Abstract
Importance of the Field
Prostate carcinoma is the most common non-cutaneous malignancy in American men. The efficacy of docetaxel and prednisone in metastatic castrate-resistant prostate cancer (mCRPC) has been shown to improve overall survival however its effect is not durable highlighting the need for new therapies.
Areas covered in this Review
We will review the development of some of the leading compounds with direct and indirect anti-angiogenic activity in prostate cancer including antibodies to vascular endothelial growth factor and its receptors, small molecule inhibitors of downstream signaling, immunomodulatory drugs with anti-angiogenic activity, and compounds thought to directly inhibit or destroy vascular endothelial cells.
What the reader will gain
The reader will gain a basic understanding of the role of angiogenesis in prostate cancer growth and metastasis. Current and potential targets of angiogenesis and their corresponding drugs under development for prostate cancer are discussed.
Take Home Message
There are now multiple early phase clinical trials of anti-angiogenic agents alone or in combination in prostate cancer. Several of these are now in phase III development. Combined therapy with two or more anti-angiogenic compounds may improve the activity of either compound alone. Multiple targets in the angiogenesis pathway continue to be elucidated and should remain an active area of investigation for the treatment of prostate cancer.
1 Introduction
1.1 Epidemiology and Natural History
In the United States, prostate cancer is the leading non-cutaneous malignancy in men with an estimated 192,280 new diagnoses and 27,360 deaths projected for 2009. 1 Approximately 80% of cases are diagnosed with localized disease and treatment strategies for these patients include active surveillance, radiation therapy or surgery. While often effective, definitive surgery with radical retropubic prostatectomy has been shown to have biochemical recurrence rates of up to 32% at 10 years. 2 For those patients receiving curative external beam radiation therapy, a study of 1,044 patients treated between 1977 and 1991 reported a 60% 10-year biochemical recurrence rate for T1-2 patients. 3 For those who progress or present at diagnosis with advanced or metastatic disease, androgen deprivation therapy (ADT) can be effective. The Medical Research Council completed a randomized trial of 938 patients with advanced or asymptomatic metastatic prostate cancer evaluating early versus late ADT and reported an improvement in overall survival for those treated with ADT early. There were also quality of life benefits including reduction in pathologic fracture, spinal cord compression and ureteral obstruction. 4 Unfortunately, the median duration of response to androgen deprivation therapy is limited to approximately 14 to 20 months. 5 There are several second line hormonal therapies available, however the vast majority of patients will eventually become castration resistant. In 2004, two phase III trials demonstrated docetaxel with either prednisone or estramustine offered castration resistant prostate cancer (CRPC) patients an improvement in overall survival and quality of life over mitoxantrone based regimens. 6, 7 Every 3 week docetaxel and daily prednisone was FDA approved in May of 2004 and is now considered to be either the backbone or comparator for trials of new agents to treat metastatic CRPC. 8 Median survival for CRPC patients treated with docetaxel is now approximately 18 to 20 months. After progression on docetaxel, CRPC patients have a very poor prognosis with median survival of approximately 6 to 10 months. 9 It is clear that more effective agents are needed in this population and targeting of the angiogenesis pathway is one strategy that is actively being pursued.
1.2 Rationale for targeting angiogenic pathway
The observation that intense neovascularization is seen surrounding growing tumor was described as early as 1939 by Ide and colleagues. 10 It was later discovered that angiogenesis is required to feed the continued growth of a malignant mass and that in the absence of neovascularization, tumor growth would halt at a diameter of 2–3 mm. 11 Once tumor cells are able to recruit their own blood supply, they may further expand and metastasize, a process that has been termed the “angiogenic switch” 12. The role of angiogenesis in tumor biology was studied intensely by Judah Folkman’s group in the early to mid 1970s. 13, 14 Several early studies suggested that there was a humoral inducer of angiogenesis that was concentrated near malignant cells. 15, 16 The subsequent isolation of basic fibroblast growth factor (bFGF) 17 and vascular permeablility factor (VPF) 18 also called vascular endothelial growth factor (VEGF) ignited further scientific interest in characterizing the angiogenesis pathway. While the activation of VEGF receptors by VEGF is important, the current model of angiogenesis involves tumor cells, the extracellular matrix and endothelial cells participating in a complex interaction with pro-and anti-angiogenic factors thought to be fueled by a hypoxic microenvironment. A detailed description of the molecular mechanisms involved in the angiogenesis pathway is outside of the scope of this review and the reader is referred to the recent excellent overview by Li and colleagues. 19 The manipulation of the dynamic process of angiogenesis with novel compounds to treat multiple malignancies is an active area of investigation..
Angiogenesis appears to playa critical role in prostate cancer with several studies correlating markers of angiogenesis with metastatic disease, higher gleason grade and clinical outcomes. Weidner showed that microvessel density (MVD)was significantly higher in prostate cancer samples for those patients with metastatic disease when compared with those without metastatic disease. 20 A 1998 study by Borre of 221 prostate cancer patients followed for a median of 15 years revealed that MVD of tumor samples at diagnosis was statistically significantly correlated with stage, grade and disease specific survival. 21 Furthermore, serum levels of the humoral ligand vascular endothelial growth factor (VEGF) were found to be significantly higher in those prostate cancer patients with metastatic disease. 22 Plasma VEGF levels have also been shown to be an independent prognostic factor in men with metastatic prostate cancer. 23, 24 Finally, a key mediator of VEGF expression, hypoxic-inducible factor (HIF), has higher expression in prostate cancer than in benign prostate tissue. 25 Based on these findings, angiogenesis inhibition has been targeted as a strategy to treat prostate cancer.
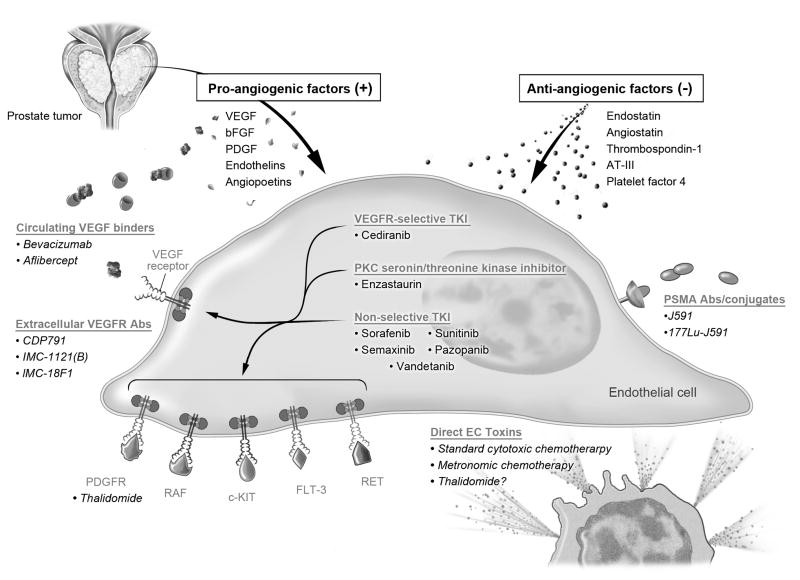
Both basic and clinical studies have shown that inhibition of angiogenesis can inhibit tumor progression and metastases. 26 There are several ways one can inhibit angiogenesis: inhibit proangiogenic factors such as VEGF, inhibit receptors of proangiogenic factors (VEGF-receptors), raise the concentration of antiangiogenic factors or directly kill tumor-related vascular endothelial cells. In this review we discuss multiple agents which are actively being developed to take advantage of several of these targets. (Fig. 1)
Figure 1.
A simplified diagram of the angiogenesis pathway with anti-angiogenic targets and select compounds exploiting them. Legend: VEGF: vascular endothelial growth factor, bFGF: fibroblast growth factor, PDGF: platelet derived growth factor, AT-III: antithrombin III, PSMA: prostate specific membrane antigen.
2 Body
2.1 Targeting VEGF
Bevacizumab (Avastin; Genentech, San Francisco, CA) is a recombinant humanized anti-VEGF antibody which has been shown to have activity in multiple cancer cell lines and is currently FDA approved for treatment of several malignancies including colorectal carcinoma, non-squamous non-small cell lung cancer, metastatic breast cancer, recurrent glioblastoma and most recently metastatic renal cell carcinoma. 27 The single agent activity of bevacizumab in prostate cancer was initially reported by Reese and colleagues in 2001. In this small phase II trial, 15 patients with metastatic castrate resistant prostate cancer (mCRPC) who had not received prior chemotherapy received 10 mg/kg of bevacizumab every 14 days. While 4 patients (27%) had PSA declines of <50%, there were no objective responses (defined by RECIST criteria or PSA declines >= 50%) and the trial was halted prior to planned second and third stages of enrollment based on not meeting predefined response goals. 28 Despite the disappointing single agent data, several trials have demonstrated activity when bevacizumab is combined with chemotherapy. CALGB 90006 was a phase II trial of docetaxel at 70 mg/m2 and bevacizumab 15 mg/kg given on day 2 every three weeks with estramustine 280 mg three times daily on days 1 through 5. Decadron was given 8 mg twice daily on days 1 through 3 and warfarin given at 2 mg daily was encouraged. 29 On the most recent follow up, 79 patients with CRPC (median Gleason score of 8)had been enrolled with 42% (14/33) of those with measurable disease achieving a partial response and 79% obtaining a >50% PSA decline. 30 More recently, DiLorenzo published a small phase II trial of bevacizumab and docetaxel in 20 docetaxel-pretreated mCRPC patients demonstrating a 37.5% overall response and median overall survival of 9 months. 31 A phase III randomized trial of every three week docetaxel (75 mg/m2) with 10 mg daily prednisone with or without 15 mg/kg bevacizumab was commenced and closed to accrual in 12/2007 (CALGB 90401). 32
Dual anti-angiogenic therapy with bevacizumab and thalidomide has also been evaluated. Thalidomide is an oral compound with immunomodulatory and anti-angiogenic effects that will be discussed later in this review. Based on data suggesting that thalidomide decreases multiple markers of angiogenesis but does not reliably decrease VEGF levels33, the National Cancer Institute developed a protocol adding thalidomide and bevacizumab to standard of care docetaxel. In this trial, 60 patients with chemotherapy naïve metastatic CRPC received bevacizumab 15 mg/kg day 1, docetaxel 75 mg/m2 day 1, thalidomide 200 mg by mouth once daily and prednisone 5 mg twice daily(ATTP) all given on an every 3 week cycle with venous thromboembolic (VTE)prophylaxis. Impressive preliminary results of the ATTP regimen were reported in 2008 and revealed a PSA decline of >50% in 88% (51/60) with durable PSA decline extending to a median of 11 cycles. Of the 32 patients with measurable disease, two patients had complete responses and 18 had a partial response for an overall response rate of 62.5%. Median progression free survival was 18.2 months and median overall survival 26.7 months. 34 The regimen was reasonably well tolerated with a median on-study duration of 12 cycles. A peer-reviewed manuscript documenting the trial and its most current updated results has been recently accepted for publication.
