Abstract
The epidemic of obesity sweeping developed nations is accompanied by an increase in atherosclerotic cardiovascular diseases. Dyslipidemia, diabetes, hypertension and obesity are risk factors for cardiovascular disease. However, delineating the mechanism of obesity-accelerated atherosclerosis has been hampered by a paucity of animal models. Similar to humans, apolipoprotein E deficient (apoE−/−) mice spontaneously develop atherosclerosis over their lifetime. To determine if apoE−/− mice would develop obesity with accelerated atherosclerosis, we fed mice diets containing 10 (LF) or 60 (HF) kcal % from fat for 17 weeks. Mice fed the HF diet had a marked increase in body weight and atherosclerotic lesion formation compared to mice fed the LF diet. There were no significant differences between groups in serum total cholesterol, triglycerides, or leptin concentrations. Plasma concentrations of the acute phase reactant serum amyloid A (SAA) are elevated in both obesity and cardiovascular disease. Accordingly, plasma SAA concentrations were increased 4.0 fold (P < 0.01) in mice fed the HF diet. SAA was associated with both pro- and anti-atherogenic lipoproteins in mice fed the HF diet compared to those fed the LF diet, in which SAA was primarily associated with the anti-atherogenic lipoprotein high density lipoprotein (HDL). Moreover, SAA was localized with apolipoprotein (apo)B-containing lipoproteins and biglycan in the vascular wall. Taken together, these data suggest male apoE deficient mice are a model of metabolic syndrome and that chronic low level inflammation associated with increased SAA concentrations may mediate atherosclerotic lesion formation.
Introduction
The prevalence of obesity is at an epidemic proportion in the U.S. and is accompanied by an increase in morbidity and mortality from cardiovascular diseases, including atherosclerosis. Obesity is often associated with high prevalence of traditional risk factors for atherosclerosis including hyperlipidemia, hypertension, and insulin resistance, as well as with emerging risk factors such as chronic inflammation. However, the mechanisms by which obesity increases atherosclerosis are not fully understood, in part due to a paucity of animal models of obesity accelerated atherosclerosis.
Although obesity is associated with increased prevalence of metabolic perturbations and increased cardiovascular risk, not all subjects with obesity have these abnormalities (often termed obese, metabolically normal), and some subjects with normal BMI have considerable metabolic abnormalities (termed normal weight, metabolically obese). Accumulating evidence suggests that the location of the excess adipose tissue is of importance to the development of metabolic dysfunction, with abdominal or visceral fat depots thought to convey the greatest risk. Clinical investigations have played a key role in identifying these associations, but animal models are necessary to elucidate the mechanisms by which obesity accelerates atherosclerosis.
Recent studies have demonstrated that obesity is associated with chronic inflammation of the adipose tissue itself, which is thought to contribute to systemic inflammation, and possibly play a role in the development and progression of atherosclerosis. A number of pro-inflammatory molecules are secreted by adipocytes and other cells resident within adipose tissue. Moreover, transplantation of visceral fat from obese mice into lean apoE−/− mice increased inflammatory markers, including leptin and monocyte chemoattractant protein-1, and atherosclerotic lesion formation(1). Leptin, an adipokine having a profound effect on appetite regulation, is a marker of total body fat. Although some investigators have proposed that leptin may play a role in atherogenesis(2, 3), this has not been supported by other studies(4). Adipose tissue macrophages have been shown to contribute to the pathogenesis of insulin resistance observed in obesity(5, 6). However, the link between insulin resistance or hyperglycemia with atherosclerosis and cardiovascular disease is also unclear. Obesity is associated with modestly but chronically elevated plasma concentrations of acute phase reactants SAA and C reactive protein (CRP) (7, 8), which have been shown to be predictive of cardiovascular disease events. SAA has been proposed to play a role in atherogenesis, whereas there is no evidence that CRP has a direct pro-atherogenic role at this time(9–11). Reductions in body weight decrease serum SAA concentrations(8, 12, 13) and the reduction in SAA concentrations in response to caloric restriction is independent of dietary composition(13) suggesting a direct link of SAA concentrations with adipose mass. Data from a small clinical study suggests that increased SAA production from adipose tissue of obese individuals significantly contributes to the systemic SAA pool(8). Therefore, adipose tissue SAA may play a critical role in the development and/or progression of atherosclerosis.
Animal models of obesity accelerated atherosclerosis are necessary to elucidate the mechanisms involved in the pathogenesis of atherosclerosis. Rodent models are desirable due to their short lifespan, sequenced genome with availability of genetic manipulation, and relative low cost. However, models that reliably develop features of the metabolic syndrome and obesity-accelerated atherosclerosis are lacking. The goal of this study was to develop a murine model of diet-induced obesity with accelerated atherosclerosis. Similar to humans, apoE−/− mice develop atherosclerosis spontaneously over their lifetime. Despite previous studies that have reported that apoE−/− mice are resistant to diet-induced obesity and accelerated atherosclerosis(14, 15), we demonstrate that diets high in saturated fat induce obesity, insulin resistance, modest systemic inflammation and accelerated atherosclerosis in apoE−/− mice.
