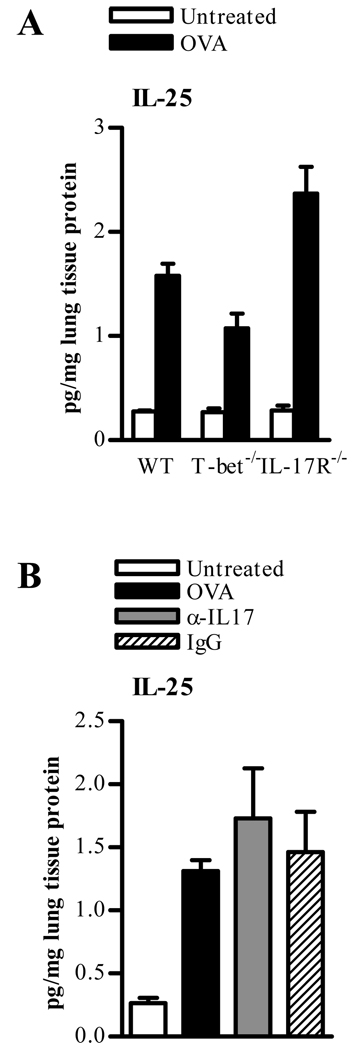
We considered the possibility that other members of the IL-17 cytokine family could have the potential to enhance airway inflammation. IL-25 (IL-17E), a Th2 cell-derived cytokine, is structurally related to IL-17 and uses the IL-17R as a receptor component (30). In addition, IL-25 enhances antigen-induced allergic airway inflammation by promoting Th2-type inflammatory responses (31). In order to determine its potential role, IL-25 was measured in WT, T-bet−/−, and IL-17R−/− OVA-induced and challenged mice. IL-25 expression in T-bet−/− mice following OVA challenge was somewhat attenuated compared with WT mice (Fig. 6A), confirming that Th2 cells are not as active in T-bet−/− mice at this stage of allergen challenge. However, a significant increase in IL-25 production in IL-17R−/− animals was observed following OVA challenge (Fig. 6A), presumably due to accumulation of the cytokine in the absence of functional signaling. Anti-IL-17A mAb treatment of T-bet−/− mice resulted in levels of IL-25 that were not significantly different from isotype control treated mice, indicating that IL-17 depletion targeted IL-17A only and not IL-25 (Fig. 6B).
FIGURE 6. IL-25 (IL-17E) production in the lungs of OVA-sensitized and challenged mice.
A. BALB/c WT, T-bet−/−, and IL-17R−/− mice were sensitized with OVA in alum, and 2 weeks later were challenged i.n. for 5 consecutive days with OVA. Twenty-four hrs after the last OVA inoculation, homogenates were prepared from lung tissue, protein levels were normalized to total lung protein, and IL-25 (IL-17E) was measured by ELISA. The data represent means ± SEM; 4 mice/group. B. IL-25 production in T-bet−/− mice sensitized and challenged with OVA. Neutralizing anti-IL-17A mAb (α-IL-17) and isotype control mAb (IgG) were administered in conjunction with OVA i.n. challenge. IL-25 (IL-17E) cytokine levels were measured by ELISA 24 hrs after the final OVA challenge. The data represent means ± SEM; 4 mice/group.
Taken together, these results indicate that IL-17 is the primary mediator of lung inflammation that is observed in the absence of T-bet and furthermore, that T-bet is essential for the inhibition of antigen-induced Th17 as well as Th2 cytokine production. Finally, neutralization of IL-17 essentially reverses airway inflammation in T-bet−/− mice.
Discussion
The purpose of this study was to investigate the regulatory role of T-bet in allergen-induced asthma since variable expression of T-bet has been identified as a major factor associated with allergic asthma. We demonstrated that airway inflammation is enhanced in T-bet−/− mice following allergen challenge and that this inflammation is mediated by Th17 cells. Allergic inflammation was significantly decreased in T-bet−/− mice following treatment with neutralizing anti-IL-17 mAb and little disease was observed in IL-17R−/− mice. These results suggest a potential novel avenue for therapeutic intervention of human asthma.
