Abstract
Introduction:
Genital shedding of herpes simplex virus type-2 (HSV-2) occurs frequently. Anatomic patterns of genital HSV-2 reactivation have not been intensively studied.
Methods:
4 HSV-2 seropositive women with symptomatic genital herpes attended clinic over a 30 day period. Daily samples were collected from 7 separate genital sites. Swabs were assayed for HSV DNA by quantitative polymerase chain reaction. Anatomic sites of clinical HSV-2 recurrences were recorded.
Results:
HSV was detected on 44 (37%) of 120 days and from 136 (16%) of 840 swabs. Lesions were documented on 35 (29%) of 120 days. HSV was detected at more than one anatomic site on 25 (57%) of 44 days with HSV shedding (median 2 sites, range 1-7), with HSV detected bilaterally on 20 (80%) of the 25 days. The presence of a lesion was significantly associated with detectable HSV from any genital site (IRR=5.41, 95%CI=1.24-23.50, p=0.02) and with the number of positive sites (IRR=1.19, 95%CI=1.01-1.40, p=0.03). The maximum HSV copy number detected was associated with the number of positive sites (IRR=1.62, 95%CI=1.44-1.82, p<0.001).
Conclusions:
HSV-2 reactivation occurs frequently at widely spaced regions throughout the genital tract. To prevent HSV-2 reactivation, suppressive HSV-2 therapy must control simultaneous viral reactivations from multiple sacral ganglia.
Keywords: HSV-2, subclinical shedding, genital ulcers
INTRODUCTION
Herpes simplex virus type 2 (HSV-2) is a common infection of the genital skin and mucosa. During primary infection, HSV infects genital epithelial cells (1), and then travels via sensory nerves to the sacral root ganglion, where lifelong latency is established (2, 3). Intermittent reactivation of HSV from the ganglia and lytic replication of virus in the epithelium is thought to result in viral shedding at the genital mucosa, either in the presence or absence of symptoms. Longitudinal studies in HSV-2 seropositive persons have shown that greater than 90% of persons reactivate HSV in the genital tract (4). Recurrences with genital ulceration and subclinical shedding are thought to occur predominantly at the site of primary acquisition (5, 6). The anatomic patterns of genital HSV reactivation and the resulting immune response to clear the virus may affect the risk of sexual transmission of HSV as well as the acquisition of viral co-pathogens, such as HIV-1.
The detection of HSV-2 reactivation depends on the frequency of sampling and the sites sampled, as well as the population under study. Among women, the most common sites of viral shedding are the vulva, cervix and perianal areas (6). Previous studies in women in which samples from cervicovaginal, vulvar, and rectal areas were grown in viral culture showed that HSV reactivation occurs over a period of days, and often involves more than one anatomic site (7). More recent studies have used polymerase chain reaction (PCR) to detect HSV from mucosal swabs, a technique that is 4 fold more sensitive for HSV detection than viral culture (8). These studies of viral shedding have used a “mixed” anogenital swab, in which swabs of the entire genital region (encompassing the vulva, cervix, perineum, and perianal region in women) were obtained. While this approach efficiently identifies HSV shedding in the genital region (9), it provides little information about specific anatomic sites and patterns of reactivation.
Recent modeling studies suggest that HSV-2 reactivates at overlapping genital sites from multiple ganglia (10). To determine the anatomic patterns of HSV reactivation and the frequency of overlapping reactivations in the presence and absence of clinically evident lesions, we conducted a study with detailed sampling of the female genitalia in a cohort of 4 women with symptomatic untreated genital HSV-2.
METHODS
Study population & data collection
Immunocompetent HSV-2 seropositive women were recruited from the University of Washington Virology Research Clinic, Seattle, WA. Participants with a history of symptomatic genital herpes who were not taking antiviral medication and who were willing to be seen daily for 30 consecutive days were eligible for the study. Participants had a daily detailed genital examination by an experienced clinician. If lesions were present, the lesion location was recorded on genital diagrams. Seven distinct sites were swabbed at each visit: right labia majora, right labia minora, left labia majora, left labia minora, cervix, urethra and perianal. Care was taken to avoid contamination from other anatomic areas during sampling. Samples were collected using Dacron swabs, which were placed into 1ml PCR tubes containing 1X digestion buffer and stored at 2 to 8°C (8). Participants kept a daily diary detailing genital symptoms (4). The Human Subjects Review Committee at the University of Washington approved the study. All subjects gave written informed consent.