Bevacizumab has also been combined with immunotherapy and other experimental agents. In a phase II study, prostatic acid phosphatase-pulsed dendritic cells (APC8015; Provenge, Dendreon Pharmaceuticals, Seattle, WA) were combined with bevacizumab for biochemical recurrence after definitive local therapy. The combination was found to induce an immune response and slow serum PSA doubling time. 35 The combination of Satraplatin, a third generation oral platinum compound (Spectrum Pharaceuticals, Irvine, CA), and bevacizumab is being studied in CRPC patients who have previously been treated with docetaxel (NCT00499694). Bevacizumab is actively being studied in several other combinations in early and late stage prostate cancer (see table 1).
Table 1.
Select active clinical trials of VEGF-directed compounds bevacizumab and aflibercept in prostate cancer
| Phase | Regimen | Goal N | Clinical Trials ID |
|---|---|---|---|
| Bevacizumab | |||
| I | Dasatinib and Bevacizumabin Treating Patients With Solid Tumors That Are Metastatic or Cannot be Removed by Surgery | 48 | NCT00792545 |
| II | Bevacizumab, Lenalidomide, Docetaxel, and Prednisone (ART-P) for Treatment of Metastatic Castrate-Resistant Prostate Cancer | 57 | NCT00942578 |
| II | Bevacizumab in Combination With Hormonal and Radiotherapy in Patients With High-Risk Prostate Cancer | 18 | NCT00349557 |
| II | Satraplatin and Bevacizumab in CRPC patients previously treated with docetaxel | 24 | NCT00499694 |
| II | Androgen Deprivation Therapy +/− Bevacizumab for PSA Recurrence of Prostate Cancer After Definitive Local Therapy | 100 | NCT00776594 |
| II | Avastin, Docetaxel and Androgen Deprivation Followed by Continued Avastin and Androgen Deprivation for Men With a Rising PSA After Local Therapy | 42 | NCT00658697 |
| III | Docetaxel and prednisone with or without bevacizumab for mCRPC (enrollment complete) | 1020 | NCT00110214 |
| Aflibercept | |||
| III | Docetaxel and prednisone with or without Aflibercept in mCRPC | 1200 | NCT00519285 |
Another promising agent Aflibercept (AVE0005) (Sanofi-Aventis/Regeneron) is a novel fusion protein of domain 2 of VEGFR-1 and domain 3 of VEGFR-2 with the Fc fragment of IgG1. This compound, also known as VEGF-Trap, acts as a soluble decoy VEGF receptor that can sequester VEGF. 36 Multiple phase II studies have been reported in patients with ovarian, non-small cell lung and other solid tumors revealing anti-tumor activity and reasonable side effect profile. 37, 38 Based on these preliminary findings and the role of angiogenesis in prostate cancer, a large multicenter, randomized, double blind placebo controlled trial is being conducted to compare the efficacy and safety of aflibercept versus placebo with docetaxel and prednisone for metastatic CRPC. (Table 1)
2.2 Targeting VEGF-receptors
2.2.1 Extracellular VEGFR agents
Compounds targeting the extracellular domain of the VEGF receptor are under study but are still relatively early in development. Ton and colleagues published phase I results of CDP791, a di-Fab’ polyethylene glycol (PEG) conjugate that inhibits VEGFR-2 in vitro and angiogenesis in vivo. 12 patients were treated in the phase I setting and the drug was felt to be biologically active and well tolerated. 39 Camidge presented preliminary data from a Phase I study of IMC-1121(B) a monoclonal antibody to VEGFR-2. 40 A large randomized phase II trial of IMC-1121 in addition to mitoxantrone and prednisone in CRPC following progression on docetaxel is underway.(NCT00683475) Another antibody to the extracellular binding domain of VEGFR-1, IMC-18F1, is also being studied in the phase I setting. 41
2.2.2 Intracellular selective VEGFR antagonists
AZD-2171 (Cediranib®, Astra Zeneca, Wilmington, DE) is an in dole-ether quinazoline with potent and selective ATP-competitive inhibition of VEGF-receptors 1 and 2. 42 This oral tyrosine kinase inhibitor (TKI) is actively being developed in multiple malignancies including prostate cancer. In the CRPC setting, a phase I study looked at 26 patients and established a MTD dose of 20 mg with grade 3 toxicity of muscle weakness and fatigue. 43 There was one objective response and several PSA declines that occurred after discontinuation of therapy based on PSA progression, challenging the use of PSA as a marker of antitumor effect for this compound. A phase II trial of Cediranib in metastatic CRPC with progression following docetaxel is ongoing at the National Cancer Institue (NCT00436956). Cediranib is given at a dose of 20 mg by mouth daily and in a second cohort given with concurrent daily oral prednisone on 28 day cycles. Progression is defined by clinical or radiographic evidence rather than PSA rise alone. Updated results of the first 34 patients were presented by Karakunnel in 2009. 44 Thirteen of the twenty-three patients with measurable disease had some tumor shrinkage with 4 meeting the criteria for partial response. PSA levels have not correlated well with response, further supporting the idea that PSA may be an unreliable marker for disease surveillance when using this compound. The main toxicities were hypertension, dysphonia and fatigue. DCE-MRI, which has been studied as a pharmacodynamic imaging strategy for cediranib 45, was performed on all patients and results of the clinical correlation of DCE-MRI will be forthcoming.
2.2.3 Intracellular non-selective inhibitors of the VEGFR
There are now multiple agents with activity that includes, but is not limited to, the intracellular domain of the VEGF family of receptors. These compounds, largely tyrosine kinase inhibitors (TKIs), are being actively studied in multiple different malignancies and are in various stages of development. (Table 2) One such agent that has been studied in prostate cancer is sorafenib (Nexavar®, Bayer HealthCare and Onyx Pharmaceuticals, Emeryville, CA). This agent is a small molecule tyrosine kinase inhibitor (TKI) which targets RAF kinase in addition to VEGFR-2 and PDGFR-beta resulting in anti-proliferative and anti-angiogenic effects. 46 The agent is currently FDA approved for hepatocellular carcinoma and renal cell carcinoma. Several phase II trials of sorafenib in prostate carcinoma have been conducted. Our group conducted a single arm open label trial of single agent sorafenib given at 400 mg by mouth twice daily continuously on 28 day cycles. Initial results from the first 22 CRPC patients enrolled showed no PSA declines >50%. There was discordance between PSA and radiographic response criteria with 2 patients progressing by PSA criteria but having a decrease in the number of lesions seen on bone scan. Of the 21 patients with progressive disease, 13 were based on PSA only and had otherwise stable clinical and radiographic disease. 47 The second stage of the study enrolled 24 additional patients with progression redefined as clinical or radiographic criteria alone. 21 of the 24 patients had previous docetaxel therapy and the median Gleason score was 8. One patient had partial response and 10 patients had stable disease. Median progression free survival was 3.7 months and median overall survival was 18.0 months. Pooled data from both stages of the trial (N=46) revealed a median survival of 18.3 months. 48 Another phase II trial in chemotherapy naïve metastatic or CRPC patients enrolled 57 patients who were to receive 400 mg twice daily sorafenib. Of the 55 evaluable patients, only 2 had PSA decline >50% and none had objective responses by RECIST. Nonetheless, 15 had stable disease and 31% of patients had not progressed by 12 weeks. 49 Chi et al reported their phase II findings in 2008 with 28 chemotherapy naïve patients with CRPC. 50 The number of patients with PSA decline >50% was only 3.6%, however PSA declines were seen post-discontinuation of therapy, again suggesting that the agent may lead to increased serum PSA levels independent of tumor growth. Since these trials have been completed there has been discussion regarding PSA as an endpoint in phase II trials of CRPC 51 and the Prostate Cancer Clinical Trials Working Group does not recommend removing patients from study based on rising PSA alone. 52 A review of the safety profile and adverse events from studies involving sorafenib combined with chemotherapies or other targeted agents was recently published. 53 Encouraging preliminary results from a phase I trial of sorafenib in combination with docetaxel and prednisone were presented by Mardjuadi demonstrating 15 of 20 patients with PSA decline >50% although a significant number of febrile neutropenia was noted. 54 Based on the preliminary studies of sorafenib in prostate cancer, the agent continues to be actively pursued alone and in combination with other therapies. (Table 3)
Table 2.
Tyrosine kinase inhibitors (TKI) with VEGF receptor activity. Based on Table 1 from Chu, Expert Opin. Biol. Ther. (2009) 9(2): 263–271. By permission of Quincy Chu).
| Drug | Mechanism of Action | Company | Current Stage of Development |
|---|---|---|---|
| Predominant class III tyrosine kinase inhibitors | |||
| ABT-869 | VEGFR-2/PDGFR beta | Abbott | II |
| Axitinib | VEGFR-1, 2, 3/C-KIT/PDGFR beta | Pfizer | II/III |
| AMG 706 | VEGFR-1, 2, 3/PDGFR alpha/C-KIT/RET | Amgen | II/III |
| BAY 57-9352 | VEGFR-2, 3/PDGFR/C-KIT | Bayer | I |
| Brivanib | VEGFR-1, 2/FGFR-1 | Bristol Meyer Squibb | II/III |
| BIBF 1120 | VEGFR-1,2,3/FGFR-3/PDGFR alpha | Boehringer-Ingelheim | II/III |
| Cediranib | VEGFR-1,2,3/PDGFR alpha/C-KIT | AstraZeneca | II/III |
| Pazopanib | VEGFR-1,2,3/PDGFR alpha/C-KIT | GlaxoSmithKline | II/III |
| Sorafenib | VEGFR-2,3/PDGFR alpha/FLT-3/C-KIT | Bayer | III and FDA approved for renal cell carcinoma and HCC |
| Sunitinib | VEGFR-1,2/C-KIT/FLT-3/PDGFR alpha | Pfizer | III and FDA approved for GIST and renal cell carcinoma |
| Vatalanib | VEGFR-1,2,3/PDGFR alpha/C-KIT/C-FOS | Novartis | III |
| Epidermal growth factor receptor (EGFR) and VEGFR inhibitors | |||
| AEE788 | EGFR/HER-2/VEGFR-2 | Novartis | II |
| BMS-690514 | Pan-HER and VEGFR | Bristol Meyer Squibb | I/II |
| Vandetanib | EGFR/VEGFR-2 | AstraZeneca | III |
| XL-647 | EGFR/VEGFR-2 | Exelixis | I |
| Miscellaneous multi-kinase inhibitors with VEGFR activity | |||
| CYC116 | Aurora kinase/VEGFR-2 | Cyclacel | I |
| MGCD265 | VEGFR/C-MET | MethylGene | I |
| OSI930 | VEGFR-2/C-KIT/LCK/C-RAF | OSI | I |
Table 3.