Materials and Methods
Animals
Male apoE −/− mice backcrossed 10 times onto a C57BL/6 background were obtained from The Jackson Laboratories (Bar Harbor, Maine). Mice were housed in specific pathogen-free rooms and fed a normal laboratory diet (Harlan Teklad diet 2918) prior to commencement of studies. All studies were approved by the University of Kentucky IACUC. Eight week old male apoE−/− mice were fed diets containing 10 or 60% kcal from fat (D124508 and D12492, respectively; Research Diets, New Brunswick, NJ). These diets contain no added cholesterol, and thus their total cholesterol content is 0.002% and 0.003% by weight respectively (compare to 0.15% cholesterol by weight in the standard atherogenic Western diet TD88137, Harlan Teklad). The mice received diets ad lib for 17 weeks and had free access to water. Systolic blood pressure was measured in conscious mice by tail cuff using a Visitech BP-2000 Platform(16). Systolic pressure was defined as the mean of 10 individual measurements for each individual mouse per recording session. Mice were acclimated for 5 days prior to data collection. Blood pressure was measured 5 days during the final week (week 17) of diet.
Metabolic analyses
Intraperitoneal glucose tolerance test (IPGGT) was performed in all mice after 16 weeks. Mice were fasted overnight (12 hrs) then injected IP with glucose (20% solution) at a dose of 2g glucose/kg body weight. Plasma glucose was measured by glucometer (Freestyle) prior to and at 30, 60, 90 and 120 min after glucose injection. After 17 weeks of diet mice were killed, blood was collected and plasma leptin, SAA, and adiponectin concentrations were measured by specific ELISAs or Milliplex Assay (Linco Research, Biosource, and Millipore respectively) according to manufacturers' directions. SAA isoforms were characterized by isoelectric focusing, as described previously(17). Serum cholesterol and triglyceride concentrations were measured by enzymatic colorimetric assay (Wako Chemical Company) as described previously. Plasma aliquots from individual mice were separated by FPLC as previously described(18) and cholesterol and triglyceride content of each fraction quantified. Equal aliquots of fractions from the peaks of each lipoprotein compartment (VLDL, LDL, and HDL) were analyzed by Western blot for SAA content (antibody from Lab Logix Inc., Belmont, CA). Liver triglyceride content was extracted by Bligh Dyer Method and triglycerides measured by enzymatic colorimetric assay (Wako Chemical).
Cytokine/Chemokine analyses
Plasma MCP-1, IL-6, and TNF-α concentrations were measured by Milliplex assay (Millipore). Total RNA was isolated from mouse liver using the Aurum Total RNA Mini Kit (BioRad). 1 μg of RNA was reverse transcribed into cDNA using an iScript cDNA Synthesis Kit (BioRad). After 20-fold dilution, 5 μl was used as a template for Q-PCR. Primers used in this study are listed in Supplemental Table I. Amplification was done for 40 cycles using iQ SYBR Green Supermix (BioRad) on a My iQ Cycler (BioRad). Both internal control (18S rRNA) and negative control (minus reverse transcriptase) were included. Values of each RNA sample were the average of triplicate assays normalized toward 18S rRNA (internal control) levels.
Atherosclerosis quantification
Mouse aortas were removed and fixed in paraformaldehyde overnight. Aortas were cut, pinned, and photographed for en face measurements of atherosclerosis. Aortic root sections were collected beginning at the appearance of the valve leaflets, stained with Oil Red O, and lesions were quantified over 400 um. Images of aortas and aortic roots were captured by a digital camera (Nikon DXM1200), and lesion area was measured by Image-Pro software. Quantitative analysis of atherosclerosis was performed as described previously(19). Adjacent sections were immunostained using antibodies against apoB (BioDesign, Saco, ME), biglycan (R&D Systems, Minneapolis, MN) and SAA (Santa Cruz Biotechnology, Santa Cruz, CA) as previously described(11, 20)
Statistical analyses
Data were analyzed by Student's t test or ANOVA with repeated measures, as appropriate. Significant interactions identified by ANOVA were analyzed using a Tukey post hoc test for all pairwise comparisons. Non-parametric data was analyzed by Mann-Whitney Rank sum test or significant interactions were analyzed using a Holm-Sidak multiple comparison, where appropriate. All data analyses were performed using SigmaStat 3.5 software (SPSS, Inc.) All data are represented as means ± standard error of means (SEM). P < 0.05 values were considered to be statistically significant.