Our results show that T-bet has a crucial role following specific allergen provocation, as demonstrated by an increase in goblet cell hyperplasia and mucus production in T-bet−/− mice challenged with OVA, as well as enhanced granulocytic cellular infiltration into the airways, specifically eosinophils, neutrophils and lymphocytes, and an increase in bronchial responsiveness. Indeed, these results mirror reports from human asthmatic patients (9–11). In addition, our results confirm previous observations that BALB/c T-bet−/− mice do not spontaneously develop airway inflammation as compared to C57BL/6 T-bet−/− mice (32). The reasons for this difference may reflect genetic background bias related to the ability to mount Th1- versus Th2-dominant responses (33) or could potentially be due to differences in animal husbandry between different laboratories (34). In any case, the observation of increased airway inflammation following allergen challenge in T-bet−/− mice, as well as the strong correlation between T-bet deficiencies and development of asthma in humans, suggests that dysfunction of T-bet expression may be involved in the pathogenesis of allergic asthma.
T-bet is considered to be the master regulator of Th1 differentiation. In addition to inducing IFN-γ production, T-bet also has the ability to suppress Th2 cytokine production (8). Surprisingly, we did not observe a correlation between Th2 cytokine production and enhanced inflammation in T-bet−/− mice following a 5 day inhalation challenge with OVA. On the other hand IL-4, IL-5 and IL-13 were significantly up regulated in T-bet+/+ mice following allergen challenge. It has previously been reported that in the absence of T-bet, IL-13 production is enhanced and is responsible for airway remodeling in the asthmatic airway (35). We also saw increases in IL-13 expression in T-bet−/− mice, increases that might have played a role in the increased bronchial hyper-responsiveness and changes in the smooth muscle cells of the airway that were observed. However, the increased infiltration of inflammatory cells into the airways, which likely was the cause of more severe pulmonary damage, is unlikely to be explained only by the changes in IL-13 production. Thus, our results suggest that BALB/c T-bet−/− mice do not develop enhanced airway inflammation solely through induction of Th2 cytokines.
In addition to enhanced eosinophil infiltration, neutrophilic infiltration was also increased in OVA-challenged T-bet−/− mice. Considering that IL-17 can induce neutrophil migration (12) and that IL-17 has been associated with severe asthma pathogenesis (36),we investigated the potential role of IL-17 in the disease phenotype of T-bet−/− animals. IL-17 cytokine levels were upregulated in both T-bet+/+ and the T-bet−/− lungs after allergen challenge and lymphocytes obtained from the lungs of these mice also demonstrated increased IL-17 production. Surprisingly, however, IL-17 expression was significantly more pronounced in T-bet−/− versus T-bet+/+ mice. The majority of the IL-17-expressing cells were CD3+. In addition to the increased presence of CD4+IL-17+ lymphocytes, there was a partial contribution of IL-17 production among the CD4−CD8− population, suggesting that in addition to Th17 cells, NKT cells may also play a role, as recently reported (37). Our data parallels previous results by McKinley et al. (38) that IL-17 producing cells are potential mediators of airway hyper-responsiveness and inflammation in the lung. These studies also suggest that Th17 cells are steroid resistant. While it is unknown whether, in the absence of T-bet, Th17 cells are steroid resistant or not, we have demonstrated that Th17 cells are more predominant. Taken together these data underscore the necessity for better therapeutics. Ultimately, these data suggest that IL-17 has a crucial role in enhanced airway inflammation in the absence of T-bet regulation. Indeed, following OVA sensitization and challenge, IL-17R−/− mice were found to have an inability to mount an immunological response to allergen compared to IL-17R+/+ mice. Previous studies have similarly shown that IL-17R signaling is crucial for optimal pulmonary defense against K. pneumoniae infection (27, 39) as well as for asthma development (40).
Since our results suggested a pivotal role for IL-17-induced neutrophil recruitment in mediating the enhanced allergic lung inflammation observed in the absence of T-bet expression, we tested whether lung inflammation could be reversed in T-bet−/− mice by pulmonary delivery of anti-IL-17 mAb during allergic challenge. It was found that administration of anti-IL-17 mAb abrogated the influx of neutrophils into the bronchial lining and essentially prevented development of pulmonary inflammation. At the same time, anti-IL-17 mAb treatment had a minor effect on eosinophilic airway infiltration and Th2 cytokine production. These results suggest that in mice lacking T-bet, IL-17 has a predominant effect on inducing recruitment of neutrophils into the airways and that this then promotes subsequent inflammatory responses. Thus, blocking IL-17 in certain individuals who are prone to asthma development may represent a novel therapeutic approach for prevention of pulmonary inflammation.