Select clinical trials of tyrosine kinase inhibitors and other anti-angiogenic compounds in prostate cancer.
| Phase | Regimen | Goal N | Clinical Trials ID |
|---|---|---|---|
| AZD-2171 (Cediranib) | |||
| II | AZD-2171 and prednisone in metastatic CRPC | 37 | NCT00436956 |
| II | Docetaxel and prednisone with or without cediranib | 104 | NCT00527124 |
| Sorafenib | |||
| I/II | Concurrent sorafenib, ADT and radiation therapy for initial treatment of intermediate and high risk localized prostate cancer | 50 | NCT00924807 |
| II | Sorafenib and docetaxel for mCRPC | 69 | NCT00589420 |
| Sunitinib | |||
| II | Sunitinib with docetaxel and prednisone in chemotherapy naïve metastatic CRPC | 30 | NCT00879619 |
| II | Docetaxel, sunitinib and prednisone followed by EBRT for men with biochemical recurrence following radical prostatectomy | 38 | NCT00734851 |
| II | Neoadjuvant sunitinib and hormonal ablation for patients who will have prostatectomy | 42 | NCT00329043 |
| III | Prednisone with or without sunitinib in CRPC patients who have failed docetaxel | 819 | NCT00676650 |
| Pazopanib | |||
| II | Pazopanib after failure of total androgen blockade | 34 | NCT00945477 |
| II | Pazopanib with or without bicalutamide in mCRPC | 74 | NCT00486642 |
| Vandetanib | |||
| II | ZD 6474 (Vandetanib) with Bicalutamide versus Bicalutamide alone in chemotherapy naïve CRPC | 74 | NCT00757692 |
| II | Randomized placebo controlled trial of vandetanib and bicalutamide versus bicalutamide alone in metastatic CRPC | 90 | NCT00659438 |
| Other anti-angiogenic agents | |||
| I | Docetaxel/Prednisone and 177 Lu-J591 antibody for mCRPC | 30 | NCT00916123 |
| II | 177Lu-J591 and ketoconazole/hydrocortisone in high risk nonmetastatic CRPC | 140 | NCT00859781 |
There are many other nonselective TKIs being developed for multiple malignancies including prostate cancer. SU5416 (Semaxinib®, Pharmacia, San Francisco, CA) is a synthetic TKI that reversibly inhibits VEGFR-2 and KIT. 55, 56 A phase II study of 36 patients with CRPC receiving SU5416 + dexamethasone pretreatment versus dexamethasone alone revealed no significant meaningful clinical activity. 57 This, in addition to inconvenient IV dosing requiring a central line, and modest toxicity led to the decision to halt further development of this agent in prostate cancer.
SU11248/Sunitinib (Sutent®, Pfizer Inc. New York, NY) is an oral multi-tyrosine kinase inhibitor with activity against VEGFR-2, PDGFRb, FLT-3 and KIT. 58 Sunitinib (SU) is currently FDA approved for gastrointestinal stromal tumor (GIST) after failure of imatinib and advanced/metastatic renal cell carcinoma. A phase I trial of SU in addition to docetaxel and prednisone in CRPC showed the regimen to be safe and tolerable with 1/7 evaluable patients having partial response and four additional with stable disease. 59 Updated results from the phase I/II trial of SU combined with docetaxel and prednisone were recently presented. 60 Patients received SU at 37.5 mg/d on days 1–14, docetaxel 75 mg/m2 on day 1 and prednisone 5 mg twice daily days 1–21 on 21 day cycles and the primary endpoint was PSA decline by PSA working group criteria. 55 patients were enrolled and 36 discontinued therapy (16 for disease progression and 13 for adverse events). Most common grade 3–4 AEs were neutropenia (75%), febrile neutropenia (15%) and fatigue (15%). Sunitinib dose reductions were required in 26% of patients and docetaxel dose reduction necessary in 33%. Thirty nine percent of evaluable patients had a partial response. PSA declines occurred in 56% and median TTP was 42 weeks. Median progression free and overall survival had not yet been reached. Another ongoing phase II study is evaluating neoadjuvant androgen deprivation therapy with concurrent daily SU at 37.5 mg daily given for 90 days prior to definitive surgery for high risk local prostate cancer. Early results were presented and showed one pathologic CR out of 30 was obtained. 61 Sunitinib is actively being studied with and without docetaxel in several other clinical trials. There is also a large phase III international trial comparing Sunitinib and prednisone with prednisone alone in CRPC patients who have failed docetaxel based therapy. (Table 3)
Vandetanib (ZD 6474, Zactima™, Astra Zeneca) is a once daily oral multi-tyrosine kinase inhibitor targeting the VEGFR, EGFR and RET pathways. 62 Vandetanib has shown promise in non-small cell lung cancer (NSCLC) with results from a randomized phase II trial revealing an improvement in progression free survival at 300 mg once daily when compared to gefitinib. 63 These results have led to several large phase III trials of vandetanib with and without docetaxel in NSCLC. Vandetanib is currently being investigated in several phase II trials for prostate cancer in both the metastatic and non-metastatic castrate resistant setting. (Table 3) Another non-selective TKI in early development for prostate cancer is GW786034 (Pazopanib®, GlaxoSmithKline, London, United Kingdom). This agent is an oral multi-targeted TKI that targets VEGF, PDGFR and KIT. Pazopanib has a robust amount of early phase data in other malignancies and is currently in phase III clinical trials for ovarian cancer, soft tissue sarcoma, inflammatory breast cancer and renal cell carcinoma. Several phase II clinical trials are actively recruiting patients in the chemotherapy naïve prostate cancer setting. (Table 3)
Downstream VEGF receptor targets have also been evaluated in prostate cancer. Ly317615 (Enzastaurin, Ely Lilly, Indianapolis, IN, USA) is a potent and selective serine/threonine kinase inhibitor that targets protein kinase C (PKC), a downstream kinase involved in VEGFR signal transduction. Preclinical studies reveal enzastaurin can lead to inhibition of new vessel growth in the rat corneal micropocket assay 64 as well as decrease MVD and VEGF levels in human tumor xenografts. 65, 66 A randomized double blind placebo controlled phase II trial of docetaxel and prednisone with and without enzastaurin for front line treatment of CRPC is actively recruiting patients. (NCT00466440)
2.3 Targeting the tumor vascular endothelium
2.3.1 Metronomic Chemotherapy (MC)
In 1991, Kerbel proposed the idea that targeting the more genetically stable tumor vascular endothelial cell may bypass the typical acquired drug resistance that has plagued traditional cytotoxic chemotherapy. 67 The more rapid cycling of immature tumor endothelial cells may also offer the specificity required to obtain a therapeutic index and avoid unwanted toxicity to normal vasculature. In 2000, Hanahan coined the term “metronomic” chemotherapy (MC) to describe the frequent administration of chemotherapy at doses well below the MTD with no prolonged drug free breaks. 68 Preclinical data has suggested that MC can overcome drug resistance. 69 This effect of overcoming resistance by using more frequent dosing has clinical precedent with etoposide in lung cancer 70 and paclitaxel in breast cancer. 71 Further preclinical studies have demonstrated the anti-angiogenic and antitumor effects of MC delivered with multiple agents including taxanes, cyclophosphamide, vinblastine, etoposide and platinums. 72 The metronomic strategy is thought to provide direct endothelial cytotoxicity as well as upregulate endogenous endothelial cell inhibitors like thrombospondin-1 73. Studies have also supported an immune-stimulatory effect with reduction of T-regulatory cells. 74
Initial evidence of the utility of MC in prostate cancer came from Gode and Colleagues in 2003. This was a retrospective case series of 34 CRPC patients treated with metronomic cyclophosphamide (mCTX) (50 mg daily) and dexamethasone (1 mg daily). Patients had a mean on-study PSA of 154 and over half had bone or soft tissue disease with 13 patients having received prior chemotherapy. Only 1 patient was taken off therapy secondary to treatment related toxicity and 69% of patients had a PSA decline >50%. 75 Following these results, a single center prospective open label phase II trial of mCTX alone enrolled 80 patients with CRPC. 76 Patients received 50 mg/d of oral CTX and were evaluated for PSA decline and objective tumor response by RECIST. 58 patients completed two cycles (8 weeks) and were included in the intent to treat analysis. Fourty five percent of patients had a WHO performance status 2. Response by traditional criteria was marginal with 5.2% (3/58) having either PSA reduction >50% or objective tumor response. An additional 39% of patients had some decline in PSA or improved PSA velocity and the median overall survival had not yet been reached. The most concerning toxicity was grade 3 lymphopenia in 32.8%, none of whom had opportunistic infections. The study was limited by short follow up and 22 patients who were not included in the intention to treat analysis, 15 of whom had rapidly progressive PSA or progressive symptoms. Recently, a prospective non-randomized phase II study published by Fontana treated 28 advanced metastatic CRPC (68% docetaxel resistant) patients with mCTX (50 mg PO daily), dexamethasone (1 mg daily) and celecoxib (200 mg twice daily) after receiving one upfront IV dose of CTX at 500 mg/m2. Thirty two percent (9/28) of patients had PSA decline >50% and median overall survival was 21 months. 77
The benefits of metronomic chemotherapy include low toxicity and low cost as well as ease of administration. Early preclinical studies suggested a benefit from combining targeted anti-angiogenesis compounds such as TNP-470 with MC. 69 A phase I study has been published by Di Lorenzo who used mCTX (50 mg/d) with concurrent thalidomide in CRPC patients who had failed docetaxel chemotherapy. MTD was 100 mg daily thalidomide and the most common adverse effects were fatigue, constipation and peripheral neuropathy. Two evaluable patients had >50% PSA decline (15%) and one other had PSA decrease <50%.78 Further studies combing MC with targeted anti-angiogenic therapies are underway in hopes of increasing anti-tumor effects.