Results
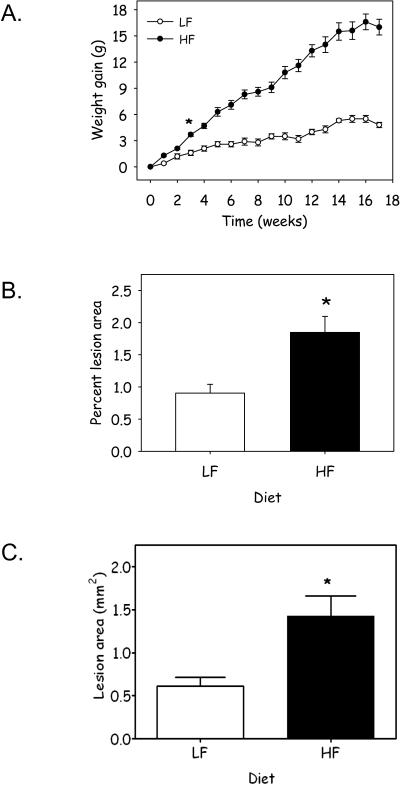
To determine the effect of obesity on atherosclerosis, 8 – 10 week old male apoE−/− mice were placed on either a low fat (LF), or high fat (HF) diet, 10 or 60 kcal % fat respectively, for 17 weeks. Body weight was measured weekly throughout the 17 week time course of the study. Body weight significantly differed between groups following 3 weeks of feeding the HF diet compared to LF diet (Figure 1A). Moreover, body weight increased linearly in mice fed the HF diet, throughout the time course of the study. Although, mice fed the LF diet consumed more food on a daily basis than those fed a HF diet; caloric consumption was increased in HF fed mice compared to those on the LF diet (Table 1). The distribution of adiposity was measured in liver, epididymal and retroperitoneal adipose tissue. Mice fed the HF diet had a marked increase in percent body weight of both epididymal and retroperitoneal adipose tissue depots; however, feeding a HF diet did not alter percent body weight of the liver (Table 1). To determine if the obese mice had increased atherosclerosis, atherosclerotic lesion area was quantified in the descending aorta and aortic sinus. Atherosclerosis was markedly increased in both sites in mice fed the HF diet (Figure 1B, P = 0.02; Figure 1C, P = 0.016; Supplemental Figure IA). Moreover, increased weight gain correlated with increased atherosclerotic lesion formation (Supplemental Figure IB; R2 = 0.287; P = 0.001.
Figure 1.
Feeding a HF diet increases (A) weight gain and atherosclerotic lesion formation in the (B) descending aorta and (C) aortic sinus in apoE−/− mice. Data represents mean ± SEM (n = 22 – 26 mice/group; *, denotes P ≤ 0.001; P = 0.02; P = 0.016, respectively).
Table 1.
Body and tissue weights
| LF Diet | HF Diet | P Value | |
|---|---|---|---|
| Body Weight (beginning, g) | 24.5 ± 0.5 | 23.8 ± 0.4 | NS |
| Body Weight (ending, g) | 29.5 ± 0.7 | 40.1 ± 1.0 | P < 0.001 |
| Epididymal Fat (Percent body weight) | 1.41 ± 0.16 | 4.30 ± 0.43 | P < 0.001 |
| Retroperitoneal Fat (Percent body weight) | 0.39 ± 0.04 | 1.85 ± 0.18 | P < 0.001 |
| Liver (Percent body weight) | 4.28 ± 0.28 | 4.11 ± 0.29 | NS |
| Food Consumption (g/day) | 3.57 ± 0.07 | 2.92 ± 0.03 | P < 0.001 |
| Caloric Density Consumption (kcal/g/day) | 13.46 ± 0.32 | 15.32 ± 0.17 | P < 0.001 |
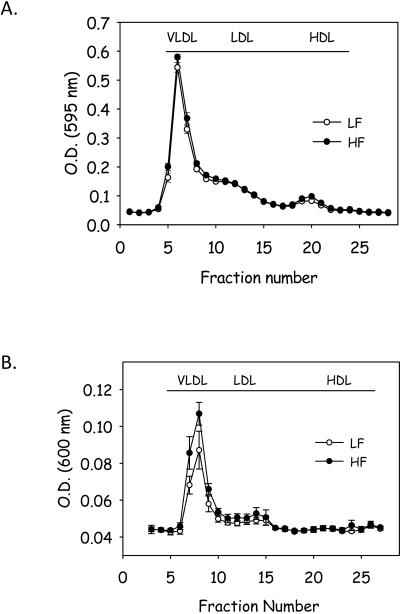
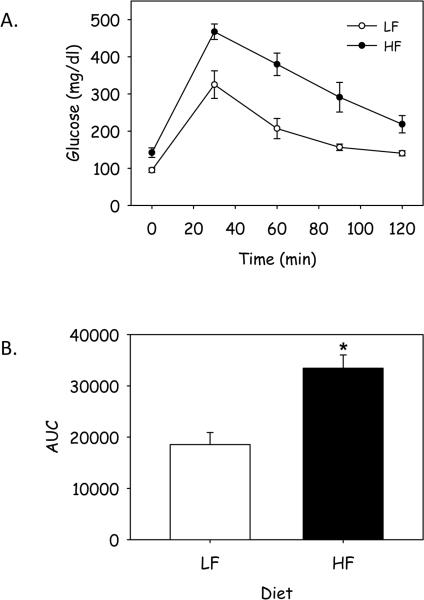
Total serum cholesterol concentrations were modestly, but not significantly increased in mice fed the HF diet (LF: 513 ± 15 vs HF: 601 ± 23 mg/dl; P = NS) and the cholesterol lipoprotein distribution was not different between the 2 groups (Figure 2A). Consumption of the HF diet for 17 weeks did not alter total plasma triglyceride concentrations compared to consumption of the LF diet (LF: 96 ± 31 vs HF: 101 ± 25 mg/dl, p = NS); however, the distribution of triglyceride among lipoproteins was modestly increased in VLDL fraction from mice fed the HF diet (Figure 2B). Moreover, liver content of triglyceride was increased in mice fed the HF diet (Supplemental Figure IIA). However, liver expression of a number of inflammatory genes was not changed in response to feeding