Abstract
Objectives
Obtaining assent and respecting dissent are widely adopted safeguards when conducting dementia research involving individuals who lack consent capacity, but there is no consensus on how assent and dissent should be defined or what procedures should be used regarding them. Our objective was to provide recommendations on these issues based on the opinions of knowledgeable key informants.
Design
Cross-sectional qualitative research.
Setting
University research institutions.
Participants
Forty informants, including (1) nationally known experts on dementia and research ethics, (2) dementia researchers, and (3) dementia caregivers and advocates.
Measurements
Semi-structured individual and focus group interviews, audio recorded, and transcribed for content analysis.
Results
Assent and dissent should be defined broadly and based on an assessment of how adults who lack consent capacity can express or indicate their preferences verbally, behaviorally, or emotionally. Assent requires the ability to indicate a meaningful choice and at least a minimal level of understanding. Assent should be required whenever an individual has the ability to assent, and dissent should be binding if it is unequivocal or sustained after an effort to relieve concerns and/or distress. Standards for seeking assent and respecting dissent should not be linked to the risks or potential benefits of a study. Lacking the ability to assent and/or dissent should not automatically preclude research participation.
Conclusions
Obtaining assent and respecting dissent from individuals who lack consent capacity for dementia research allows them to participate, to the extent possible, in the consent process. Assent and dissent are important independent ethical constructs.
Keywords: dementia research, human subjects, assent, dissent, ethics
Objective
Individuals with dementia are at significant risk of lacking the capacity to give informed consent to enroll in research (1–3). The high prevalence of non-cognitive disturbances, such as agitation and suspiciousness (4–6), both lessen the likelihood that individuals can provide informed consent (7) and might indicate objections to research participation. Simply excluding from research individuals who lack consent capacity would dramatically and perhaps inappropriately limit the ability to address important research questions. Thus, enrolling participants who lack consent capacity or express objections to research procedures raises ethical, legal, and practical challenges for researchers.
Two safeguards—obtaining assent and respecting dissent—have been widely recommended as means of addressing these concerns (8–10). Assent has been referred to as an affirmative agreement to participate in research (10), while dissent has been described as an objection to participation (8). However, no consensus has evolved on how these concepts should be defined for dementia research or what procedures researchers should use in practice (9–13). This has the potential drawback of delaying or preventing research to understand and treat disorders such as agitation, since it can be difficult to discern if the behavior is an indication of dissent or a symptom of the disease that needs to be treated, and for persons with severe cognitive impairment in whom the ability to clearly state a willingness to participate may be impaired.
To improve this situation, we sought the opinions of key informants to determine whether consensus exists on answers to the following questions: 1) how should assent and dissent for dementia research be defined for persons who lack consent capacity, 2) what abilities are needed to provide assent, 3) should standards for assent and dissent vary by the risks or benefits of a study, 4) under what circumstances should assent be required, and 5) when should dissent be binding?
Methods
This study was approved by the Institutional Review Boards (IRB) of the Johns Hopkins Medical Institutions and the University of Pennsylvania. Written informed consent was obtained from all participants. Qualitative research methods were used to collect and analyze data derived through a series of interviews and focus groups with key informants. These methods are based on the principles of grounded theory (14) where the analysis of data iteratively compares the subjective views of participants. Using both individual interviews and group discussions provides triangulation of data to support the trustworthiness of our findings (15).