2.3.2: Prostate Specific Membrane Antigen (PSMA)
Prostate specific membrane antigen (PSMA) is a type 2 integral transmembrane glycoprotein expressed preferentially on prostate epithelial cells. Although its role in malignancy is unclear, it is currently an active candidate for research into both the diagnosis and treatment of prostate cancer. 19 PSMA is preferentially and highly expressed in advanced prostate cancer versus benign prostatic tissue. 79 Because PSMA has been shown to be expressed on the neovasculature of multiple tumors but not on normal vasculature80, it has been identified as a target for anti-angiogenic therapy. Several PSMA antibodies have been developed 81, the most studied has been human recombinant monoclonal antibody J591 (MLN J591, BZL Biologics, Millenium, Cambridge, MA). Initial phase I studies of the antibody showed excellent targeting and a phase II trial of the naked antibody given with interleukin 2 demonstrated acceptable tolerability but no PSA declines >50%. 82 Results with J591 radio-labeled with 177-lutetium 83 and 90-Yttrium 84 have revealed specific targeting of prostate cancer metastases with acceptable toxicity and more appreciable biologic activity. A trial of 177-Lu-J591 with Docetaxel and Prednisone in CRPC is actively recruiting patients (Table 3).
2.4 Antiangiogenic/Immunomodulatory Agents: Thalidomide and Lenalidomide
Thalidomide (Thalomid®, Celgene Corporation, Summit NJ) is an oral agent with anti-tumor efficacy demonstrated in several malignancies and is currently FDA approved for newly diagnosed Multiple Myeloma. While its mechanism is not clearly elucidated, Thalidomide and its analogs appear to inhibit angiogenesis in part by affecting the platelet derived growth factor receptor pathway. 85 Furthermore, preclinical data has demonstrated that thalidomide may increase the number of apoptotic circulating endothelial cells. 86 Aside from its possible anti-angiogenic activity, thalidomide and its derivatives lenalidomide and pomalidomide may have immunomodulatory effects including T-cell co-stimulation, T-regulatory cell inhibition (lenalidomide and pomalidomide) and enhanced NK cell activity. 87, 88 The agent was initially used as a sedative/anti-emetic until it was discovered to be teratogenic and was taken off the market in the 1960s. 89, 90 In 1994 D’Amato revealed that thalidomide inhibits angiogenesis in a rabbit cornea micropocket assay 91, prompting further study of this agent as a anti-angiogenic compound to combat malignancy.
An early phase II open label trial of 100 mg daily of thalidomide in CRPC patients reported by Drake and colleagues resulted in >50% PSA reduction in 15% (3/20) of patients. 92 Another open label randomized phase II trial at the National Cancer Institute compared thalidomide at 200 mg per day with higher doses up to 1200 mg daily in 63 patients (50 low dose and 13 high dose). Eighteen percent of patients had a PSA decline of >50% and 27% had declines of >40%. 33 The authors noted that preclinical studies had shown that thalidomide may increase PSA secretion 93 and thus the activity was thought to warrant further study. Additionally, there were decreases in PET uptake on experimental PET scans performed during the trial, suggesting anti-tumor activity. 94, 95
Thalidomide has also been used in combination with cytotoxic agents. A phase 2 randomized study of weekly docetaxel (30 mg IV per week for 3 weeks on 28 day cycles) with or without 200 mg of daily thalidomide was completed in chemotherapy-naïve metastatic CRPC. 96 At a median 26 month follow up, 75 patients had been enrolled (25 docetaxel alone and 50 docetaxel + thalidomide). While not reaching statistical significance, the endpoints of >50% PSA reduction (53% vs. 37%) and median PFS (5.9 mo vs. 3.7 mo) favored the combined arm. 97 In an updated analysis with median follow-up of 46.7 months, median overall survival met statistical significance with a median OS of 25.9 months for the combined arm versus 14.7 months for docetaxel alone P2=0.0407. 98 However, 12 of the first 43 patients in the combined group suffered thromboembolic events. As such, prophylactic anticoagulation with low-molecular weight heparin was offered to patients for the remainder of the study. Other important toxicities included a higher number of patients with fatigue, depression, neuropathy and pleural effusions in the combined arm. Several other studies have shown excellent antitumor activity combining thalidomide with estramustine and taxanes, 99, 100 however excess toxicity has been a problem with estramustine based regimens. Thalidomide has also been looked at in patients earlier in the course of prostate cancer with PSA recurrence following definitive local therapy in several phase II trials with encouraging results. 101, 102
Because of the excess thrombotic events, fatigue and neuropathy associated with thalidomide, there is interest in developing other potent thalidomide derivatives such as lenalidomide in hopes of building on thalidomides activity while improving its toxicity profile. Lenalidomide (Revlamid®, Celgene Corporation, Summit, NJ) is a thalidomide analog currently FDA approved for multiple myeloma and low risk 5q-myelodysplastic syndrome. In a phase I trial of lenalidomide alone in patients with refractory solid tumors, doses as high as 35 mg per day for 21 days with a 7 day rest period were tolerated (a dose well above the dose for multiple myeloma or MDS). Stable disease was documented in 12 of 44 evaluable patients, 9 of whom had prostate cancer. 103, 104 Another phase I study looked at lenalidomide in addition to docetaxel. 33 patients with advanced solid tumors received lenalidomide on day 1–14 and docetaxel on day 1 in 21 day cycles. 105 Pegfilgrastim was added on day 2 secondary to myelosuppression. The trial reached an MTD of 75 mg/m2 of docetaxel with 25 mg of lenalidomide given daily on days 1–14 and further follow up demonstrated a 3% response rate with 69% stable disease. 106 Moss and colleagues administered lenalidomide combined with every 3 week docetaxel and prednisone in a phase I trial of 19 CRPC patients. This trial revealed a 38.5% partial response (5/13) in those with measurable disease. An additional 7 of the 13 patients had stable disease and 47% (9/19) of the patients had a PSA decline >50%. 107 A phase I study of weekly paclitaxel and lenalidomide 21 out of 28 days in metastatic CRPC with prior taxane treatment had minimal activity with high levels of dose limiting toxicities. 108
Lenalidomide has been added to ketoconazole in the chemotherapy-naïve CRPC setting. Preliminary phase II results of 18 evaluable patients were reported in 2008. After a median of 2 cycles, 10 of 18 patients had a PSA decline >50% and 3 of 4 patients with soft tissue disease had a partial response. 109 Building on the results of the ATTP trial with dual anti-angiogenesis agents thalidomide and bevacizumab, a phase II trial of ART-P (Avastin, Revlamid, Taxotere and Prednisone) is currently underway. (Table 1) Other thalidomide analogs including CPS 11 and CPS 49 have shown some preclinical activity. 110
2.5 Hypoxia and Angiogenesis
Hypoxia in the tumor microenvironment plays a significant role in prostate cancer by stimulating angiogenesis as well as protecting prostate tumor cells from androgen deprivation therapy, chemotherapy and radiation cytotoxicity. Hypoxia-inducible factor 1 (HIF-1) alpha is a transcriptional regulator that responds to hypoxic conditions and has been implicated in angiogenesis in part by activating VEGF and VEGF receptor transcription. 111, 112 HIF-1 alpha is overexpressed in prostate cancer113 and is considered a potential therapeutic target. Preclinical data on RX-0447, an anti-sense HIF-1 alpha inhibitor, has demonstrated inhibition of growth in PC-3 prostate cancer cell lines as well as prostate xenografts114. Further development of HIF-1 alpha targeted compounds in prostate cancer may be promising. Cyclooxygenase 2 (COX-2) is an inducible enzyme which is upregulated in prostate cancer and involved in hypoxia-induced VEGF expression via upregulation of HIF-1 alpha. 115 While other mechanisms for COX-2 inhibition’s role in anti-tumor activity have been proposed, including BCL-2 downregulation and possible AKT pathway inhibition, a recently published pilot study of 45 prostate cancer patients randomized to celecoxib or placebo prior to prostatectomy revealed a trend toward decreased markers of angiogenesis including HIF-1 and VEGF as well as decreased proliferation (MIB-1) on their prostatectomy specimens. 116 The use of celecoxib in addition to metronomic cyclophosphamide and dexamethasone in mCRPC has been discussed in the metronomic chemotherapy section. 77 Another phase II trial looked at celecoxib at 400 mg twice daily given to 40 patients with biochemical recurrence (no evidence of metastasis) following radical prostatectomy or radiation. In this study, 90% of patients had slowing of their rate of PSA increase after 3 months of treatment indicating a potential use for celecoxib in delaying time to androgen deprivation therapy in this population. 117 The potential anti-tumor effect of COX-2 inhibitors must be weighed against the increased cardiac risk of the use of these medications. 118
3 Expert Opinion
There is now ample early phase clinical data to support the continued investigation of angiogenesis inhibitors in prostate cancer. (Table 4) While initial results of single agent trials of anti-angiogenic (AA) therapies alone have suggested only modest benefit, combining 2 AA agents (Bevacizumab and Thalidomide) or combining AA agents with chemotherapy appears to have a more robust anti-tumor effect. Our experience with dual-anti-angiogenic therapy in the ATTP trial 34 supports further research into this strategy and we eagerly await accrual and results from the ART-P trial using lenalidomide in place of thalidomide with the hope that anti-tumor efficacy is maintained with an improved adverse event profile. The phase III trial of docetaxel and prednisone with or without bevacizumab will also be instructive and may change the standard of care for metastatic or clinically progressive CRPC. Further study of newer agents targeting the angiogenic pathway both alone and in combination are underway. Early stage trial design for targeted therapies must take into account the lack of reliability of PSA as a surrogate marker. While PSA decline has been used in evaluation of cytotoxic agents with some success, there are now several examples of the limitations of this endpoint in trials of small molecules and immunotherapy. In two phase II studies of sorafenib, PSA declines were seen after discontinuation of therapy and bone disease was seen to improve in the face of rising PSA. 47,50 There are also examples of vaccine trials where progression free survival endpoints were not met despite overall survival being statistically in favor of the experimental arm. 119, 120 We strongly encourage investigators to follow the recommendations of the PSA working group and discourage taking patients off trial for PSA progression alone. Further development of markers of anti-tumor activity including circulating tumor cell analysis and dynamic imaging should also be explored. Finally, the idea of exerting a constant pressure on prostate cancer may be a promising strategy. To this end, metronomic chemotherapy (MC) may be a cost-effective and well tolerated option as one part of a multi-agent regimen. While clinical trials of anti-angiogenic compounds have shown some promise in prostate cancer, mCRPC continues to be incurable with the goal being improvement in the quality and quantity of life for these patients. To this end, continued investigation into multiple combination therapies involving single or dual agent AA agents with or without cytotoxic chemotherapy, MC or immunotherapy should continue to be explored.