a HF diet for 17 weeks (Supplemental Figure IIB). Previous studies have demonstrated that neither Western nor diabetogenic diets altered serum glucose concentrations in apoE−/− mice(15). In marked contrast, fasting glucose concentrations were markedly increased in male apoE−/− mice fed the HF diet for 17 weeks (LF: 103 ± 11 vs HF: 147 ± 7 mg/dl; P <0.001). To further characterize the hyperglycemia, we performed an IPGTT following 16 weeks of feeding the diets. Glucose levels peaked 30 minutes following IP loading of glucose (Figure 3A). There was a marked increase in the area under the curve (AUC) in mice fed the HF diet compared to LF diet demonstrating impaired glucose tolerance in mice fed the HF diet (Figure 3B, P = 0.003). These data suggest that obesity due to consumption of a HF diet induces glucose intolerance in male apoE−/− mice.
Figure 2.
Feeding a HF diet does not alter (A) cholesterol lipoprotein distribution, but (B) increased the distribution of triglyceride into VLDL in apoE−/− mice. Data represent mean ± SEM (n = 10 mice/group).
Figure 3.
Glucose tolerance is altered in apoE−/− mice fed a HF diet. A) Mice were injected with a 20% glucose solution (2 g glucose/kg body weight) and plasma glucose levels were measured by glucometer every 30 minutes over a 120 min time period. B) AUC was measured in individual mice. Data represents mean ± SEM (n = 13 mice/group). *, denotes P = 0.003.
Systolic blood pressure was measured during the final week of diets and did not differ between the 2 groups of mice (LF: 118 ± 3 vs HF: 113 ± 1 mm Hg, P = NS). As expected, leptin concentrations were increased in obese HF-fed mice compared to lean mice (Table 2), although this did not reach statistical significance (P = 0.068). However, as expected increases in body weight are highly correlated (R2 = 0.9132; P < 0.001) with increases in leptin concentrations (data not shown). Obesity is associated with increased levels of inflammatory markers. Cytokines and chemokines, known to be associated with obesity, including adiponectin, MCP-1, IL-6 and TNF-α were measured after 17 weeks of diet, and no significant differences between groups were observed (Table 2). However, serum SAA concentrations were markedly elevated in mice fed the HF diet compared with LF diet (Figure 4A). Isoelectric focusing demonstrated that both acute phase SAAs (SAA1.1 and SAA2.1) were increased in serum from mice fed the HF diet compared to the LF diet (Figure 4B). Although SAA is primarily carried on HDL, previous studies have suggested that high fat diets may promote a redistribution of SAA to the pro-atherogenic lipoproteins VLDL and LDL(9, 21, 22). In agreement with these findings, western blot analyses demonstrated a marked increase in SAA content in VLDL and LDL fractions in mice fed the HF diet (Figure 4C).
Table 2.
Serum Adipokine Concentrations
| LF Diet | HF Diet | P Value | |
|---|---|---|---|
| Leptin (ng/ml) | 5.1 ± 1.4 | 17.4 ± 3.4 | P = 0.068 |
| Adiponectin (mg/ml) | 1.0 ± 0.5 | 1.1 ± 0.5 | P = 0.181 |
| MCP-1 (pg/ml) | 27.4 ± 5.0 | 18.3 ± 4.6 | P = 0.179 |
| IL-6 (pg/ml) | 26.3 ± 7.0 | 13.5 ± 2.0 | P = 0.249 |
| TNF-α (pg/ml) | 5.5 ± 0.6 | 5.5 ± 0.5 | P = 0.949 |
Figure 4.