Laboratory Methods
Serum was assayed by HSV Western Blot to detect antibodies to HSV-1 and HSV-2 (11). Quantitative HSV polymerase chain reaction (PCR) was performed on DNA extracted from genital swabs (12).
Statistical methods
Generalized estimating equations (GEE) with Poisson link and small-sample adjusted standard errors (13) were used to calculate incident rate ratios for the association between number of genital sites with HSV DNA detected or the maximum log10 copy number HSV DNA with the presence of lesions (outcome). Similar models were also used to examine whether maximum log10 copy number of HSV DNA was associated with the number of genital sites with HSV DNA detected (outcome). Small-sample adjustment was made since only 4 women and 120 observations were used in these regressions. Two-sided P values <0.05 were considered statistically significant. SAS for Windows (Version 9.1.3) was used for data analysis.
RESULTS
Characteristics of study population
Four HSV-2 seropositive, HIV seronegative women enrolled in the study. Women ranged in age from 22 to 26 years. All participants were Caucasian. Two participants were also HSV-1 seropositive. The median age at the first sexual encounter was 16 years (range 13-19), and the median number of lifetime partners was 14 (range 2-30). All participants had a history of symptomatic genital herpes, with a median of 1 year since documented primary infection (range 254 days-5.4 years). The median number of genital recurrences in the 6 months prior to study participation was 4 (range 0-9). None of the participants took antiviral medication during the study period.
HSV shedding and lesions by anatomic site
All four women attended the clinic for examination and swab collection every day for 30 consecutive days. HSV DNA was detected in 136 (16%) of 840 swabs and on 44 (37%) of the 120 days sampled. HSV was detected from the labial region on 27 (23%) of days, the perianal region on 25 (21%) of days, the cervix on 17 (14%) of days, and urethra on 15 (13%) of days sampled (Table 1). Of the 27 days with shedding from the labia, 6 (22%) were positive from only one site, 6 (22%) were positive from two or three sites, and 15 (56%) were positive from all 4 sites.
Table 1.
Site specific HSV-2 detection, by overall number of swabs collected and on days with lesions
| Site | Days with HSV detection (N= 120 days) |
Days with lesion (N=120 days) |
|---|---|---|
| Any labial | 27 (23) | 32 (27) |
| Cervix | 17 (14) | 0 (0) |
| Perianal | 25 (21) | 7 (6) |
| Periurethral | 15 (13) | 0 (0) |
| Any site | 44 (37) | 35 (29) |
Note: Values are listed as number (%)
Lesions were observed at one or more sites on 35 (29%) of 120 days at one or more sites (Table 1), with five distinct recurrences noted by clinicians. The majority of the recurrences involved the labia (32 (91%) of 35 days with lesions). HSV was detected on 26 (74%) of 35 days when a lesion was present. There were 9 days when an ulceration was clinically present but HSV DNA was not detected; these reflect a prolonged healing ulcer on the right labia minora in a single participant.
Of 44 days with HSV shedding, clinically apparent lesions were present on 26 (59%) days and absent on 18 (41%) days. HSV shedding was confined to one site on 18 (41%) of the positive days, and lesions were present on 12 (67%) of those days. The perianal area was the most common location with isolated shedding, representing 12 (67%) of the 18 days with a single site positive. HSV DNA was detected from a vulvar site on 4 (22%) and from the cervix on 2 (11%) of the 18 days with a single site positive.