Table 4.
Selected studies of anti-angiogenic compounds in the treatment of prostate cancer.
| Agent (Author, Year) | Phase-Population | N= | Results | Grade 3 or 4 toxicity |
|---|---|---|---|---|
| Bevacizumab (Reese, 2001) |
II mCRPC |
15 | OR: 0% (0/15) PSA: 0% (0/15) |
2 hyperglycemia, 1 anemia, 3 hyperkalemia (all thought to be unrelated) |
| Bevacizumab + Docetaxel + Estramustine (Picus, 2004) |
II mCRPC |
79 | OR: 42% (14/33) PSA: 79% |
5 VTE with 1 death mesenteric vein thrombus, 1 perforated sigmoid diverticulum, <3% febrile neutropenia |
| Bevacizumab + Docetaxel (DiLorenzo, 2008) |
II Doc-resistant mCRPC |
20 | OR: 37.5% (3/8) PSA: 55% (11/20) mPFS=4 mo, mOS=9 mo |
Grade 4: 1 neutropenia and 1 thrombocytopenia. Grade 3: 3 neutropenia, 2 nausea/vomiting, 1 neuropathy |
| Bevacizumab + Docetaxel + Thalidomide + Prednisone (Ning, 2008) |
II Chemo-naïve mCRPC |
60 | OR: 62% (20/32) PSA: 88% (51/60) mPFS=18.2 mo, mOS=26.7 mo |
febrile neutropenia (5/60), syncope (5/60), GI perforation or fistula (3/60), thrombosis (3/60), grade 3 bleeding (2/60) |
| AZD-2171 (Cediranib) (Karakunnel, 2009) |
II Doc-resistant mCRPC |
34 | OR: 17% (4/23) PSA not reported mPFS and mOS not reported |
vomiting (2), prolonged QTc interval (1) and muscle weakness (3), weight loss (3), dehydration (4), fatigue (6), hypoxia (1), renal failure (1), transaminitis (3), and anorexia (1) |
| Sorafenib (Aragon-Ching, 2009) |
II 88% Doc-resistant mCRPC |
24 | OR: 8% (1/13) PSA not reported mPFS =3.7 mo, mOS=18 mo |
Lab abnormalities (9), Dermatologic (4), Fatigue (2), Nausea (1), Anemia (1), Cath DVT (1), Infxn (1) |
| Sorafenib (Steinbild, 2007) |
II Chemo-naïve CRPC and mCRPC |
55 | OR: 0% (0/55), PSA: 3.6% (2/55) mPFS= 2 mo (8 weeks) mOS= had not been reached |
Dermatologic (5), Hypertension (3), Fatigue (2), Constipation (2) |
| Sorafenib + Docetaxel + Prednisone (Mardjuadi, 2009) |
I Chemo-naïve CRPC |
24 | OR: not reported PSA: 75% (15/20) PFS and OS not reported |
febrile neutropenia (8), uncomplicated neutropenia (5), and hand-foot syndrome (4) |
| Sunitinib + Docetaxel + Prednisone (Zurita, 2009) |
I/II Chemo-naïve mCRPC |
55 | OR: 39% (13/33) PSA: 56% (31/55) mPFS 42 weeks |
neutropenia (75%), febrile neutropenia (15%), fatigue (15%), stomatitis (7%), and anorexia (7%). 33% patients required docetaxel dose reduction. |
| Metronomic Cyclophosphamide (mCTX) (Lord, 2007) |
II Chemo-naïve CRPC and mCRPC |
58 | OR: 1.7% (1/58) PSA: 3.4% (2/58) mOS had not yet been reached |
Grade 3 lymphopenia in 33%. Required dose reduction in 5 patients. |
| mCTX + Celecoxib + Dexamethasone (Fontana, 2009) |
II 68% Doc-resistant mCRPC |
28 | OR: 20% (1/5) PSA: 32% (9/28) mPFS=3 mo, mOS=21 mo |
No grade 3 or 4 adverse events were reported. |
| Thalidomide (Figg, 2001) |
II 74% chemo naïve CRPC and mCRPC |
63 | OR: 0% (0/35) PSA: 14% (9/63) mTTF: 2.1–2.2 mo mOS 15.8 mo |
18 events >=grade 3. Suicide within 30 days of study drug discontinuation in one patient. One patient developed acute AML (hx of prolonged prior cytotoxic exposure. |
| Thalidomide + Docetaxel (Dahut, 2004) |
II Chemo naïve mCRPC |
47 | OR: 35% (7/20) PSA: 53% (25/47) mPFS: 5.9 mo, mOS: 25.9 mo |
All grade 3: neutropenia (4), anemia (2), Thromboembolism (9), fatigue (2), Hyperglycemia (7) |
| Lenalidomide + Docetaxel + Prednisone (Moss, 2007) |
I mCRPC w/<2 prior Chemo regimens |
19 | OR 38.5% (5/13) PSA: 47% (9/19) PFS and OS not reported |
Grade 3 neutropenia (3), Thromboembolism (1) |
Definitions: N=: Total patients reported on study. OR: Overall Response (partial responses +complete responses). OR is only available for those patients with measurable disease and thus may report less patients than the total study number (N=). PSA: >50% decline from baseline. mPFS: median progression free survival. mOS: is median overall survival. Doc-resistant: patients had progressed on prior docetaxel therapy. When possible, grade 3 and 4 toxicity was limited to toxicity that was thought to be drug-related. mTTF: median time to failure.
Article Highlights Box.
- Angiogenesis plays an important role in prostate cancer growth and metastasis.
- Increased levels of VEGF correlate with metastasis and survival in prostate cancer.
- CALGB 90401, a large phase III trial of Docetaxel and Prednisone with or without Bevacizumab, has completed enrollment. Forthcoming results may lead to a change in the standard of care for treatment of metastatic castrate resistant prostate cancer.
- Phase 2 data on thalidomide and bevacizumab in addition to standard chemotherapy supports the potential role for dual anti-angiogenic therapy in castrate resistant prostate cancer.
- Treatment of mCRPC with agents targeting the angiogenesis pathway continues to be an area of active investigation.
Acknowledgments
This project has been supported by the Intramural Research Program of the National Cancer Institute, Center for Cancer Research, National Institutes of Health. The content of this publication does not reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Declaration of interest:
The authors state no conflict of interest and have received no payment in preparation of this manuscript
References
- 1.Horner MJRL, Krapcho M, Neyman N, Aminou R, Howlader N, et al. SEER Cancer Statistics Review. 2009 [cited 7/9/2009]; Available from: http://seer.cancer.gov/statfacts/html/prost.html.