A HF diet increases (A) plasma concentrations of SAA. Data represent the mean ± SEM (n = 22 – 26 mice/group). *, denotes P = 0.01. (B) IEF demonstrates that both SAA 1.1 and 2.1 expression are increased in plasma from mice fed a HF diet. Shown are representative blots with each lane representing one mouse from each group. (C) Immunoblot analysis demonstrates that SAA is highly associated with plasma VLDL and LDL in mice fed a HF diet. Shown are representative blots from one mouse from each group. Lanes were loaded with equal aliquots of (B) plasma and (C) FPLC fractions. (D) Immunocytochemistry demonstrates SAA co-localizes with biglycan and apoB. Shown are representative adjacent sections from an atherosclerotic lesion from an apoE−/− mice fed a HF diet. (200× magnification).
Recently, we demonstrated that SAA stimulated the synthesis of vascular proteoglycans, increased their LDL binding affinity, and specifically up-regulated biglycan(11). We proposed that this would be pro-atherogenic as increased vascular proteoglycan (biglycan) content would be expected to lead to increased lipoprotein retention(23–26). Accordingly, immunohistochemical analyses of atherosclerotic lesions demonstrated co-localization of SAA with biglycan and apoB within atherosclerotic lesions from mice fed a HF diet (Figure 4D).
Discussion
Taken together, these data demonstrate that consumption of a HF diet promotes obesity accelerated atherosclerosis in apoE−/− mice, accompanied by development of a metabolic syndrome phenotype. Similar to humans, the obese mice developed obesity, impaired fasting glucose, impaired glucose tolerance, modest dyslipidemia and increased levels of the inflammatory marker SAA. The single major feature of metabolic syndrome lacking in this model is that of hypertension. Plasma leptin concentrations were increased in mice that became obese as expected, albeit this increase did not reach statistical significance. Thus, apoE−/− mice fed HF diets are an animal model of obesity with accelerated atherosclerosis.
Furthermore, this murine model allows some understanding of mechanisms by which obesity may accelerate atherosclerosis development. The LF and HF-fed mice did not differ in many of the major known risk factors for atherosclerosis formation including total cholesterol levels, lipoprotein cholesterol distribution, or blood pressure. However, HF-fed mice did have impaired glucose tolerance, increased triglyceride-rich lipoprotein particles, a trend towards increased leptin levels, and markedly increased plasma SAA levels with altered SAA lipoprotein distribution compared to LF-fed mice. Any or all of these metabolic factors may have contributed to the atherosclerosis development. Although the impaired glucose tolerance observed in HF-fed mice may contribute towards the increased atherosclerosis observed, large clinical trials have struggled to clearly identify a link between glucose concentrations and cardiovascular diseases. Similarly, clinical studies are controversial as to whether elevated plasma triglycerides are a risk factor for cardiovascular disease. Although we did not find a difference in total plasma triglycerides between groups, we did observe increased triglyceride rich lipoproteins and increased hepatic triglycerides in HF-fed mice, and thus we cannot exclude a contribution of these lipoproteins to the increased atherosclerosis observed. The role of leptin in atherosclerosis formation is also controversial. The leptin receptor is expressed on vascular cells under both normal and pathological conditions, as well as on inflammatory cells that have migrated into the vascular wall during the development of atherosclerosis(27). Previous studies have demonstrated that exogenous administration of leptin augments atherosclerosis(28). Paradoxically, deficiency of either leptin or its receptor also increased lesion formation(29, 30). However, deficiency of leptin or its receptor resulted in marked increases in both plasma cholesterol and triglyceride concentrations that may have confounded interpretation of the direct effect of leptin on atherosclerotic lesion formation. Therefore, we can't exclude the possibility that increases in leptin may have contributed to the increase in atherosclerotic lesion formation observed in mice fed the HF diet. However, the most marked difference observed between HF and LF fed mice was in the total SAA concentration and its lipoprotein distribution. This increase in SAA was due to increased amounts of both acute phase isoforms, SAA1.1 and SAA2.1. During an acute phase response plasma SAA levels can increase up to a 1,000 fold. Interestingly, the 4 fold increase in plasma SAA observed in the obese mice is similar to the chronic modest elevations of SAA observed in obese patients (8, 12, 31).
Clinical studies have demonstrated an association between elevated levels of the acute phase proteins SAA and CRP with increased risk of cardiovascular events(32–34). CRP is not an acute phase protein in mice, but SAA protein and mRNA have been detected in both human and mouse atherosclerotic lesions(35). Moreover, SAA mRNA expression has been localized to smooth muscle cells, endothelial cells, and macrophages derived from atherosclerotic plaques(35). Previous studies demonstrated that plasma SAA concentrations correlate with atherosclerotic lesion size in hyperlipidemic mice(9, 21), and this correlation was independent of plasma cholesterol concentrations. While these studies do not prove a causal role for SAA in the development and progression of atherosclerosis, they are suggestive that SAA may play a fundamental role.