Simultaneous HSV shedding from multiple anatomic sites
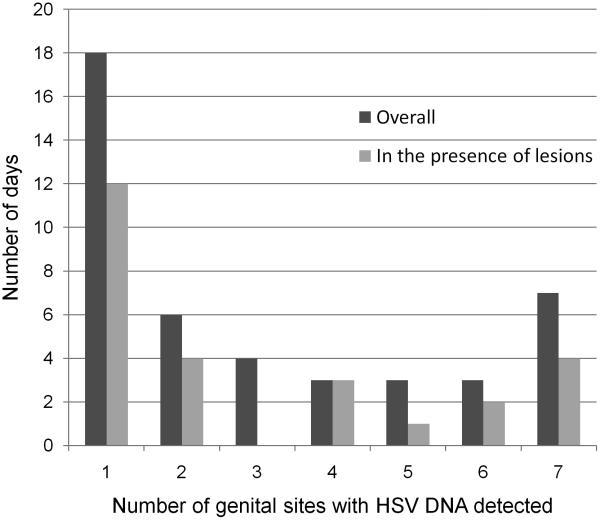
HSV was detected from more than one site on 26 (58%) of the 44 days with HSV shedding. The distribution of days with one or more sites with HSV detected overall and in the presence of lesions is shown in Figure 1. On 20 (77%) of 26 days, HSV DNA was detected from both the right and the left vulva: on 10 (50%) of these 20 days, lesions were present and on 10 (50%) of days lesions were absent (Table 2). HSV was found at all 7 sites on 7 (27%) of the 26 days with HSV shedding. In 4 of 7 (57%) days lesions were noted at one or more sites. Having detectable HSV shedding from any genital site was significantly associated with presence of a lesion (IRR=5.41, 95% CI=1.24-23.5, p=0.024). Similarly, the lesion detection rate increased for each additional genital site positive for HSV DNA (IRR=1.19, 95% CI 1.01-1.40, p=0.034). A one log increase in maximum copy number detected from the genital region was associated with a 1.62-fold increase in the number of sites positive (95%CI=1.44-1.82, p<0.001) and with a 1.27-fold increase risk in the probability of having lesions detected (95% CI=1.07-1.50, p=0.006). Figure 2 illustrates a representative anatomic pattern of HSV detection throughout the female genitalia over a 4 day period, prior to the development of a lesion and in the presence of a lesion. These areas of reactivation involve multiple, bilateral branches of the pudendal nerve.
Figure 1.
Distribution of number of genital sites with HSV detected
Table 2.
Pattern and amount of HSV DNA detected on days with more than one site positive for HSV.
| ID | Day | Urethra | LMN | LMJ | RMN | RMJ | Cervix | PA |
|---|---|---|---|---|---|---|---|---|
| 1 | 9 | 2.3 | 2.3 | 7.3 | ||||
| 10 | 2.3 | 3.3 | 3.3 | 3.3 | 2.3 | 6.3* | ||
| 11 | 3.3 | 5.3* | ||||||
| 19 | 2.3 | 6.3* | ||||||
| 20 | 2.3 | 3.3* | ||||||
| 23 | 2.3 | 6.3 | 5.3* | 4.3 | 4.3* | |||
| 25 | 2.3 | 3.3 | ||||||
| 26 | 7.3 | 5.3 | 4.3* | 5.3 | 3.3* | 3.3 | 3.3 | |
| 27 | 2.3 | 2.3 | 2.3 | |||||
| 30 | 5.3 | 3.3 | 6.3 | 5.3 | 6.3 | 2.3 | ||
| 2 | 1 | 4.3 | 6.3* | 4.3 | 6.3 | 3.3 | 4.3 | 3.3 |
| 2 | 2.3 | 2.3* | 2.3 | 2.3 | 2.3 | 6.3 | ||
| 3 | 2.3 | 3.3 | 2.3 | 3.3 | 2.3 | 3.3 | 2.3 | |
| 4 | 2.3 | 2.3 | 2.3 | 2.3 | 3.3 | |||
| 5 | 2.3 | 2.3 | 2.3 | |||||
| 6 | 2.3 | 2.3 | 3.3 | |||||
| 29 | 5.3 | 5.3 | 4.3 | 2.3 | 2.3 | |||
| 3 | 1 | 2.3 | 2.3 | 2.3 | 2.3* | 3.3 | 2.3 | 2.3 |
| 16 | 3.3 | 4.3 | 4.3 | 5.3 | 6.3 | 3.3 | 2.3 | |
| 17 | 2.3 | 5.3 | 3.3 | 4.3 | 5.3 | 2.3 | 2.3 | |
| 18 | 2.3 | 3.3 | ||||||
| 4 | 16 | 2.3 | 2.3 | 2.3* | 2.3 | |||
| 17 | 3.3 | 3.3 | 3.3* | 3.3 | ||||
| 18 | 2.3 | 3.3 | 3.3 | 3.3* | 3.3 | 3.3 | 2.3 | |
| 19 | 2.3 | 2.3 | 2.3* | 2.3 | ||||
| 27 | 2.3 | 2.3 |
Log10 copies/ml indicated in color:  No HSV-2 detected,
No HSV-2 detected,  =102,
=102,  =103,
=103,  =104,
=104,  =105,
=105,  =106,
=106,  =107
=107
Each row represents one day of sampling.