- 2.Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004 Sep;172(3):910–4. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 3.Zietman AL, Coen JJ, Dallow KC, Shipley WU. The treatment of prostate cancer by conventional radiation therapy: an analysis of long-term outcome. Int J Radiat Oncol Biol Phys. 1995 May 15;32(2):287–92. doi: 10.1016/0360-3016(95)00123-G. [DOI] [PubMed] [Google Scholar]
- 4.Immediate versus deferred treatment for advanced prostatic cancer: initial results of the Medical Research Council Trial. The Medical Research Council Prostate Cancer Working Party Investigators Group. Br J Urol. 1997 Feb;79(2):235–46. doi: 10.1046/j.1464-410x.1997.d01-6840.x. [DOI] [PubMed] [Google Scholar]
- 5.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005 Jul 13;294(2):238–44. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 6.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004 Oct 7;351(15):1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 7.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004 Oct 7;351(15):1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong AJ, Carducci MA. Novel therapeutic approaches to advanced prostate cancer. Clin Adv Hematol Oncol. 2005 Apr;3(4):271–82. [PubMed] [Google Scholar]
- 9.Rosenberg JE, Weinberg VK, Kelly WK, Michaelson D, Hussain MH, Wilding G, et al. Activity of second-line chemotherapy in docetaxel-refractory hormone-refractory prostate cancer patients: randomized phase 2 study of ixabepilone or mitoxantrone and prednisone. Cancer. 2007 Aug 1;110(3):556–63. doi: 10.1002/cncr.22811. [DOI] [PubMed] [Google Scholar]
- 10.Ide A, Baker N, Warren S. Vascularization of the Brown-Pearce Rabbit Epithelioma Transplant As Seen in the Transparent Ear Chamber. Am J Roentgen. 1939;42(891) [Google Scholar]
- 11.Folkman J, Cole P, Zimmerman S. Tumor behavior in isolated perfused organs: in vitro growth and metastases of biopsy material in rabbit thyroid and canine intestinal segment. Ann Surg. 1966 Sep;164(3):491–502. doi: 10.1097/00000658-196609000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banerjee S, Dowsett M, Ashworth A, Martin LA. Mechanisms of disease: angiogenesis and the management of breast cancer. Nat Clin Pract Oncol. 2007 Sep;4(9):536–50. doi: 10.1038/ncponc0905. [DOI] [PubMed] [Google Scholar]
- 13.Folkman J. Anti-angiogenesis: new concept for therapy of solid tumors. Ann Surg. 1972 Mar;175(3):409–16. doi: 10.1097/00000658-197203000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Folkman J. Tumor angiogenesis: a possible control point in tumor growth. Ann Intern Med. 1975 Jan;82(1):96–100. doi: 10.7326/0003-4819-82-1-96. [DOI] [PubMed] [Google Scholar]
- 15.Ehrmann RL, Knoth M. Choriocarcinoma. Transfilter stimulation of vasoproliferation in the hamster cheek pouch. Studied by light and electron microscopy. J Natl Cancer Inst. 1968 Dec;41(6):1329–41. [PubMed] [Google Scholar]
- 16.Greenblatt M, Shubi P. Tumor angiogenesis: transfilter diffusion studies in the hamster by the transparent chamber technique. J Natl Cancer Inst. 1968 Jul;41(1):111–24. [PubMed] [Google Scholar]
- 17.Shing Y, Folkman J, Sullivan R, Butterfield C, Murray J, Klagsbrun M. Heparin affinity: purification of a tumor-derived capillary endothelial cell growth factor. Science. 1984 Mar 23;223(4642):1296–9. doi: 10.1126/science.6199844. [DOI] [PubMed] [Google Scholar]
- 18.Klagsbrun M, Soker S. VEGF/VPF: the angiogenesis factor found? Curr Biol. 1993 Oct 1;3(10):699–702. doi: 10.1016/0960-9822(93)90073-w. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Cozzi PJ. Angiogenesis as a strategic target for prostate cancer therapy. Med Res Rev. 2009 Jun 18; doi: 10.1002/med.20161. [DOI] [PubMed] [Google Scholar]
- 20.Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993 Aug;143(2):401–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Borre M, Offersen BV, Nerstrom B, Overgaard J. Microvessel density predicts survival in prostate cancer patients subjected to watchful waiting. Br J Cancer. 1998 Oct;78(7):940–4. doi: 10.1038/bjc.1998.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duque JL, Loughlin KR, Adam RM, Kantoff PW, Zurakowski D, Freeman MR. Plasma levels of vascular endothelial growth factor are increased in patients with metastatic prostate cancer. Urology. 1999 Sep;54(3):523–7. doi: 10.1016/s0090-4295(99)00167-3. [DOI] [PubMed] [Google Scholar]
- 23.George DJ, Halabi S, Shepard TF, Vogelzang NJ, Hayes DF, Small EJ, et al. Prognostic significance of plasma vascular endothelial growth factor levels in patients with hormone-refractory prostate cancer treated on Cancer and Leukemia Group B 9480. Clin Cancer Res. 2001 Jul;7(7):1932–6. [PubMed] [Google Scholar]
- 24.El-Gohary YM, Silverman JF, Olson PR, Liu YL, Cohen JK, Miller R, et al. Endoglin (CD105) and vascularendothelial growth factor as prognostic markers in prostatic adenocarcinoma. Am J Clin Pathol. 2007 Apr;127(4):572–9. doi: 10.1309/X6NXYE57DLUE2NQ8. [DOI] [PubMed] [Google Scholar]
- 25.Du Z, Fujiyama C, Chen Y, Masaki Z. Expression of hypoxia-inducible factor 1alpha in human normal, benign, and malignant prostate tissue. Chin Med J (Engl) 2003 Dec;116(12):1936–9. [PubMed] [Google Scholar]
- 26.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005 Dec 15;438(7070):967–74. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 27.FDA. FDA label information for bevacizumab. 2009 [cited 2009 8/3/2009]; Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/125085s0168lbl.pdf.
- 28.Reese DM, Fratesi P, Corry M, Novotny W. A Phase II Trial of Humanized Anti-Vascular Endothelial Growth Factor Antibody for the Treatment of Androgen-Independent Prostate Cancer. The Prostate Journal. 2002;3(2):65–70. [Google Scholar]
- 29.Picus JHS, Rini B. The use of bevacizumab (B) with docetaxel (D) and estramustine (E) in hormone refractory prostate cancer (HRPC): Initial results of CALGB 90006. Proc Am Soc Clin Oncol. 2003 [abstract] 1578. [Google Scholar]
- 30.Picus J. Docetaxel/Bevacizumab (Avastin) in prostate cancer. Cancer Invest. 2004;Supp 1(Abstract 46):60. [Google Scholar]
- 31.Di Lorenzo G, Figg WD, Fossa SD, Mirone V, Autorino R, Longo N, et al. Combination of bevacizumab and docetaxel in docetaxel-pretreated hormone-refractory prostate cancer: a phase 2 study. Eur Urol. 2008 Nov;54(5):1089–94. doi: 10.1016/j.eururo.2008.01.082. [DOI] [PubMed] [Google Scholar]
- 32.Phase III Randomized Study of Docetaxel and Prednisone With Versus Without Bevacizumab in Patients With Hormone-Refractory Metastatic Adenocarcinoma of the Prostate. [cited 2009 9/9/2009]; Available from: http://www.cancer.gov/clinicaltrials/CALGB-90401.
- 33.Figg WD, Dahut W, Duray P, Hamilton M, Tompkins A, Steinberg SM, et al. A randomized phase II trial of thalidomide, an angiogenesis inhibitor, in patients with androgen-independent prostate cancer. Clin Cancer Res. 2001 Jul;7(7):1888–93. [PubMed] [Google Scholar]
- 34.Ning YM, Arlen PM, Gulley WD, Stein A. A phase II trial of thalidomide, bevacizumab, and docetaxel in patients with metastatic castration-refractory prostate cancer (CRPC) J Clin Oncol. 2008;26(May 20 Suppl) Abstract #5000. [Google Scholar]
- 35.Rini BI, Weinberg V, Fong L, Conry S, Hershberg RM, Small EJ. Combination immunotherapy with prostatic acid phosphatase pulsed antigen-presenting cells (provenge) plus bevacizumab in patients with serologic progression of prostate cancer after definitive local therapy. Cancer. 2006 Jul 1;107(1):67–74. doi: 10.1002/cncr.21956. [DOI] [PubMed] [Google Scholar]
- 36.Chu QS. Aflibercept (AVE0005): an alternative strategy for inhibiting tumour angiogenesis by vascular endothelial growth factors. Expert Opin Biol Ther. 2009 Feb;9(2):263–71. doi: 10.1517/14712590802666397. [DOI] [PubMed] [Google Scholar]
- 37.Masarelli E, Miller V, Leighl N. Phase II study of the efficacy and safety of intravenous (IV) AVE0005 (VEGF-Trap) given every 2 weeks in patients (pts) with platinum-and erlotinib-resistant adenocarcinoma of the lung (NSCLA) J Clin Oncol. 2007;25(18s) Abstract # 7627. [Google Scholar]
- 38.Tew W, Colombo N, Ray-Coquard A. VEGF-Trap for patients (pts) with recurrent platinum-resistant epithelial ovarian cancer (EOC): Preliminary results of a randomized, multicenter phase II study. J Clin Oncol. 2007;25(18s) Abstract # 5508. [Google Scholar]
- 39.Ton NC, Parker GJ, Jackson A, Mullamitha S, Buonaccorsi GA, Roberts C, et al. Phase I evaluation of CDP791, a PEGylated di-Fab’ conjugate that binds vascular endothelial growth factor receptor 2. Clin Cancer Res. 2007 Dec 1;13(23):7113–8. doi: 10.1158/1078-0432.CCR-07-1550. [DOI] [PubMed] [Google Scholar]
- 40.Camidge D, Eckhardt S, Diab S, Gore L. A phase I dose-escalation study of weekly IMC-1121B, a fully human anti-vascular endothelial growth factor receptor 2 (VEGFR2) IgG1 monoclonal antibody (Mab), in patients (pts) with advanced cancer. J Clin Oncol. 2006;24(18s) Abstract # 3032. [Google Scholar]
- 41.Krishnamurthi S, LoRusso P, Goncalves F, Fox E, Rowinsky J. Phase I study of weekly anti-vascular endothelial growth factor receptor-1 (VEGFR-1) monoclonal antibody IMC-18F1 in patients with advanced solid malignancies. J Clin Oncol. 2008;26(May 20 Suppl) Abstract # 14630. [Google Scholar]
- 42.Wedge SR, Kendrew J, Hennequin LF, Valentine PJ, Barry ST, Brave SR, et al. AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005 May 15;65(10):4389–400. doi: 10.1158/0008-5472.CAN-04-4409. [DOI] [PubMed] [Google Scholar]