Study Sample
The study included three categories of participants: 1) a panel of nationally known experts on dementia, dementia research, decisional capacity, and research ethics, 2) two panels of local dementia researchers (in Baltimore and Philadelphia), and 3) a panel (in Baltimore) of local caregivers and advocates for dementia patients. Sample selection was based on an a priori decision to have representative coverage of variables (i.e., phenomenal variation) (16) likely to be important for understanding the process of how researchers seek assent and respect dissent in dementia research. For example, participants were selected to reflect relevant disciplines (e.g., geriatric medicine, neurology, psychiatry, neuropsychology), research roles (e.g., principal investigators, research assistants), and relationships to dementia patients (e.g., family caregivers, proxy decision-makers). Participants were identified by the investigators through their knowledge of experts in the field and their professional networks of individuals conducting dementia research. National experts were selected based on their relevant publications, training, research, and clinical experience, and were drawn from major public, private, and governmental research institutions across the United States. Local researchers were selected based on their training, research experience, and the role they have in conducting dementia research. They were drawn from two universities and a private health care facility in Baltimore and from two universities and a government health care facility in Philadelphia. Caregivers and advocates were family caregivers for dementia patients, proxy decision-makers or study partners for dementia research participants, or worked in relevant educational and advocacy organizations such as the Alzheimer’s Association. Participants were recruited by sending a personal letter from the principal investigator (BSB), with the consent form, requesting the individual’s participation in the project. Of the 62 individuals approached, 44 (71%) agreed to participate. Those who declined were distributed fairly evenly (3–7 individuals) across the four panels and cited prior commitments as the most common reason.
Data Collection and Analysis
We used a multi-staged approach to obtain participants’ opinions on assent and dissent for dementia research. First, using a semi-structured guide, national panel members were interviewed individually by telephone. The interview guide included open-ended questions developed to address issues on assent and dissent that have been raised in prior published recommendations, existing guidelines, the extant literature, and the investigators’ experiences. Questions focused on how to define assent and dissent, what abilities are needed to provide assent, whether a study’s potential risks or benefits should influence assent or dissent standards, when assent is required, and when dissent is binding. Participants were not asked explicitly to differentiate their responses by an individual’s degree of cognitive impairment. Interviews lasted approximately 1 hour each. We then conducted a series of focus groups—one with each of the three local panels of informants. These discussions followed the same line of questions addressed in the interviews with national panel members. Each focus group lasted approximately 2½ hours. Thereafter, we convened the national panel to review the major findings from the prior interviews and focus groups and determine if they could reach consensus on recommendations regarding assent and dissent for dementia research. The national experts met in person for their discussion, which lasted approximately 3 hours.
Each interview and focus group discussion was audio recorded and transcribed verbatim. All transcripts were verified, coded (14), summarized, and content analyzed (17) using QSR NUD*IST (18) qualitative data software. Codes were created initially to answer central study questions (e.g., Assent defined); additional codes were included as new themes emerged from the data (e.g., Dissent challenges). We analyzed the data using a four step approach. First, the national panel members’ interview data were analyzed using the constant comparative method within and across interviews (19). No new major themes emerged from the final interview, suggesting that we had achieved theoretical saturation (20). Second, as the three local focus groups were completed, their data were analyzed using the constant comparative method. Third, a matrix was developed to compare findings on each study question across the four panels derived from the interviews and focus groups. Based on that comparison, preliminary findings on the key study questions were itemized. To indicate the extent to which participants within and across groups agreed on these issues, each item was categorized according to one of three levels of agreement. Category A signified consensus or near consensus, level B indicated majority agreement, and C denoted there was no clear majority opinion on the issue. This categorized list of preliminary findings was sent to the national experts for review. For the national panel’s focus group, all items on the list of preliminary findings were discussed, but most of the discussion focused on items on which there had been less agreement (levels B and C). Finally, based on content analysis of their discussion we concluded that data saturation on the study’s key questions was achieved. The national panel served as final arbiters for the guidance offered in this report.
Results
Forty of the 44 individuals enrolled in the study participated in interviews and/or focus groups. Table 1 shows characteristics of the total sample and each panel. Participants were most likely to be research co-investigators (45.5%) or principal investigators (35.0%). The most common types of dementia research in which participants had been involved were drug (47.5%) and non-drug clinical trials (47.5%), followed by epidemiologic (40%) and natural history (30%) studies. Most local researchers (90–91%) and national experts (75%) had conducted the informed consent process for research.
Table 1.