LMN=left labia minora, LMJ=left labia majora, RMN=right labia minora, RMJ=right labia majora, PA=perianal
=lesion present
Figure 2. Appearance and progression of HSV-2 lesion and genital shedding over a 4 day period.
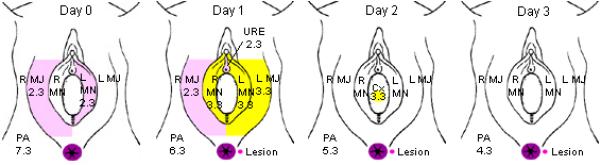
On the day before the appearance of lesion (Day 0), HSV shedding was present at 107 copies/ml in the perianal area, and also on the right labia majora and left labia minora at 102 copies/ml. On Day 1, the lesion appeared, shedding was detected at all of the sampled sites, with highest level of shedding at the site of lesion. In the following days, shedding from the lesion persisted, at decreasing copy number each day. HSV shedding continued after the perianal lesion healed for an additional 2 days (data not shown).
 no HSV DNA detected
no HSV DNA detected  2-3 log10copies/ml HSV DNA detected,
2-3 log10copies/ml HSV DNA detected,  3-4 log10copies/ml HSV DNA detected,
3-4 log10copies/ml HSV DNA detected,  site of lesion, with log10copies/ml HSV DNA detected.
site of lesion, with log10copies/ml HSV DNA detected.
L=left, R=right. MJ=Labia majora, MN=labia minora, PA=perianal, URE=urethra.
Numbers represent log10 copy number/ml detected.
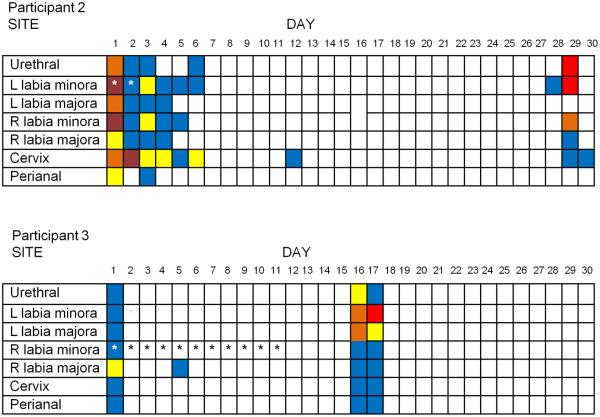
Figure 3 demonstrates the shedding patterns over the 30 day observation period for two participants who had widespread anatomic HSV detected at high copy number during both asymptomatic and lesional shedding episodes. On multiple days in both women, HSV was detected from both the right and left vulva, either in the presence or the absence of lesions.
Figure 3.