Our results in mice lacking the IL-17R clearly show that IL-17 is required for the initiation of allergic asthma and confirm previous studies of others (40). OVA-sensitized and challenged IL-17R−/− mice exhibited a deficiency in pulmonary cytokine production and airway inflammation compared to OVA-sensitized and challenged WT animals. Administration of anti-IL-17 mAb to T-bet−/− mice during the challenge phase also had dramatic effects on neutrophil recruitment and on the peripheral airway response to methacholine. However, neutralization of IL-17 did not suppress Th2 responses as was seen in IL-17R−/− animals. The possibility that Th17 cells can convert to Th2 cells following anti-IL-17 mAb treatment cannot be ruled out in our model. However, the Th2 cytokine levels do not appear to be restored to WT levels following OVA challenge (compare Fig 2 and Fig 5). It is interesting to note that the levels of IL-25 (IL-17E) were elevated in IL-17R−/− mice. IL-25 is known to enhance Th2 responses and since it signals through a receptor complex consisting of the IL-17RB subunit (41), it would lose this capability in the absence of IL-17R. Thus, while signaling by IL-17 and IL-25 would be lost in IL-17R−/− mice, IL-25 would still be functionally active in anti-IL-17 treated animals. This may explain the differential effects on Th2 cytokine expression observed in IL-17R−/− mice compared to mice treated with neutralizing anti-IL-17A mAb. Indeed our data suggest that neutralizing IL-17 would be beneficial in reducing allergic asthma, however, Schnyder-Candrian et al. demonstrate that eosinophilic asthma was exacerbated by neutralizing IL-17A (40). The model used is a Th2 cell-mediated and eosinophil-dominant murine asthma model. Our results suggest that in the absence of T-bet, our asthma model is mediated by IL-17 and that this production of IL-17 is more potent than a Th2 cytokine production (see Fig. 2). These data demonstrate the effector role of IL-17 in regulating neutrophilic as well as eosinophilic infiltration and subsequent inflammation. By blocking effector role of IL-17 in the early stages alleviates allergic lung inflammation. Considering that asthmatic patients demonstrate decreased levels of T-bet expression (9), our model becomes relevant to human asthmatics. In addition to IL-17, IL-6 may also be a potential upstream target for alleviation of allergic lung inflammation. IL-6 is known to be necessary for Th17 cell differentiation and there is an increase of Th17 cells in our model. On the other hand, there is no apparent role for CD8 T cells in this model. Ultimately, these results further highlight the role of IL-17 in mediating enhancement of allergen induced airway inflammation during the challenge phase of experimental allergic asthma in the absence of T-bet.
IL-17 has been shown to be produced in the airways in an allergic lung inflammation model and to be necessary for neutrophilic recruitment (14). Our results confirm these results and furthermore, clearly define the role of IL-17 in the increased severity of inflammation that is observed in the absence of T-bet. The importance of T-bet in allergic asthma is underscored by recent studies from asthmatic patients who as a group, have decreased T-bet expression (10, 11). Previously it was shown that production of IL-17 can occur in T-bet−/− mice (42). Our study is now the first to demonstrate that neutralization of IL-17 during allergen challenge in the absence of T-bet regulation strongly inhibits airway inflammation. Thus, T-bet in wild-type animals appears to potently inhibit not only Th2 cell-mediated pulmonary inflammation but also IL-17-mediated neutrophilic inflammation. In fact, our results demonstrate that the enhanced airway inflammation following allergen challenge in the absence of T-bet is dependant upon IL-17 function. These findings suggest that therapeutic strategies aimed at reducing IL-17 production will be useful for suppressing allergic inflammatory responses and other pulmonary disorders characterized by inappropriate T cell activation. Targeting both Th2 cytokines as well as IL-17 may ultimately lead to a more effective therapeutic intervention.
Supplementary Material
Acknowledgments
We would like to thank the Immunology Core of the Center for Immunology and Microbial Disease for histology services and flow cytometry. We also thank Amgen for IL-17R−/− mice and neutralizing IL-17 mAb.
Footnotes
This work was supported by NIH grants RO1 AI41715 (to D.W.M.) and RO1 AR054389 and DE018822 (to S.L.G.)
Disclosures
The authors have no financial conflict of interest.