Study Participants
| Characteristics | Total (n=40) a | National Experts (n=8) b | Baltimore Researchers (n=11) | Philadelphia Researchers (n=10) | Caregivers/Advocates (n=11) |
|---|---|---|---|---|---|
| Demographics | |||||
| Female (%) | 57.5 | 37.5 | 54.5 | 40.0 | 90.9 |
| White (%) | 82.5 | 87.5 | 72.7 | 90.0 | 81.8 |
| Years of Education, Mean (SD) | 19.1 (2.8) | 20.8 (2.6) | 19.8 (3.6) | 18.3 (2.1) | 17.8 (2.1) |
| Training in Bioethics c (%) | 45.0 | 75.0 | 54.5 | 40.0 | 18.2 |
| Involvement in Research | |||||
| Role d (%) | |||||
| IRB Member e | 15.0 | 37.5 | 0.0 | 20.0 | 9.1 |
| Investigator | 50.0 | 87.5 | 63.6 | 60.0 | 0.0 |
| Research Coordinator/Assistant | 25.0 | 0.0 | 54.6 | 40.0 | 0.0 |
| Proxy Dec. Maker | 17.5 | 0.0 | 9.1 | 0.0 | 54.6 |
| Advocates | 10.0 | 0.0 | 9.1 | 0.0 | 27.3 |
| Other | 25.0 | 12.5 | 9.1 | 30.0 | 45.5 |
| Conducted Consent Process (%) | 67.5 | 75.0 | 90.9 | 90.0 | 18.2 |
| Years Involved in Research, Mean (SD) | 11.4 (10.5) | 17.9 (7.6) | 11.8 (9.3) | 7.3 (7.3) | 10.1 (14.3) |
Excludes 4 individuals who agreed to participate but later could not attend their focus group meeting.
Includes 1 individual who was interviewed but later could not attend the focus group meeting.
Training in bioethics ranged from short courses to having majored in philosophy.
Some participants reported multiple roles in research.
IRB – Institutional review board.
Importance of Obtaining Assent and Respecting Dissent
Several participants raised the broad issue of why the concepts of assent and dissent are important. The national panel’s discussion provided clarity on this question. The primary justification for obtaining assent is respecting individuals’ remaining autonomy and providing the opportunity for cognitively impaired adults to be involved, to the extent possible, in the decision-making process. Seeking assent acknowledges that many individuals who lack consent capacity have residual abilities to understand, appreciate, reason, and express a choice at some level and demonstrates that their remaining abilities and opinions have value and should be respected. The primary justification for respecting dissent is to provide a measure of protection from risks or burdens that an individual would not want to accept and to make sure that the person’s dignity is maintained. It is a mechanism for shielding a person from distress or unwanted research activities.
Findings on our research questions are organized below beginning with those on assent, followed by those on dissent. For each issue, we describe the extent to which participants agreed and what the national panel concluded.
Assent
Assent Defined
All panels acknowledged that individuals who lack consent capacity for dementia research have a range of abilities to express or indicate assent. There was general agreement that the definition of assent for dementia research should reflect a broad conception of assent that includes an affirmative agreement to participate as expressed verbally (i.e., orally) or a non-verbal indication of willingness to cooperate with study procedures, both at the time of enrollment and over the course of the study.
Participants were shown the Common Rule’s definition of assent for research with childrena and asked if it should apply to cognitively impaired adults. Responses in each group varied, but the national panel concluded that regulations for children should not be applied to adults who lack consent capacity since the overwhelming majority of these individuals once had intact decisional capacity and many are likely to still have partial capacity. While the essence of the code’s definition of assent—willingness to participate—applies to both children and decisionally impaired adults, the national panel felt that it does not adequately capture the distinct differences between these adults and children. For example, prior attitudes, past preferences and life-long values, which would be lacking in children, are relevant when considering research participation by cognitively impaired adults. Also, many individuals with dementia who are unable to express their willingness verbally could do so behaviorally.