Clinical and subclinical HSV-2 shedding at site-specific locations in 2 participants
NOTE: R=Right, L =Left *indicates the presence of a lesion
Log 10 copies/ml indicated in color:  No HSV-2 detected,
No HSV-2 detected,  =102,
=102,  =103,
=103,  =104,
=104,  =105,
=105,  =106
=106
DISCUSSION
This study describes HSV-2 genital shedding patterns measured at defined anatomic sites in combination with a detailed daily clinical assessment for HSV related genital signs and symptoms in women. HSV was detected from more than one anatomic site on 56% of days with any HSV shedding and was found on bilateral genital surfaces on most days on which more than one site had virus detected. The number of sites with HSV DNA detected was associated with the maximum amount of HSV detected and the presence of a genital lesion. These results demonstrate that genital HSV reactivation may occur simultaneously from multiple sacral ganglia; the mechanism explaining these observations requires further study.. This study utilized a novel, very detailed sampling method in combination with a sensitive PCR assay to illustrate an important new concept in HSV-2 pathogenesis: that both clinical and subclinical HSV-2 reactivations are often multifocal and occur in a wide anatomic distribution in the genital tract. Furthermore, such reactivations are often bilateral in their anatomic distribution, even though clinical lesions typically emanate from one anatomic focus.
In both men and women, the external genitalia are innervated by branches of the pudendal nerve, which originates from the S2, S3 and S4 ganglia of the sacral plexus (14). It is therefore perhaps not surprising that careful sampling of the external genitalia reveals simultaneous HSV reactivations at multiple sites, in that reactivation from the ganglia that form the pudendal nerve could result in the potential appearance of HSV throughout the branches of the nerve distribution. What is remarkable is the high frequency of bilateral reactivations, both in the presence and absence of lesions, perhaps due to a high frequency of simultaneous sacral ganglionic reactivation. Infection of contralateral ganglia may occur during primary or recurrent infection. Guinea pig models have demonstrated that virus spreads from the site ipsilateral to inoculation to the contralateral dorsal root ganglia and peripheral nerves by five days post infection (15). Alternatively, HSV viremia may lead to infection of contralateral sacral ganglia (16). Modeling studies performed by Crespi et al have suggested that episodes of HSV genital shedding measured once daily with a mixed anogenital swab may actually represent multiple, overlapping ganglionic reactivations, particularly in the setting of a high shedding rate (10). The results from this study support this dynamic model of viral reactivation, demonstrating the detection of multiple distinct areas of simultaneous HSV reactivation throughout the genital mucosa.
The observation that HSV reactivation is widespread throughout the genital tract is intriguing because it suggests that the virus is rapidly cleared from some areas of the genital mucosa, whereas other areas have prolonged shedding and progress to ulceration. Mark et al have shown that the HSV reactivation rate has been underestimated with once daily sampling, and that HSV reactivations last a median of 13 hours (17). The clearance of virus from mucosal surfaces is likely dependent upon a number of factors, including the amount of HSV which reaches the mucosa and local immunologic factors that facilitate viral clearance. The infiltration of HSV-specific cytotoxic T cells has been shown to be correlated with resolution of HSV related genital ulcers (18). Zhu et al have demonstrated that HSV specific CD8+ T-cells persist at the site of a genital ulceration for at least 6 months (19). The persistence of activated HSV specific-T cells in areas of the genital mucosa may explain why some episodes of HSV shedding are asymptomatically cleared within hours while others progress to genital lesions.
One limitation of this study is the small sample size and the unique features of our cohort. All participants had a history of symptomatic genital herpes, and three of the four participants had documented acquisition of HSV-2 within the past year, which is associated with high viral reactivation (20, 21) and high lesion rates (22). While we observed a relatively high proportion of days with lesions during the study period (35 (29%) of 120), two of the four participants with recently acquired genital herpes contributed the majority of lesion days. Despite the high lesion rate, nearly half of HSV shedding days were asymptomatic, a similar proportion to larger previously reported cohorts with symptomatic disease (7). The frequency and distribution of widespread genital reactivation in the presence and absence of lesions in other groups (men, persons with longstanding infection, and persons with asymptomatic infection) will require further study.
It is possible that our observation that HSV was detected simultaneously in different anatomic areas represents cross contamination from adjacent sites rather than distinct ganglionic reactivations. While contamination cannot be ruled out, we believe it is unlikely, based both on the careful collection techniques performed by our clinicians and on the fact that on 18 (41%) of days with genital shedding, HSV DNA was found from only one site. One approach to overcome this limitation would be to utilize localized tissue biopsies to demonstrate the simultaneous detection of HSV antigen or specific immune response in widely separated anatomic areas. We have initiated a study to further explore this issue.