- 43.Ryan CJ, Stadler WM, Roth B, Hutcheon D, Conry S, Puchalski T, et al. Phase I dose escalation and pharmacokinetic study of AZD2171, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinase, in patients with hormone refractory prostate cancer (HRPC) Invest New Drugs. 2007 Oct;25(5):445–51. doi: 10.1007/s10637-007-9050-y. [DOI] [PubMed] [Google Scholar]
- 44.Karakunnel J, Gulley J, Arlen P, Mulquin M, Wright J. Cediranib (AZD2171) in docetaxel-resistant, castration-resistant prostate cancer (CRPC) J Clin Oncol. 2009;17(15s) Abstract # 5141. [Google Scholar]
- 45.Bradley DP, Tessier JJ, Lacey T, Scott M, Jurgensmeier JM, Odedra R, et al. Examining the acute effects of cediranib (RECENTIN, AZD2171) treatment in tumor models: a dynamic contrast-enhanced MRI study using gadopentate. Magn Reson Imaging. 2009 Apr;27(3):377–84. doi: 10.1016/j.mri.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 46.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004 Oct 1;64(19):7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 47.Dahut WL, Scripture C, Posadas E, Jain L, Gulley JL, Arlen PM, et al. A phase II clinical trial of sorafenib in androgen-independent prostate cancer. Clin Cancer Res. 2008 Jan 1;14(1):209–14. doi: 10.1158/1078-0432.CCR-07-1355. [DOI] [PubMed] [Google Scholar]
- 48.Aragon-Ching JB, Jain L, Gulley JL, Arlen PM, Wright JJ, Steinberg SM, et al. Final analysis of a phase II trial using sorafenib for metastatic castration-resistant prostate cancer. BJU Int. 2009 Jun;103(12):1636–40. doi: 10.1111/j.1464-410X.2008.08327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steinbild S, Mross K, Frost A, Morant R, Gillessen S, Dittrich C, et al. A clinical phase II study with sorafenib in patients with progressive hormone-refractory prostate cancer: a study of the CESAR Central European Society for Anticancer Drug Research-EWIV. Br J Cancer. 2007 Dec 3;97(11):1480–5. doi: 10.1038/sj.bjc.6604064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chi KN, Ellard SL, Hotte SJ, Czaykowski P, Moore M, Ruether JD, et al. A phase II study of sorafenib in patients with chemo-naive castration-resistant prostate cancer. Ann Oncol. 2008 Apr;19(4):746–51. doi: 10.1093/annonc/mdm554. [DOI] [PubMed] [Google Scholar]
- 51.Colloca G, Checcaglini F, Venturino A. About sorafenib in castration-resistant prostate cancer. Ann Oncol. 2008 Oct;19(10):1812–3. doi: 10.1093/annonc/mdn546. author reply 3–4. [DOI] [PubMed] [Google Scholar]
- 52.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008 Mar 1;26(7):1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takimoto CH, Awada A. Safety and anti-tumor activity of sorafenib (Nexavar) in combination with other anti-cancer agents: a review of clinical trials. Cancer Chemother Pharmacol. 2008 Apr;61(4):535–48. doi: 10.1007/s00280-007-0639-9. [DOI] [PubMed] [Google Scholar]
- 54.Mardjuadi F, Medioni J, Kerger J, Canon L, Duck S. A phase I study of Sorafenib in association with docetaxel-prednisone in chemonaive metastatic castrate-resistant prostate cancer. J Clin Oncol. 2009;27(15s) Abstract # 5153. [Google Scholar]
- 55.Fong TA, Shawver LK, Sun L, Tang C, App H, Powell TJ, et al. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 1999 Jan 1;59(1):99–106. [PubMed] [Google Scholar]
- 56.Mendel DB, Laird AD, Smolich BD, Blake RA, Liang C, Hannah AL, et al. Development of SU5416, a selective small molecule inhibitor of VEGF receptor tyrosine kinase activity, as an anti-angiogenesis agent. Anticancer Drug Des. 2000 Feb;15(1):29–41. [PubMed] [Google Scholar]
- 57.Stadler WM, Cao D, Vogelzang NJ, Ryan CW, Hoving K, Wright R, et al. A randomized Phase II trial of the antiangiogenic agent SU5416 in hormone-refractory prostate cancer. Clin Cancer Res. 2004 May 15;10(10):3365–70. doi: 10.1158/1078-0432.CCR-03-0404. [DOI] [PubMed] [Google Scholar]
- 58.Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol. 2007 Mar 1;25(7):884–96. doi: 10.1200/JCO.2006.06.3602. [DOI] [PubMed] [Google Scholar]
- 59.Zurita A, Shore M, Kozloff C, Ryan C, Beer TM. Phase I study of sunitinib in combination with docetaxel and prednisone in patients (pts) with metastatic hormone refractory prostate cancer (mHRPC). 2007 Prostate Cancer Symposium; 2007. Abstract # 230. [Google Scholar]
- 60.Zurita A, Liu G, Hutson T, Kozloff M, Shore N. Sunitinib in combination with docetaxel and prednisone in patients (pts) with metastatic hormone-refractory prostate cancer (mHRPC) J Clin Oncol. 2009;27(15s) Abstract # 5166. [Google Scholar]
- 61.Zurita A, Ward J, Araujo C, Pettaway L, Pisters L. Presurgical sunitinib malate and androgen ablation in patients with localized prostate cancer at high risk for recurrence. J Clin Oncol. 2008;26(May 20 Suppl) Abstract # 16004. [Google Scholar]
- 62.Herbst RS, Heymach JV, O’Reilly MS, Onn A, Ryan AJ. Vandetanib (ZD6474): an orally available receptor tyrosine kinase inhibitor that selectively targets pathways critical for tumor growth and angiogenesis. Expert Opin Investig Drugs. 2007 Feb;16(2):239–49. doi: 10.1517/13543784.16.2.239. [DOI] [PubMed] [Google Scholar]
- 63.Natale RB, Bodkin D, Govindan R, Sleckman BG, Rizvi NA, Capo A, et al. Vandetanib versus gefitinib in patients with advanced non-small-cell lung cancer: results from a two-part, double-blind, randomized phase ii study. J Clin Oncol. 2009 May 20;27(15):2523–9. doi: 10.1200/JCO.2008.18.6015. [DOI] [PubMed] [Google Scholar]
- 64.Teicher BA, Alvarez E, Menon K, Esterman MA, Considine E, Shih C, et al. Antiangiogenic effects of a protein kinase Cbeta-selective small molecule. Cancer Chemother Pharmacol. 2002 Jan;49(1):69–77. doi: 10.1007/s00280-001-0386-2. [DOI] [PubMed] [Google Scholar]
- 65.Graff JR, McNulty AM, Hanna KR, Konicek BW, Lynch RL, Bailey SN, et al. The protein kinase Cbeta-selective inhibitor, Enzastaurin ( LY317615.HCl), suppresses signaling through the AKT pathway, induces apoptosis, and suppresses growth of human colon cancer and glioblastoma xenografts. Cancer Res. 2005 Aug 15;65(16):7462–9. doi: 10.1158/0008-5472.CAN-05-0071. [DOI] [PubMed] [Google Scholar]
- 66.Keyes KA, Mann L, Sherman M, Galbreath E, Schirtzinger L, Ballard D, et al. LY317615 decreases plasma VEGF levels in human tumor xenograft-bearing mice. Cancer Chemother Pharmacol. 2004 Feb;53(2):133–40. doi: 10.1007/s00280-003-0713-x. [DOI] [PubMed] [Google Scholar]
- 67.Kerbel RS. Inhibition of tumor angiogenesis as a strategy to circumvent acquired resistance to anti-cancer therapeutic agents. Bioessays. 1991 Jan;13(1):31–6. doi: 10.1002/bies.950130106. [DOI] [PubMed] [Google Scholar]
- 68.Hanahan D, Bergers G, Bergsland E. Less is more, regularly: metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Invest. 2000 Apr;105(8):1045–7. doi: 10.1172/JCI9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O’Reilly MS, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000 Apr 1;60(7):1878–86. [PubMed] [Google Scholar]
- 70.Kakolyris S, Samonis G, Koukourakis M, Vlachonicolis I, Chalkiadakis G, Kalbakis K, et al. Treatment of non-small-cell lung cancer with prolonged oral etoposide. Am J Clin Oncol. 1998 Oct;21(5):505–8. doi: 10.1097/00000421-199810000-00018. [DOI] [PubMed] [Google Scholar]
- 71.Alvarez A, Mickiewicz E, Brosio C, Giglio R, Cinat G. Reinduction of Response with Weekly Taxol (T) in Advanced Breast Cancer (ABC) 1999. ASCO Annual Meeting; 1999. Abstract #636. [Google Scholar]
- 72.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004 Jun;4(6):423–36. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 73.Hamano Y, Sugimoto H, Soubasakos MA, Kieran M, Olsen BR, Lawler J, et al. Thrombospondin-1 associated with tumor microenvironment contributes to low-dose cyclophosphamide-mediated endothelial cell apoptosis and tumor growth suppression. Cancer Res. 2004 Mar 1;64(5):1570–4. doi: 10.1158/0008-5472.can-03-3126. [DOI] [PubMed] [Google Scholar]
- 74.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005 Apr 1;105(7):2862–8. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 75.Glode LM, Barqawi A, Crighton F, Crawford ED, Kerbel R. Metronomic therapy with cyclophosphamide and dexamethasone for prostate carcinoma. Cancer. 2003 Oct 15;98(8):1643–8. doi: 10.1002/cncr.11713. [DOI] [PubMed] [Google Scholar]
- 76.Lord R, Nair S, Schache A, Spicer J, Somaihah N, Khoo V, et al. Low dose metronomic oral cyclophosphamide for hormone resistant prostate cancer: a phase II study. J Urol. 2007 Jun;177(6):2136–40. doi: 10.1016/j.juro.2007.01.143. discussion 40. [DOI] [PubMed] [Google Scholar]
- 77.Fontana A, Galli L, Fioravanti A, Orlandi P, Galli C, Landi L, et al. Clinical and pharmacodynamic evaluation of metronomic cyclophosphamide, celecoxib, and dexamethasone in advanced hormone-refractory prostate cancer. Clin Cancer Res. 2009 Aug 1;15(15):4954–62. doi: 10.1158/1078-0432.CCR-08-3317. [DOI] [PubMed] [Google Scholar]
- 78.Di Lorenzo G, Autorino R, De Laurentiis M, Forestieri V, Romano C, Prudente A, et al. Thalidomide in combination with oral daily cyclophosphamide in patients with pretreated hormone refractory prostate cancer: a phase I clinical trial. Cancer Biol Ther. 2007 Mar;6(3):313–7. doi: 10.4161/cbt.6.3.3664. [DOI] [PubMed] [Google Scholar]
- 79.Sweat SD, Pacelli A, Murphy GP, Bostwick DG. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology. 1998 Oct;52(4):637–40. doi: 10.1016/s0090-4295(98)00278-7. [DOI] [PubMed] [Google Scholar]
- 80.Chang SS, Reuter VE, Heston WD, Bander NH, Grauer LS, Gaudin PB. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999 Jul 1;59(13):3192–8. [PubMed] [Google Scholar]
- 81.Liu H, Moy P, Kim S, Xia Y, Rajasekaran A, Navarro V, et al. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res. 1997 Sep 1;57(17):3629–34. [PubMed] [Google Scholar]