Abilities Needed to Provide Assent
Participants agreed that the ability to express or indicate a meaningful choice is essential for assent, and individuals who cannot communicate meaningfully cannot give assent. The word “meaningful” is difficult to define, but the implication is that the choice should be an authentic reflection of willingness to participate. Most thought that the absence of a minimal level of understanding precludes providing assent. The national panel recommended, from a procedural perspective, that the conversation a researcher has with an individual and the information the researcher provides about the study should occur at the highest level of the individual’s abilities to understand, appreciate, and reason. During that conversation, researchers should gauge what potential participants are capable of understanding and then determine whether they in fact do understand, appreciate, and reason at the level of their capacity. A majority of the national panel agreed that the ability to assent should not be linked to the potential risks of a study and protective measures related to a study’s risks should be handled external to the assent process.
Expressions or Indications of Assent
Participants agreed that assent can be expressed or indicated verbally (e.g., saying “yes”), behaviorally (e.g., acting agreeably), or emotionally (e.g., having a positive facial expression). Some participants suggested that individuals with greater impairment may be more likely to indicate their assent behaviorally than verbally. Informants on each panel noted that some individuals with dementia have an impaired ability to express themselves verbally (e.g., saying “no” when meaning yes or “yes” when meaning no) as the result of an aphasia and highlighted the challenges these impairments pose for researchers in seeking assent. While verbal expressions may be the most desirable form of assent, behavior (e.g., cooperation) may be the clearest indication of willingness to participate if an individual is unable to express a meaningful choice verbally or if his verbal expressions or affect are unreliable. The national panel recommended that if a person has the ability to express a meaningful choice verbally, verbal agreement should be required. Participants agreed unanimously that a person’s statement indicating he would “go along with” whatever the proxy decision-maker consents to should be considered assent, but there was less agreement on whether passivity or lack of objection should be regarded as an implied form of assent. Ultimately, the national panel concluded that assent should be an affirmative expression or indication of willingness and that assent is not the same as lack of objection or dissent.
When Assent Is or Is Not Required
There was general agreement that assent is required when an individual has the ability to assent. All agreed that research focused on individuals who have behavioral problems (e.g., resistance, agitation) can present major challenges for researchers in obtaining assent. Some informants thought that assent should not be required for observational studies with behaviorally disturbed individuals, a few were uncertain whether assent should be required for drug trials for behavioral disturbances, and others worried that some types of research could not be done if assent is always required. The national panel concluded that assent should not be required of individuals who cannot provide assent and that IRBs should determine whether individuals who lack the ability to assent can be enrolled in a specific study with proxy consent alone.
Dissent
Dissent Defined
Participants agreed that individuals who lack consent capacity also have a range of abilities to express or indicate dissent and that dissent is a verbal or non-verbal indication of unwillingness to participate in study procedures. While the clarity with which individuals express or indicate their unwillingness depends on their remaining abilities, all informants agreed that dissent does not require a reason or justification. The vast majority of informants thought that dissent can occur either initially at study recruitment or at any time during a study, and there was wide agreement that an individual may assent to some aspects of a study and dissent to other elements if that is allowed by the study’s protocol.
Expressions or Indications of Dissent
As with assent, informants agreed that dissent can be expressed or indicated verbally (e.g., saying “no”), behaviorally (e.g., not cooperating, being agitated, wanting to leave), or emotionally (e.g., showing distress, unhappiness). Some participants suggested that individuals with higher cognitive function may have a greater ability to articulate a firm or “hard no.” Because some individuals are unable, or find it difficult, to express their thoughts or feelings verbally and there can be different degrees of clarity in an individual’s behaviors or emotions, a researcher is obliged to try to determine the meaning of an individual’s behavior, mood, or expression that might indicate dissent. For example, aphasia can lead to a person’s repeatedly saying “no” when the person may not necessarily mean “no” or an agnosia can lead to emotional distress or saying “go away” because of the misperception that the person is being threatened. Discrepancies may also occur between an individual’s verbal, behavioral, or emotional responses. If the meaning of an individual’s statements or behaviors is initially unclear, most informants thought that the researcher should seek assistance from a knowledgeable informant, such as a caregiver, who understands the meaning of the individual’s communication. The national panel concluded that an inability to clarify ambiguity should result in a conservative approach by excluding the individual from the study or study procedure.