These data should inform how patients are counseled about risk of HSV transmission. Patients should be aware that one is unlikely to be able to predict not only when, but also where, one is shedding, and that HSV shedding may not be restricted to areas where lesions are currently or have previously been present. The relationship between shedding frequency and extent, and the risk of transmission to sexual partners, has not been quantified.
In conclusion, we demonstrate that HSV-2 reactivation occurs frequently and at widely spaced anatomic regions throughout the genital tract in women with a history of symptomatic genital herpes, suggesting that latent HSV-2 ganglionic infection is present in bilateral sacral ganglia and that control of viral replication at the level of the sacral ganglia is incomplete. Furthermore, these data suggest that the genital skin and mucosa play an essential immunologic role in clearance of HSV-2. Whether widespread subclinical reactivation occurs in HSV-2 seropositive persons without a clinical history of HSV-2, or in persons with longstanding HSV-2 infection, requires further study. However, these patterns of widespread reactivation may help explain the role of HSV-2 in increasing the risk of HIV-1 acquisition, particularly if each reactivation episode elicits a persistent immune response to clear the virus. To prevent HSV-2 transmission and, potentially, acquisition of viral co-pathogens such as HIV-1, HSV-2 therapy will need to more effectively suppress simultaneous viral reactivations from bilateral sacral ganglia.
Acknowledgments
We are indebted to the dedicated participants, as well as study clinician Gail Barnum, who performed the examinations and collected the samples.
Funding: This work was supported by the National Institutes of Health/NIAID grants P01-AI030731 (LC, AW), and K24-AI071113 (AW), K23-AI079394 (CJ)
Abbreviations
- HSV-2
Herpes simplex virus type 2
Footnotes
Conflicts of Interest: ST, MLH, SS, AM, no conflicts. CJ and AW have received grant support from the National Institutes of Health and GlaxoSmithKline. AW has also received grant support from Antigenics, and Astellas and has been a consultant for Aicuris. LC directs the University of Washington Virology Laboratory which has acted as a reference laboratory for the diagnosis of HSV infections. LC has received grant support from NIH and is a consultant to Immune Design Corporation regarding HSV and VZV candidate vaccines.
REFERENCES
- 1.Blank H, Haines HG. Experimental human reinfection with herpes simplex virus. J Invest Dermatol. 1973 Oct;61(4):223–5. doi: 10.1111/1523-1747.ep12676442. [DOI] [PubMed] [Google Scholar]
- 2.Baringer JR. Recovery of herpes simplex virus from human sacral ganglions. N Engl J Med. 1974 Oct 17;291(16):828–30. doi: 10.1056/NEJM197410172911606. [DOI] [PubMed] [Google Scholar]
- 3.Cook ML, Stevens JG. Pathogenesis of herpetic neuritis and ganglionitis in mice: evidence for intra-axonal transport of infection. Infect Immun. 1973 Feb;7(2):272–88. doi: 10.1128/iai.7.2.272-288.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wald A, Zeh J, Selke S, Warren T, Ryncarz AJ, Ashley R, et al. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med. 2000;342(12):844–50. doi: 10.1056/NEJM200003233421203. [DOI] [PubMed] [Google Scholar]
- 5.Krone M, Wald A, Tabet S, Paradise M, Corey L, Celum C. Herpes Simplex Virus Type 2 Shedding in Human Immunodeficiency Virus-Negative Men Who Have Sex with Men: Frequency, Patterns, and Risk Factors. Clin Infect Dis. 2000;30:261–67. doi: 10.1086/313647. [DOI] [PubMed] [Google Scholar]
- 6.Gupta R, Wald A, Krantz E, Selke S, Warren T, Vargas-Cortes M, et al. Valacyclovir and acyclovir for suppression of shedding of herpes simplex virus in the genital tract. J Infect Dis. 2004 Oct 15;190(8):1374–81. doi: 10.1086/424519. [DOI] [PubMed] [Google Scholar]