References
- 1.Eapen SS, Busse WW. Asthma. Clin Allergy Immunol. 2002;16:325–353. [PubMed] [Google Scholar]
- 2.Herrick CA, Bottomly K. To respond or not to respond: T cells in allergic asthma. Nat Rev Immunol. 2003;3:405–412. doi: 10.1038/nri1084. [DOI] [PubMed] [Google Scholar]
- 3.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 4.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 5.Iwamoto I, Kumano K, Kasai M, Kurasawa K, Nakao A. Interleukin-12 prevents antigen-induced eosinophil recruitment into mouse airways. Am J Respir Crit Care Med. 1996;154:1257–1260. doi: 10.1164/ajrccm.154.5.8912732. [DOI] [PubMed] [Google Scholar]
- 6.Gavett SH, O'Hearn DJ, Li X, Huang SK, Finkelman FD, Wills-Karp M. Interleukin 12 inhibits antigen-induced airway hyperresponsiveness, inflammation, and Th2 cytokine expression in mice. J Exp Med. 1995;182:1527–1536. doi: 10.1084/jem.182.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 8.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 9.Finotto S, Neurath MF, Glickman JN, Qin S, Lehr HA, Green FH, Ackerman K, Haley K, Galle PR, Szabo SJ, Drazen JM, De Sanctis GT, Glimcher LH. Development of spontaneous airway changes consistent with human asthma in mice lacking T-bet. Science. 2002;295:336–338. doi: 10.1126/science.1065544. [DOI] [PubMed] [Google Scholar]
- 10.Raby BA, Hwang ES, Van Steen K, Tantisira K, Peng S, Litonjua A, Lazarus R, Giallourakis C, Rioux JD, Sparrow D, Silverman EK, Glimcher LH, Weiss ST. T-bet polymorphisms are associated with asthma and airway hyperresponsiveness. Am J Respir Crit Care Med. 2006;173:64–70. doi: 10.1164/rccm.200503-505OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munthe-Kaas MC, Carlsen KH, Haland G, Devulapalli CS, Gervin K, Egeland T, Carlsen KL, Undlien D. T cell-specific T-box transcription factor haplotype is associated with allergic asthma in children. J Allergy Clin Immunol. 2008;121:51–56. doi: 10.1016/j.jaci.2007.07.068. [DOI] [PubMed] [Google Scholar]
- 12.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawaguchi M, Onuchic LF, Li XD, Essayan DM, Schroeder J, Xiao HQ, Liu MC, Krishnaswamy G, Germino G, Huang SK. Identification of a novel cytokine, ML-1, and its expression in subjects with asthma. J Immunol. 2001;167:4430–4435. doi: 10.4049/jimmunol.167.8.4430. [DOI] [PubMed] [Google Scholar]
- 14.Hellings PW, Kasran A, Liu Z, Vandekerckhove P, Wuyts A, Overbergh L, Mathieu C, Ceuppens JL. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am J Respir Cell Mol Biol. 2003;28:42–50. doi: 10.1165/rcmb.4832. [DOI] [PubMed] [Google Scholar]
- 15.Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Page N, Olivenstein R, Elias J, Chakir J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001;108:430–438. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 16.Shen F, Zhao MW, He B, Wang YZ, Yao WZ. The levels and clinical implications of induced sputum interleukin-17 in chronic obstructive pulmonary disease and asthma. Zhonghua Nei Ke Za Zhi. 2004;43:888–890. [PubMed] [Google Scholar]
- 17.Sharkhuu T, Matthaei KI, Forbes E, Mahalingam S, Hogan SP, Hansbro PM, Foster PS. Mechanism of interleukin-25 (IL-17E)-induced pulmonary inflammation and airways hyper-reactivity. Clin Exp Allergy. 2006;36:1575–1583. doi: 10.1111/j.1365-2222.2006.02595.x. [DOI] [PubMed] [Google Scholar]
- 18.Liang SC, Long AJ, Bennett F, Whitters MJ, Karim R, Collins M, Goldman SJ, Dunussi-Joannopoulos K, Williams CM, Wright JF, Fouser LA. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J Immunol. 2007;179:7791–7799. doi: 10.4049/jimmunol.179.11.7791. [DOI] [PubMed] [Google Scholar]
- 19.Barczyk A, Pierzchala W, Sozanska E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respir Med. 2003;97:726–733. doi: 10.1053/rmed.2003.1507. [DOI] [PubMed] [Google Scholar]
- 20.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 21.Mathur AN, Chang HC, Zisoulis DG, Kapur R, Belladonna ML, Kansas GS, Kaplan MH. T-bet is a critical determinant in the instability of the IL-17-secreting T-helper phenotype. Blood. 2006;108:1595–1601. doi: 10.1182/blood-2006-04-015016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Langrish CL, McKenzie B, Joyce-Shaikh B, Stumhofer JS, McClanahan T, Blumenschein W, Churakovsa T, Low J, Presta L, Hunter CA, Kastelein RA, Cua DJ. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006;116:1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alouani S, Juillard P, Chvatchko Y. Murine model of allergic lung inflammation. Methods Mol Biol. 2000;138:285–293. doi: 10.1385/1-59259-058-6:285. [DOI] [PubMed] [Google Scholar]