Respecting Dissent
Participants agreed that researchers should respect an individual’s objection whenever it occurs by not initiating the study or study procedure (or it may occur during a study procedure) and then immediately assessing its meaning. Determining what expressions or indications are truly reflective of dissent can be challenging in persons with dementia and requires the judgment of a person with expertise in dementia. When individuals have characteristics (e.g., aphasia, apathy, impulsivity, difficulty regulating emotions) that can impair the ability to communicate, researchers should have the flexibility to determine whether an initial expression or indication of possible dissent can be addressed and resolved so that study procedures can proceed then or at a subsequent time if it is appropriate to do so.
When Dissent is Binding
All informants felt strongly that dissent is binding (i.e., meaningful) if an individual’s unwillingness is unequivocal or is sustained after an effort is made to relieve the person’s concerns and/or distress. This led the national panel to caution that having flexibility to try to resolve the individual’s initial objection or distress cannot be regarded as sanction for repeated efforts to obtain the person’s willingness or cooperation. When the judgment is made that dissent is being expressed, the individual should be excluded or withdrawn from the study or procedure except when immediate discontinuation would place the individual at risk of harm (e.g., suddenly stopping a medication when the dosage should be reduced gradually).
Opinions differed in each group on whether there are circumstances in which dissent should not be binding (e.g., if suffering is great, potential direct benefit is available only in the research study, and there is no significant risk), but the national panel rejected this idea for four reasons. First, this scenario is currently almost inconceivable in dementia research. Second, even were there a high likelihood of benefit, investigators could include other eligible individuals who would cooperate with and adhere to the protocol for the individual’s safety. Third, the imposition of an intervention on a person who is dissenting would violate the dignity of the individual. Fourth, forcing an objecting individual to participate in research would be troubling, if not traumatic, for study team members.
Guidance for Researchers
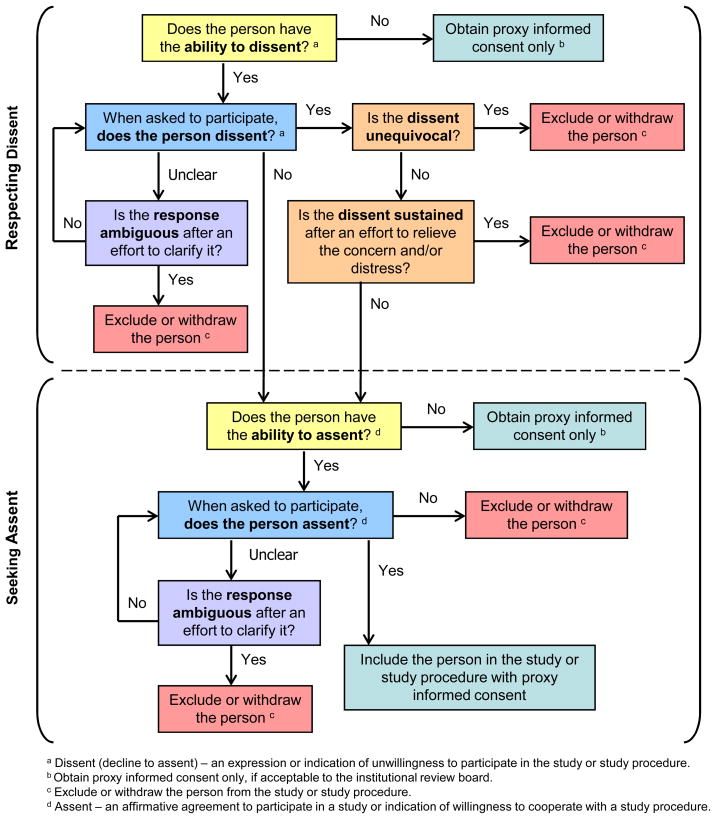
A decision tree for respecting dissent and seeking assent is presented in Figure 1. The process depicted reflects the findings described above and begins with the issue of dissent to highlight the primacy of respecting an individual’s objection to participating in research.