- 7.Wald A, Corey L, Cone R, Hobson A, Davis G, Zeh J. Frequent genital herpes simplex virus 2 shedding in immunocompetent women. Effect of acyclovir treatment. J Clin Invest. 1997 Mar 1;99(5):1092–7. doi: 10.1172/JCI119237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wald A, Huang ML, Carrell D, Selke S, Corey L. Polymerase chain reaction for detection of herpes simplex virus (HSV) DNA on mucosal surfaces: comparison with HSV isolation in cell culture. J Infect Dis. 2003 Nov 1;188(9):1345–51. doi: 10.1086/379043. [DOI] [PubMed] [Google Scholar]
- 9.Hobson A, Wald A, Wright N, Corey L. Evaluation of a quantitative competitive PCR assay for measuring herpes simplex virus DNA content in genital tract secretions. J Clin Microbiol. 1997 Mar;35(3):548–52. doi: 10.1128/jcm.35.3.548-552.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crespi CM, Cumberland WG, Wald A, Corey L, Blower S. Longitudinal study of herpes simplex virus type 2 infection using viral dynamic modelling. Sex Transm Infect. 2007 August 1 5;83:359–64. doi: 10.1136/sti.2006.022020. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashley RL, Militoni J, Lee F, Nahmias A, Corey L. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol. 1988 Apr;26(4):662–7. doi: 10.1128/jcm.26.4.662-667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cone RW, Hobson AC, Brown Z, Ashley R, Berry S, Winter C, et al. Frequent detection of genital herpes simplex virus DNA by polymerase chain reaction among pregnant women. Jama. 1994 Sep 14;272(10):792–6. [PubMed] [Google Scholar]
- 13.Mancl LA, DeRouen TA. A covariance estimator for GEE with improved small-sample properties. Biometrics. 2001 Mar;57(1):126–34. doi: 10.1111/j.0006-341x.2001.00126.x. [DOI] [PubMed] [Google Scholar]
- 14.Shafik A, el-Sherif M, Youssef A, Olfat ES. Surgical anatomy of the pudendal nerve and its clinical implications. Clin Anat. 1995;8(2):110–5. doi: 10.1002/ca.980080205. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein DI, Stanberry LR. Zosteriform spread of herpes simplex virus type 2 genital infection in the guinea-pig. J Gen Virol. 1986 Sep;67(Pt 9):1851–7. doi: 10.1099/0022-1317-67-9-1851. [DOI] [PubMed] [Google Scholar]
- 16.Johnston C, Magaret A, Selke S, Remington M, Corey L, Wald A. Herpes Simplex Virus Viremia during Primary Genital Infection. The Journal of Infectious Diseases. 2008;198(1):31–4. doi: 10.1086/588676. [DOI] [PubMed] [Google Scholar]
- 17.Mark K, Wald A, Magaret A, Selke S, Olin L, Huang ML, et al. Rapidly Cleared Episodes of Herpes Simplex Virus Reactivation in Immunocompetent Adults. J Infect Dis. 2008;198(8):1141–9. doi: 10.1086/591913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koelle DM, Posavad CM, Barnum GR, Johnson ML, Frank JM, Corey L. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J Clin Invest. 1998 Apr 1;101(7):1500–8. doi: 10.1172/JCI1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu J, Koelle DM, Cao J, Vazquez J, Huang ML, Hladik F, et al. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med. 2007 Mar 19;204(3):595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koelle DM, Benedetti J, Langenberg A, Corey L. Asymptomatic reactivation of herpes simplex virus in women after the first episode of genital herpes. Ann Intern Med. 1992 Mar 15;116(6):433–7. doi: 10.7326/0003-4819-116-6-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wald A, Zeh J, Barnum G, Davis LG, Corey L. Suppression of subclinical shedding of herpes simplex virus type 2 with acyclovir. Ann Intern Med. 1996 Jan 1;124(1 Pt 1):8–15. doi: 10.7326/0003-4819-124-1_part_1-199601010-00002. [DOI] [PubMed] [Google Scholar]
- 22.Benedetti J, Corey L, Ashley R. Recurrence rates in genital herpes after symptomatic first-episode infection. Ann Intern Med. 1994 Dec 1;121(11):847–54. doi: 10.7326/0003-4819-121-11-199412010-00004. [DOI] [PubMed] [Google Scholar]