During an acute phase response the liver is the primary source of SAA production. The acute phase response in humans results in SAA association primarily with HDL. Interestingly, our data demonstrates that a large concentration of SAA associates with the pro-atherogenic lipoproteins VLDL and LDL in apoE−/− mice with diet-induced obesity. SAA association with VLDL and LDL is also markedly increased in LDL receptor deficient mice fed diets enriched in saturated fat(9, 22). We propose that both the elevated SAA and SAA lipoprotein redistribution observed in HF-fed mice may contribute to the increased atherosclerosis observed.
The response to retention hypothesis suggests that lipoprotein binding to proteoglycans in the subendothelial space is a key step in the development and/or progression of atherosclerosis(24, 36). This concept is supported by data demonstrating that atherosclerotic lesion formation is attenuated in mice expressing proteoglycan-binding-defective lipoproteins(36). SAA itself is able to bind proteoglycans, which is likely relevant to its role in vascular pathology(9, 21). The basic C-terminal residues of SAA are particularly important for its binding to proteoglycans. Additionally, we recently demonstrated that SAA increased proteoglycan synthesis by vascular smooth muscle cells, and in particular, up-regulated biglycan(11). This is of particular interest as we and others have demonstrated that biglycan is the proteoglycan most closely associated with apoB, thus may have a major role in atherosclerosis development(11, 20, 37, 38). In addition, SAA itself can bind to proteoglycans, and HDL containing SAA has greater proteoglycan binding than HDL without SAA(9, 21). Previous work by Flood et al has demonstrated that proteoglycan binding proteins can act co-operatively to increase lipoprotein-proteoglycan interactions(39). In the present study we demonstrate the co-localization of SAA in the subendothelial space with biglycan and apoB. These findings could be due to either increased retention of apoB-containing lipoprotein particles that also contain SAA (as in Figure 4C), or increased retention of lipoprotein particles due to SAA-induced upregulation of biglycan, or a combination of the above.
Previous studies have suggested that apoE−/− mice are resistant to the development of obesity; however, those studies used a diabetogenic diet(14, 15). Conversely, our data demonstrates that as early as 3 weeks following consumption of a diet enriched in lard, apoE−/− mice gain significant body weight, which increased linearly for the 17 week time course of these studies The diabetogenic diet, which did not cause obesity, contains 35.5 gm% fat and 36.3 gm% carbohydrate, primarily sucrose. The HF diet we used, which did cause obesity, was fairly similar in composition, containing 34.9 gm % fat and 26.3 gm% carbohydrate, also primarily sucrose. The concentration and the primary source of fat (lard) is similar between our HF diet and the previous diabetogenic diets, suggesting that the increase in body weight observed in the present study is not solely attributable to the fat in the diet and may be accounted for by differences in other constituents in the diet (14, 15). An important feature of the diets used in the present study is that they only differed in concentrations of constituents, rather than diet composition. In contrast, previous studies investigating the effect of diabetogenic diets on obesity and atherosclerotic lesion formation compared diets which had a marked difference in dietary constituents.
In summary, feeding apoE−/− mice a HF diet enriched in lard induces a metabolic phenotype which is characterized by obesity, modest dyslipidemia, impaired glucose tolerance and accelerated atherosclerosis. Similar to humans, the increase in obesity and increased cardiovascular disease is associated with increases in plasma SAA concentrations. We speculate that the increase in SAA and the association of SAA with pro-atherogenic lipoproteins in obese mice contributes to the increase in atherosclerotic lesion formation observed.
Supplementary Material
Acknowledgments
We would like to gratefully acknowledge the excellent technical support provided by Joanne Wroblewski, Ph.D. and Nathan Whitaker, M.B.A.
Sources of Funding: This work was supported in part by grants P20RR021954 (VLK), HL082772 (LRT) P01HL086670 (FCD).