- 24.Doherty TA, Soroosh P, Broide DH, Croft M. CD4+ cells are required for chronic eosinophilic lung inflammation but not airway remodeling. Am J Physiol Lung Cell Mol Physiol. 2009;296:L229–L235. doi: 10.1152/ajplung.90543.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 26.Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol. 1992;72:168–178. doi: 10.1152/jappl.1992.72.1.168. [DOI] [PubMed] [Google Scholar]
- 27.Bates JH, Wagers SS, Norton RJ, Rinaldi LM, Irvin CG. Exaggerated airway narrowing in mice treated with intratracheal cationic protein. J Appl Physiol. 2006;100:500–506. doi: 10.1152/japplphysiol.01013.2005. [DOI] [PubMed] [Google Scholar]
- 28.Tomioka S, Bates JH, Irvin CG. Airway and tissue mechanics in a murine model of asthma: alveolar capsule vs. forced oscillations. J Appl Physiol. 2002;93:263–270. doi: 10.1152/japplphysiol.01129.2001. [DOI] [PubMed] [Google Scholar]
- 29.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM, Rennick DM. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 31.Tamachi T, Maezawa Y, Ikeda K, Kagami S, Hatano M, Seto Y, Suto A, Suzuki K, Watanabe N, Saito Y, Tokuhisa T, Iwamoto I, Nakajima H. IL-25 enhances allergic airway inflammation by amplifying a TH2 cell-dependent pathway in mice. J Allergy Clin Immunol. 2006;118:606–614. doi: 10.1016/j.jaci.2006.04.051. [DOI] [PubMed] [Google Scholar]
- 32.Fujiwara M, Hirose K, Kagami S, Takatori H, Wakashin H, Tamachi T, Watanabe N, Saito Y, Iwamoto I, Nakajima H. T-bet inhibits both TH2 cell-mediated eosinophil recruitment and TH17 cell-mediated neutrophil recruitment into the airways. J Allergy Clin Immunol. 2007;119:662–670. doi: 10.1016/j.jaci.2006.12.643. [DOI] [PubMed] [Google Scholar]
- 33.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 34.Fallon PG. Why does work on same mouse models give different results? Nature. 2008;454:691. doi: 10.1038/454691c. [DOI] [PubMed] [Google Scholar]
- 35.Finotto S, Hausding M, Doganci A, Maxeiner JH, Lehr HA, Luft C, Galle PR, Glimcher LH. Asthmatic changes in mice lacking T-bet are mediated by IL-13. Int Immunol. 2005;17:993–1007. doi: 10.1093/intimm/dxh281. [DOI] [PubMed] [Google Scholar]
- 36.Molet SM, Hamid QA, Hamilos DL. IL-11 and IL-17 expression in nasal polyps: relationship to collagen deposition and suppression by intranasal fluticasone propionate. Laryngoscope. 2003;113:1803–1812. doi: 10.1097/00005537-200310000-00027. [DOI] [PubMed] [Google Scholar]
- 37.Kim HY, Pichavant M, Matangkasombut P, Koh YI, Savage PB, DeKruyff RH, Umetsu DT. The development of airway hyperreactivity in T-bet-deficient mice requires CD1d-restricted NKT cells. J Immunol. 2009;182:3252–3261. doi: 10.4049/jimmunol.0803339. [DOI] [PubMed] [Google Scholar]
- 38.McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, Henry A, Irvin CG, Piganelli JD, Ray A, Kolls JK. Th17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181:4089–4097. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001;25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 40.Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, Fossiez F, Ryffel B, Schnyder B. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006;203:2715–2725. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rickel EA, Siegel LA, Yoon BR, Rottman JB, Kugler DG, Swart DA, Anders PM, Tocker JE, Comeau MR, Budelsky AL. Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities. J Immunol. 2008;181:4299–4310. doi: 10.4049/jimmunol.181.6.4299. [DOI] [PubMed] [Google Scholar]
- 42.Furuta S, Kagami S, Tamachi T, Ikeda K, Fujiwara M, Suto A, Hirose K, Watanabe N, Saito Y, Iwamoto I, Nakajima H. Overlapping and distinct roles of STAT4 and T-bet in the regulation of T cell differentiation and allergic airway inflammation. J Immunol. 2008;180:6656–6662. doi: 10.4049/jimmunol.180.10.6656. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.