Figure 1.
Decision Tree for Respecting Dissent and Seeking Assent for Dementia Research
Conclusions
This study confirms the importance of seeking the assent and respecting the dissent of adults who lack consent capacity for dementia research, and it provides guidance for researchers charged with fulfilling these duties as well as for IRBs who are charged with ethical oversight of this research. Assent should be obtained explicitly from all those who lack consent capacity and have the ability to assent, but it should not be required of those who lack the ability to assent. Dissent should be respected whenever it occurs, and no reason or justification is needed for the individual’s objections to be honored. While maximizing remaining autonomy is important, it is the protective function of respecting dissent that makes it hierarchically more important from an ethical perspective than obtaining assent.
Participants in this study felt strongly that individuals who lack the ability to assent and/or dissent should not be precluded from involvement in all dementia research. To do so would place unnecessary and perhaps insurmountable barriers to the conduct of research that addresses important issues for dementia patients, particularly those in the moderate to late stages when these abilities may be lacking. They recommended that local IRBs determine whether individuals who lack one or both of these abilities can participate in a specific study and whether any protections, beyond proxy informed consent (e.g., an advance directive for research, an independent monitor), are needed for their inclusion. Investigators should be required to provide justification for the inclusion of such individuals in the protocol and should delineate the methods that will be used to ensure the well-being of these participants.
The range of remaining cognitive abilities of adults who lack consent capacity, the presence of neuropsychiatric symptoms, and changes in the individual’s temperament pose challenges when seeking assent and respecting dissent. Judgments must be based on the researcher’s observation of that individual and the situation at hand, a rigorous evaluation of the person’s abilities, probing to understand what an expression or indication really means, and addressing when possible any unrelated distress or concerns of the individual. Explicit discussion of these issues when an inability to consent is likely might reassure IRBs that adequate steps are being taken to fulfill the ethical obligations inherent in conducting dementia research. Because of the uniqueness of each situation, participants recommended that researchers should seek the assistance of caregivers in deciphering the responses of the individual who has dementia. Informants agreed that researchers must be given the flexibility to both clarify the meaning of behaviors, verbalizations, and emotional indications that potentially indicate dissent or assent and be allowed to address and resolve initial objections or indications of distress when the intent is not absolutely clear. If the response remains ambiguous, if the objection is unequivocal or sustained, or if an individual who has the ability to assent does not do so, researchers should exclude or withdraw the person from the research.
Caregivers who serve as proxy decision-makers for decisionally impaired adults are critical to the conduct of dementia research. The recommendation that researchers should seek caregivers’ assistance to understand an individual’s responses is based on the assumption that caregivers are, in most instances, family members who have known the individual for many years prior to their cognitive impairment and since then have provided care to the individual on a regular basis. Their knowledge of the individual’s prior attitudes and preferences and their caregiving experiences may enable them to provide an accurate interpretation of the individual’s responses. Conversely, researchers must be cognizant that a caregiver could misrepresent the individual’s responses. If there is any reason to doubt a caregiver’s motivations, researchers are obliged to act in what seems to be the patient’s best interests. Conflicts about research participation may arise between the proxy decision-maker and the potential study participant. If the individual who lacks consent capacity wishes to participate but the proxy does not consent, the proxy’s wishes prevail according to the doctrine of informed consent. Based on our findings, which are in line with prior recommendations (10), if the individual expresses/indicates an unequivocal or sustained dissent, the patient’s wishes prevail over the proxy’s consent.