Literature Cited
- 1.Ohman MK, Shen Y, Obimba CI, et al. Visceral Adipose Tissue Inflammation Accelerates Atherosclerosis in Apolipoprotein E-Deficient Mice. 2008:798–805. doi: 10.1161/CIRCULATIONAHA.107.717595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bodary PF, Gu S, Shen Y, Hasty AH, Buckler JM, Eitzman DT. Recombinant leptin promotes atherosclerosis and thrombosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:e119–22. doi: 10.1161/01.ATV.0000173306.47722.ec. [DOI] [PubMed] [Google Scholar]
- 3.Chiba T, Shinozaki S, Nakazawa T, et al. Leptin deficiency suppresses progression of atherosclerosis in apoE-deficient mice. Atherosclerosis. 2008;196:68–75. doi: 10.1016/j.atherosclerosis.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 4.Wu KK, Wu TJ, Chin J, et al. Increased hypercholesterolemia and atherosclerosis in mice lacking both ApoE and leptin receptor. Atherosclerosis. 2005;181:251–9. doi: 10.1016/j.atherosclerosis.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 5.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tannock LR, O'Brien KD, Knopp RH, et al. Cholesterol feeding increases C-reactive protein and serum amyloid A levels in lean insulin-sensitive subjects. Circulation. 2005;111:3058–62. doi: 10.1161/CIRCULATIONAHA.104.506188. [DOI] [PubMed] [Google Scholar]
- 8.Yang RZ, Lee MJ, Hu H, et al. Acute-Phase Serum Amyloid A: An Inflammatory Adipokine and Potential Link between Obesity and Its Metabolic Complications. PLoS Med. 2006;3:e287. doi: 10.1371/journal.pmed.0030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis KE, Kirk EA, McDonald TO, et al. Increase in serum amyloid a evoked by dietary cholesterol is associated with increased atherosclerosis in mice. Circulation. 2004;110:540–5. doi: 10.1161/01.CIR.0000136819.93989.E1. [DOI] [PubMed] [Google Scholar]
- 10.Tennent GA, Hutchinson WL, Kahan MC, et al. Transgenic human CRP is not pro-atherogenic, pro-atherothrombotic or pro-inflammatory in apoE−/− mice. Atherosclerosis. 2008;196:248–55. doi: 10.1016/j.atherosclerosis.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Wilson PG, Thompson JC, Webb NR, de Beer FC, King VL, Tannock LR. Serum amyloid A, but not C-reactive protein, stimulates vascular proteoglycan synthesis in a pro-atherogenic manner. Am J Pathol. 2008;173:1902–10. doi: 10.2353/ajpath.2008.080201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poitou C, Viguerie N, Cancello R, et al. Serum amyloid A: production by human white adipocyte and regulation by obesity and nutrition. Diabetologia. 2005;48:519–28. doi: 10.1007/s00125-004-1654-6. [DOI] [PubMed] [Google Scholar]
- 13.O'Brien KD, Brehm BJ, Seeley RJ, et al. Diet-induced weight loss is associated with decreases in plasma serum amyloid a and C-reactive protein independent of dietary macronutrient composition in obese subjects. J Clin Endocrinol Metab. 2005;90:2244–9. doi: 10.1210/jc.2004-1011. [DOI] [PubMed] [Google Scholar]
- 14.Schreyer SA, Lystig TC, Vick CM, LeBoeuf RC. Mice deficient in apolipoprotein E but not LDL receptors are resistant to accelerated atherosclerosis associated with obesity. Atherosclerosis. 2003;171:49–55. doi: 10.1016/j.atherosclerosis.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Schreyer SA, Vick C, Lystig TC, Mystkowski P, LeBoeuf RC. LDL receptor but not apolipoprotein E deficiency increases diet-induced obesity and diabetes in mice. Am J Physiol Endocrinol Metab. 2002;282:E207–14. doi: 10.1152/ajpendo.2002.282.1.E207. [DOI] [PubMed] [Google Scholar]
- 16.King VL, Trivedi DB, Gitlin JM, Loftin CD. Selective cyclooxygenase-2 inhibition with celecoxib decreases angiotensin II-induced abdominal aortic aneurysm formation in mice. Arterioscler Thromb Vasc Biol. 2006;26:1137–43. doi: 10.1161/01.ATV.0000216119.79008.ac. [DOI] [PubMed] [Google Scholar]
- 17.Beach CM, De Beer MC, Sipe JD, Loose LD, De Beer FC. Human serum amyloid A protein. Complete amino acid sequence of a new variant. Biochem J. 1992;282(Pt 2):615–20. doi: 10.1042/bj2820615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cole TG, Kitchens R, Daugherty A, Schonfeld G. An improved method for separation of triglyceride-rich lipoproteins by FPLC. Pharmacia Biocommunique. 1990;4:4–6. [Google Scholar]
- 19.Daugherty A, Whitman SC. Quantification of atherosclerosis in mice. Methods Mol Biol. 2003;209:293–309. doi: 10.1385/1-59259-340-2:293. [DOI] [PubMed] [Google Scholar]