- 82.Jeske S, Milowsky MI, Smith C, Smith KA, Bander NH. Phase II trial of the anti-prostate specific membrane antigen (PSMA) monoclonal antibody (mAb) J591 plus low-dose interleukin-2 (IL-2) in patients (pts) with recurrent prostate cancer (PC) J Clin Oncol. 2007;25(18s) Abstract # 15558. [Google Scholar]
- 83.Bander NH, Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ. Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J Clin Oncol. 2005 Jul 20;23(21):4591–601. doi: 10.1200/JCO.2005.05.160. [DOI] [PubMed] [Google Scholar]
- 84.Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ, Bander NH. Phase I trial of yttrium-90-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for androgen-independent prostate cancer. J Clin Oncol. 2004 Jul 1;22(13):2522–31. doi: 10.1200/JCO.2004.09.154. [DOI] [PubMed] [Google Scholar]
- 85.Ng SS, MacPherson GR, Gutschow M, Eger K, Figg WD. Antitumor effects of thalidomide analogs in human prostate cancer xenografts implanted in immunodeficient mice. Clin Cancer Res. 2004 Jun 15;10(12 Pt 1):4192–7. doi: 10.1158/1078-0432.CCR-03-0700. [DOI] [PubMed] [Google Scholar]
- 86.Li H, Raia V, Bertolini F, Price DK, Figg WD. Circulating endothelial cells as a therapeutic marker for thalidomide in combined therapy with chemotherapy drugs in a human prostate cancer model. BJU Int. 2008 Apr;101(7):884–8. doi: 10.1111/j.1464-410X.2007.07342.x. [DOI] [PubMed] [Google Scholar]
- 87.Paravar T, Lee DJ. Thalidomide: mechanisms of action. Int Rev Immunol. 2008;27(3):111–35. doi: 10.1080/08830180801911339. [DOI] [PubMed] [Google Scholar]
- 88.Galustian C, Dalgleish A. Lenalidomide: a novel anticancer drug with multiple modalities. Expert Opin Pharmacother. 2009 Jan;10(1):125–33. doi: 10.1517/14656560802627903. [DOI] [PubMed] [Google Scholar]
- 89.Kelsey FO. Thalidomide update: regulatory aspects. Teratology. 1988 Sep;38(3):221–6. doi: 10.1002/tera.1420380305. [DOI] [PubMed] [Google Scholar]
- 90.Mellin GW, Katzenstein M. The saga of thalidomide. Neuropathy to embryopathy, with case reports of congenital anomalies. N Engl J Med. 1962 Dec 13;267:1238–44. doi: 10.1056/NEJM196212132672407. concl. [DOI] [PubMed] [Google Scholar]
- 91.D’Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):4082–5. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Drake MJ, Robson W, Mehta P, Schofield I, Neal DE, Leung HY. An open-label phase II study of low-dose thalidomide in androgen-independent prostate cancer. Br J Cancer. 2003 Mar 24;88(6):822–7. doi: 10.1038/sj.bjc.6600817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dixon SC, Kruger EA, Bauer KS, Figg WD. Thalidomide up-regulates prostate-specific antigen secretion from LNCaP cells. Cancer Chemother Pharmacol. 1999;43( Suppl):S78–84. doi: 10.1007/s002800051103. [DOI] [PubMed] [Google Scholar]
- 94.Kurdziel K, Bacharach S, Carrasquillo J, Huebsch S, Whatley M, Sellers D, et al. 8:45–9:00. Using PET 18F-FDG, 11CO, and 15O-water for Monitoring Prostate Cancer During a Phase II Anti-angiogenic Drug Trial with Thalidomide. Clin Positron Imaging. 2000 Jul;3(4):144. doi: 10.1016/s1095-0397(00)00056-x. [DOI] [PubMed] [Google Scholar]
- 95.Kurdziel KA, Figg WD, Carrasquillo JA, Huebsch S, Whatley M, Sellers D, et al. Using positron emission tomography 2-deoxy-2-[18F]fluoro-D-glucose, 11CO, and 15O-water for monitoring androgen independent prostate cancer. Mol Imaging Biol. 2003 Mar-Apr;5(2):86–93. doi: 10.1016/s1536-1632(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 96.Figg WD, Arlen P, Gulley J, Fernandez P, Noone M, Fedenko K, et al. A randomized phase II trial of docetaxel (taxotere) plus thalidomide in androgen-independent prostate cancer. Semin Oncol. 2001 Aug;28(4 Suppl 15):62–6. doi: 10.1016/s0093-7754(01)90157-5. [DOI] [PubMed] [Google Scholar]
- 97.Dahut WL, Gulley JL, Arlen PM, Liu Y, Fedenko KM, Steinberg SM, et al. Randomized phase II trial of docetaxel plus thalidomide in androgen-independent prostate cancer. J Clin Oncol. 2004 Jul 1;22(13):2532–9. doi: 10.1200/JCO.2004.05.074. [DOI] [PubMed] [Google Scholar]
- 98.Figg W, Retter A, Steinberg S, Dahut W. In Reply. J Clin Oncol. 2005;23(9):2113–4. [Google Scholar]
- 99.Daliani DD, Dieringer C, Papandreou L, Pagliaro L. A tolerance and efficacy study of thalidomide, paclitaxel, and estramustine for patients with chemotherapy refractory androgen independent prostate cancer. Cancer Invest. 2004;22(62) Abstract # 48. [Google Scholar]
- 100.Figg WD, Li H, Sissung T, Retter A, Wu S, Gulley JL, et al. Pre-clinical and clinical evaluation of estramustine, docetaxel and thalidomide combination in androgen-independent prostate cancer. BJU Int. 2007 May;99(5):1047–55. doi: 10.1111/j.1464-410X.2007.06763.x. [DOI] [PubMed] [Google Scholar]
- 101.Figg WD, Hussain MH, Gulley JL, Arlen PM, Aragon-Ching JB, Petrylak DP, et al. A double-blind randomized crossover study of oral thalidomide versus placebo for androgen dependent prostate cancer treated with intermittent androgen ablation. J Urol. 2009 Mar;181(3):1104–13. doi: 10.1016/j.juro.2008.11.026. discussion 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Amato RJ, Hernandez-McClain J, Henary H. Phase 2 study of granulocyte-macrophage colony-stimulating factor plus thalidomide in patients with hormone-naive adenocarcinoma of the prostate. Urol Oncol. 2009 Jan–Feb;27(1):8–13. doi: 10.1016/j.urolonc.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 103.Tohnya TM, Ng SS, Dahut WL, Wright JJ, Arlen PM, Gulley JL, et al. A phase I study of oral CC-5013 (lenalidomide, Revlimid), a thalidomide derivative, in patients with refractory metastatic cancer. Clin Prostate Cancer. 2004 Mar;2(4):241–3. doi: 10.3816/cgc.2004.n.006. [DOI] [PubMed] [Google Scholar]
- 104.Dahut WL, Aragon-Ching JB, Woo S, Tohnya TM, Gulley JL, Arlen PM, et al. Phase I study of oral lenalidomide in patients with refractory metastatic cancer. J Clin Pharmacol. 2009 Jun;49(6):650–60. doi: 10.1177/0091270009335001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sanborn S, Cooney M, Gibbons J. Phase I trial of daily lenalidomide and docetaxel given every three weeks in patients with advanced solid tumors. J Clin Oncol. 2007:25. Abstract # 3570. [Google Scholar]
- 106.Sanborn SL, Gibbons J, Krishnamurthi S, Brell JM, Dowlati A, Bokar JA, et al. Phase I trial of docetaxel given every 3 weeks and daily lenalidomide in patients with advanced solid tumors. Invest New Drugs. 2008 Nov 15; doi: 10.1007/s10637-008-9200-x. [DOI] [PubMed] [Google Scholar]
- 107.Moss R, Mohile S, Shelton G, Melia J. A phase I open-label study using lenalidomide and docetaxel in adnrogen-independent prostate cancer (AIPC). Prostate Cancer Symposium; 2007. Abstract # 89. [Google Scholar]
- 108.Pagliaro L, Tannir N, Tu S, Moomey C. A modular phase I study of lenalidomide and paclitaxel in metastatic castrate resistant prostate cancer with prior taxane therapy. J Clin Oncol. 2008;26(May 20 Suppl) doi: 10.1007/s00280-009-1237-9. Abstract # 13545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Garcia JA, Triozzi P, Elson P, Cooney M. Clinical activity of ketoconazole and lenolidomide in castrate progressive prostate carcinoma (CPPCA): Preliminary results of a Phase II trial. J Clin Oncol. 2008;26(May 20 Suppl) Abstract # 5143. [Google Scholar]
- 110.Kumar S, Raje N, Hideshima T, Ishitsuka K, Roccaro A, Shiraishi N, et al. Antimyeloma activity of two novel N-substituted and tetraflourinated thalidomide analogs. Leukemia. 2005 Jul;19(7):1253–61. doi: 10.1038/sj.leu.2403776. [DOI] [PubMed] [Google Scholar]
- 111.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996 Sep;16(9):4604–13. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling -in control of vascular function. Nat Rev Mol Cell Biol. 2006 May;7(5):359–71. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 113.Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999 Nov 15;59(22):5830–5. [PubMed] [Google Scholar]
- 114.Dikmen ZG, Gellert GC, Dogan P, Yoon H, Lee YB, Ahn CH, et al. In vivo and in vitro effects of a HIF-1alpha inhibitor, RX-0047. J Cell Biochem. 2008 Jun 1;104(3):985–94. doi: 10.1002/jcb.21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marignol L, Coffey M, Lawler M, Hollywood D. Hypoxia in prostate cancer: a powerful shield against tumour destruction? Cancer Treat Rev. 2008 Jun;34(4):313–27. doi: 10.1016/j.ctrv.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 116.Sooriakumaran P, Coley HM, Fox SB, Macanas-Pirard P, Lovell DP, Henderson A, et al. A randomized controlled trial investigating the effects of celecoxib in patients with localized prostate cancer. Anticancer Res. 2009 May;29(5):1483–8. [PubMed] [Google Scholar]
- 117.Pruthi RS, Derksen JE, Moore D, Carson CC, Grigson G, Watkins C, et al. Phase II trial of celecoxib in prostate-specific antigen recurrent prostate cancer after definitive radiation therapy or radical prostatectomy. Clin Cancer Res. 2006 Apr 1;12(7 Pt 1):2172–7. doi: 10.1158/1078-0432.CCR-05-2067. [DOI] [PubMed] [Google Scholar]
- 118.Kearney PM, Baigent C, Godwin J, Halls H, Emberson JR, Patrono C. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ. 2006 Jun 3;332(7553):1302–8. doi: 10.1136/bmj.332.7553.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006 Jul 1;24(19):3089–94. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 120.Kantoff P, Schuetz T, Blumenstein B, Glode M, Bilhartz D, Gulley J, et al. Overall survival (OS) analysis of a phase II randomized controlled trial (RCT) of a poxviral-based PSA targeted immunotherapy in metastatic castration-resistant prostate cancer (mCRPC) J Clin Oncol. 2009;27(15s) doi: 10.1200/JCO.2009.25.0597. suppl; abstract 5013. [DOI] [PMC free article] [PubMed] [Google Scholar]