On the question of whether the dissent of an individual to participate in research should ever be overridden if the study provides the potential for a direct benefit available only in research, findings from this study differ from the positions taken by some previous groups (8, 11, 21, 22). The national panel concluded that superseding fundamental ethical principles and overriding dissent is unjustified and unnecessary. That is, best interest does not override dissent, even when a person cannot understand the potential benefit of research.
This study has a number of limitations. First, the project focused only on persons with dementia. While some findings may be applicable to research involving adults whose lack of consent capacity is due to other disorders (e.g., focal impairment due to stroke, traumatic brain injury, intellectual disability), the guidance offered here is not directly applicable to clinical care. Second, because it is a qualitative study, the sample is small and local participants were drawn from only two geographic areas in the United States. Third, since participants were identified because of their involvement in dementia research, all would likely be favorable toward research. Finally, only a small number of focus groups were conducted.
Some issues addressed in this study require more in-depth examination. For example, how can researchers determine whether the expression or indication of a choice is meaningful (i.e., authentic)? Sugarman and colleagues (23) highlight the challenge of determining authenticity when individuals who have dementia contribute little to the conversation in the informed consent encounter and caution that affirmative statements may only be a means of engagement rather than a deliberate cognitive act. Related to this is our finding that both assent and dissent can be expressed verbally, behaviorally, or emotionally (or some combination thereof). While others have noted that assent can be expressed verbally or indicated behaviorally (10, 24), an emotional indication of assent may be harder to judge. More research is needed to determine how individuals who have dementia actually respond to requests to participate in research, how researchers judge the authenticity of and consistency between an individual’s verbal, behavioral, and emotional responses, and the role that caregivers play in that encounter (25, 26).
Acknowledgments
The authors wish to thank the national experts, dementia researchers, caregivers, and patient advocates who participated in this project for their thoughtful discussions and recommendations.
Support: National Institute on Aging, grant number R21AG30036; Dr. Black is supported in part by the Stempler Fund for Dementia Research.
Footnotes
Disclosures: Dr. Rabins previously served on the speaker’s bureau of Pfizer and Forest; Dr. Karlawish is the site investigator for an NIA-Pfizer sponsored clinical trial for persons with mild to moderate Alzheimer disease; Drs. Black and Sugarman have no disclosures.
Assent means a child’s affirmative agreement to participate in research. Mere failure to object should not, absent affirmative agreement, be construed as assent (45 CFR 46.402).
References
- 1.Karlawish JHT, Kim SYH, Knopman D, et al. Interpreting the clinical significance of capacity scores for informed consent in Alzheimer disease clinical trials. Am J Geriatr Psychiatry. 2008;16:568–574. doi: 10.1097/JGP.0b013e318172b406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warner J, McCarnery R, Griffin M, et al. Participation in dementia research: Rates and correlates of capacity to give informed consent. J Med Ethics. 2008;34:167–170. doi: 10.1136/jme.2006.019786. [DOI] [PubMed] [Google Scholar]
- 3.Black BS, Brandt J, Rabins PV, et al. Predictors of providing informed consent or assent for research participation in assisted living residents. Am J Geriatr Psychiatry. 2008;16:83–91. doi: 10.1097/JGP.0b013e318157cabd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyketsos C, Steinberg M, Tschanz JT, et al. Mental and behavioral disturbances in dementia: Findings from the Cache County Study on Memory in Aging. Am J Psychiatry. 2000;157:708–714. doi: 10.1176/appi.ajp.157.5.708. [DOI] [PubMed] [Google Scholar]