- 20.Huang F, Thompson JC, Wilson PG, Aung HH, Rutledge JC, Tannock LR. Angiotensin II increases vascular proteoglycan content preceding and contributing to atherosclerosis development. J Lipid Res. 2008;49:521–30. doi: 10.1194/jlr.M700329-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.O'Brien KD, McDonald TO, Kunjathoor V, et al. Serum amyloid A and lipoprotein retention in murine models of atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:785–90. doi: 10.1161/01.ATV.0000158383.65277.2b. [DOI] [PubMed] [Google Scholar]
- 22.Subramanian S, Han CY, Chiba T, et al. Dietary cholesterol worsens adipose tissue macrophage accumulation and atherosclerosis in obese LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:685–91. doi: 10.1161/ATVBAHA.107.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gustafsson M, Levin M, Skalen K, et al. Retention of low-density lipoprotein in atherosclerotic lesions of the mouse: evidence for a role of lipoprotein lipase. Circ Res. 2007;101:777–83. doi: 10.1161/CIRCRESAHA.107.149666. [DOI] [PubMed] [Google Scholar]
- 24.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15:551–61. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–44. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 26.Tannock LR, King VL. Proteoglycan mediated lipoprotein retention: a mechanism of diabetic atherosclerosis. Reviews in endocrine & metabolic disorders. 2008;9:289–300. doi: 10.1007/s11154-008-9078-0. [DOI] [PubMed] [Google Scholar]
- 27.Schroeter MR, Schneiderman J, Schumann B, et al. Expression of the leptin receptor in different types of vascular lesions. Histochemistry and cell biology. 2007;128:323–33. doi: 10.1007/s00418-007-0319-1. [DOI] [PubMed] [Google Scholar]
- 28.Bodary PF, Gu S, Shen Y, Hasty AH, Buckler JM, Eitzman DT. Recombinant Leptin Promotes Atherosclerosis and Thrombosis in Apolipoprotein E-Deficient Mice. 2005:e119–22. doi: 10.1161/01.ATV.0000173306.47722.ec. [DOI] [PubMed] [Google Scholar]
- 29.Hasty AH, Shimano H, Osuga J, et al. Severe hypercholesterolemia, hypertriglyceridemia, and atherosclerosis in mice lacking both leptin and the low density lipoprotein receptor. J Biol Chem. 2001;276:37402–8. doi: 10.1074/jbc.M010176200. [DOI] [PubMed] [Google Scholar]
- 30.Wu KK, Wu T-J, Chin J, et al. Increased hypercholesterolemia and atherosclerosis in mice lacking both ApoE and leptin receptor. Atherosclerosis. 2005;181:251–9. doi: 10.1016/j.atherosclerosis.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 31.Gomez-Ambrosi J, Salvador J, Rotellar F, et al. Increased serum amyloid A concentrations in morbid obesity decrease after gastric bypass. Obesity surgery. 2006;16:262–9. doi: 10.1381/096089206776116525. [DOI] [PubMed] [Google Scholar]
- 32.Johnson BD, Kip KE, Marroquin OC, et al. Serum amyloid A as a predictor of coronary artery disease and cardiovascular outcome in women: the National Heart, Lung, and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109:726–32. doi: 10.1161/01.CIR.0000115516.54550.B1. [DOI] [PubMed] [Google Scholar]
- 33.Jousilahti P, Salomaa V, Rasi V, Vahtera E, Palosuo T. The association of c-reactive protein, serum amyloid a and fibrinogen with prevalent coronary heart disease--baseline findings of the PAIS project. Atherosclerosis. 2001;156:451–6. doi: 10.1016/s0021-9150(00)00681-x. [DOI] [PubMed] [Google Scholar]
- 34.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. The New England journal of medicine. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 35.Meek RL, Urieli-Shoval S, Benditt EP. Expression of apolipoprotein serum amyloid A mRNA in human atherosclerotic lesions and cultured vascular cells: implications for serum amyloid A function. Proc Natl Acad Sci U S A. 1994;91:3186–90. doi: 10.1073/pnas.91.8.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skalen K, Gustafsson M, Rydberg EK, et al. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature. 2002;417:750–4. doi: 10.1038/nature00804. [DOI] [PubMed] [Google Scholar]
- 37.O'Brien KD, Olin KL, Alpers CE, et al. Comparison of apolipoprotein and proteoglycan deposits in human coronary atherosclerotic plaques: colocalization of biglycan with apolipoproteins. Circulation. 1998;98:519–27. doi: 10.1161/01.cir.98.6.519. [DOI] [PubMed] [Google Scholar]
- 38.Kunjathoor VV, Chiu DS, O'Brien KD, LeBoeuf RC. Accumulation of biglycan and perlecan, but not versican, in lesions of murine models of atherosclerosis. Arterioscler Thromb Vasc Biol. 2002;22:462–8. doi: 10.1161/hq0302.105378. [DOI] [PubMed] [Google Scholar]
- 39.Flood C, Gustafsson M, Richardson PE, Harvey SC, Segrest JP, Boren J. Identification of the proteoglycan binding site in apolipoprotein B48. J Biol Chem. 2002;277:32228–33. doi: 10.1074/jbc.M204053200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.