- 5.Lyketsos CG, Lopez O, Jones B, et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: Results from the Cardiovascular Health Study. JAMA. 2002;288:1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- 6.Steinberg M, Sheppard J, Tschanz J, et al. The incidence of mental and behavioral disturbances in dementia: The Cache County Study. J Neuropsychiatry Clin Neurosci. 2003;15:340–345. doi: 10.1176/jnp.15.3.340. [DOI] [PubMed] [Google Scholar]
- 7.Kim SYH, Karlawish JHT, Caine ED. Current state of research on decision-making competence of cognitively impaired elderly persons. Am J Geriatr Psychiatry. 2002;10:151–165. [PubMed] [Google Scholar]
- 8.National Commission for the Protection of Human Subjects in Biomedical and Behavioral Research. Report and Recommendations: Research Involving those Institutionalized as Mentally Infirm. 1978 [PubMed] [Google Scholar]
- 9.National Bioethics Advisory Commission. Report and Recommendations of the National Bioethics Advisory Commission. I. Rockville, MD: 1998. Research involving persons with mental disorders that may affect decisionmaking capacity. [Google Scholar]
- 10.Alzheimer’s Association. Research consent for cognitively impaired adults: Recommendations for institutional review boards and investigators. Alzheimer Dis Assoc Disord. 2004;18:171–175. doi: 10.1097/01.wad.0000137520.23370.56. [DOI] [PubMed] [Google Scholar]
- 11.American Geriatrics Society Ethics Committee. Position Statement: Informed consent for research on human subjects with dementia. J Am Geriatr Soc. 1998;46:1308–1310. doi: 10.1111/j.1532-5415.1998.tb04551.x. [DOI] [PubMed] [Google Scholar]
- 12.Karlawish JHT. Research involving cognitively impaired adults. N Engl J Med. 2003;348:1389–1392. doi: 10.1056/NEJMsb030172. [DOI] [PubMed] [Google Scholar]
- 13.Wendler D, Prasad K. Core safeguards for clinical research with adults who are unable to consent. Ann Intern Med. 2001;135:514–523. doi: 10.7326/0003-4819-135-7-200110020-00011. [DOI] [PubMed] [Google Scholar]
- 14.Corbin J, Strauss A. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Los Angeles: Sage Publications; 2008. [Google Scholar]
- 15.Flick U. An Introduction to Qualitative Research. London: SAGE Publications; 2006. [Google Scholar]
- 16.Sandelowski M. Sample size in qualitative research. Res Nurs Health. 1995;18:179–183. doi: 10.1002/nur.4770180211. [DOI] [PubMed] [Google Scholar]
- 17.Patton MQ. Qualitative Research and Evaluation Methods. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- 18.QSR NUD*IST. Software for Qualitative Data Analysis. Thousand Oaks, CA: Scolari Sage Publications, Inc; 1997. [Google Scholar]
- 19.Boeije H. A purposeful approach to the constant comparative method in the analysis of qualitative interviews. Qual Quant. 2002;36:391–409. [Google Scholar]
- 20.Creswell JW. Qualitative Inquiry and Research Design: Choosing Among Five Traditions. Thousand Oaks: SAGE Publications; 1998. [Google Scholar]
- 21.National Commission for the Protection of Human Subjects in Biomedical and Behavioral Research. The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research. 1979 [Google Scholar]
- 22.High DM, Whitehouse PJ, Post SG, et al. Guidelines for addressing ethical and legal issues in Alzheimer disease research: A position paper. Alzheimer Disease and Associated Disorders. 1994;8:66–74. [PubMed] [Google Scholar]
- 23.Sugarman J, Roter D, Cain C, et al. Proxies and consent discussions for dementia research. J Am Geriatr Soc. 2007;55:556–561. doi: 10.1111/j.1532-5415.2007.01101.x. [DOI] [PubMed] [Google Scholar]
- 24.Dresser R. Dementia research: Ethics and policy for the twenty-first century. Georgia Law Rev. 2001;35:661–690. [PubMed] [Google Scholar]
- 25.Black B, Kass N, Fogarty L, et al. Informed consent for dementia research: The study enrollment encounter. IRB. 2007;29:7–14. [PubMed] [Google Scholar]
- 26.Hougham GW, Sachs GA, Danner D, et al. Empirical research on informed consent with the cognitively impaired. IRB. 2003;25:S26–S32. [PubMed] [Google Scholar]