Abstract
Mechanisms of androgen dependence of the prostate are critical to understanding prostate cancer progression to androgen independence associated with disease mortality. A transient elevation of transforming growth factor-beta (TGF-ß) expression after androgen ablation suggested cooperation of the pathways in prostate regression. To determine the role of TGF-ß on prostate response to androgen ablation, conditional TGF-ß type II receptor knockout mouse models of the epithelia (Tgfbr2NKX3.1KO) and stromal fibroblasts (Tgfbr2fspKO) were used. Following castration, the prostates of Tgfbr2NKX3.1KO mice had apoptosis levels similar to those expected for control Tgfbr2FloxE2/FloxE2 mice. Prostates of Tgfbr2fspKO mice, however, had reduced regression and high levels of proliferation associated with canonical Wnt activity through out the glandular epithelia regardless of androgen status. In contrast, Tgfbr2FloxE2/FloxE2 prostates had epithelial canonical Wnt activity only in the surviving proximal ducts following castration. In vitro studies showed that androgen antagonist, bicalutamide, transiently elevated both Tgfbr2FloxE2/FloxE2 and Tgfbr2fspKO stromal expression of Wnt-2, Wnt-3a, and Wnt-5a. The neutralization of Wnt signaling by the expression of SFRP-2 resulted in decreased LNCaP prostate epithelial cell proliferation in stromal conditioned media transfer experiments. In vivo tissue recombination studies using Tgfbr2fspKO prostatic stromal cells in combination with wild type or SV40 large T antigen expressing epithelia resulted in prostates that were refractile to androgen ablation. The expression of SFRP-2 restored the Tgfbr2fspKO-associated prostate responsiveness to androgen ablation. The studies reveal a novel TGF-ß, androgen, and Wnt paracrine signaling axis that enables prostatic regression of the distal ducts following androgen ablation while supporting proximal duct survival.
Introduction
Prostate cancer continues to be a major cause of death in aging men (1). Since the prostate is an androgen-dependent organ, a major mechanism for prostate cancer treatment includes the inhibition of androgen signaling. Regardless of the initial positive response to androgen ablation, the prostate cancer invariably overcomes its dependence on androgens and results in a drug-resistant cancer with few options for treatment. Although androgen ablation therapy is intended to target the prostate epithelia, the influence of the prostatic stroma on androgen responsiveness of the adjacent epithelia is likely to be critical. Mature and differentiated prostate tissue is formed and maintained by effects on androgen receptors within the stromal compartment (2, 3). The concept of mesenchymal cells relaying androgen sensitivity to epithelia through paracrine interactions is supported by tissue recombination experiments. Viable prostate glands develop from epithelia expressing a non-functional androgen receptor derived from testicular feminized mice when allografted with wild-type urogenital mesenchyme (4, 5). The resulting prostate glands were equally sensitive to androgen ablation carried out by castration of host mice when compared to the control tissue recombinations generated with wild-type mesenchyme and wild-type epithelia (5). This suggests that the prostatic stroma plays an instructive role in glandular development and potentially influences responsiveness to androgen ablation in prostate cancer progression (5). Prostate cancer refractile to androgen ablation therapy is the primary cause of mortality due to the disease (6, 7). As a basis to understanding androgen independent prostate cancer, we investigated the mechanism for prostatic proximal duct survival in the absence of androgens.
Following androgen ablation the prostate undergoes apoptotic regression. Subsequent spontaneous recurrence of prostate cancer often results in androgen independent regrowth, a predominant contributor to disease mortality. In mouse models, reintroduction of androgens following androgen ablation results in prostate regrowth originating from the proximal ducts. This observation indicates that the proximal ducts are inherently refractory to androgen ablation (8-10). Canonical Wnt signaling is known to play a role in cell survival in many tissues (11-13). Thus, we wanted to investigate a potential role for Wnt signaling in the survival of proximal ductal tissue upon castration. Another growth factor, TGF-ß, is thought to support prostatic apoptosis as its expression coincides with androgen ablation in benign and cancer tissues (14-16). TGF-ß binds the TGF-ß type II receptor (Tgfbr2) at the cell surface to phosphorylate the TGF-ß type I receptor and activate cytoplasmic proteins, predominantly Smad2 and Smad3 (17). Both cooperative and antagonistic interactions of Wnt, androgen, and TGF-ß signaling pathways occur in the prostatic epithelia (18-21). However, the role of Wnt and TGF-ß signaling on androgen dependence of the prostate is unknown.
We tested the hypothesis that paracrine Wnt signaling regulates stromal to epithelial interactions in response to androgen ablation in a TGF-ß-dependent manner using mouse and allografting models. Canonical Wnt signaling involves the activation of cognate frizzled receptors at the cell surface that mediate ß-catenin accumulation leading to transcriptional activity in the nucleus (22). Here we report that canonical Wnt activity is present in the prostatic epithelia of the proximal ducts of the prostate following androgen ablation. Previously, we generated a Tgfbr2 conditional fibroblast knockout mouse through FSP-1 Cre-mediated recombination of Tgfbr2 exon 2 (Tgfbr2fspKO), which resulted in the development of pre-neoplastic lesions (prostatic intraepithelial neoplasia) by five to seven weeks of age (23). To further study the role of TGF-ß on the prostatic epithelia following androgen ablation, here we developed a conditional epithelial Tgfbr2 knockout by crossing NKX3.1-Cre mice with Tgfbr2FloxE2/FloxE2 mice (24), termed Tgfbr2NKX3.1KO. Another valuable tool was the Tg(Fos-lacZ)34Efu/J mouse model, termed TOPGal, which enabled the visualization of active canonical Wnt signaling (25). Interestingly, the prostates of Tgfbr2fspKO mice had constitutive Wnt signaling throughout the prostate and were resistant to androgen ablation induced regression. This suggested that TGF-ß responsiveness of the prostatic stroma influences the androgen responsiveness of adjacent prostatic epithelia through the secretion of paracrine Wnts. The 12T7f LADY mouse model, expressing SV40 Large T antigen in the prostatic epithelia, was used since it undergoes cancer progression in a similar fashion to human prostate cancer (26). Tissue recombination allografting of the Tgfbr2fspKO prostatic stroma was able to convert the androgen-dependent 12T7f LADY prostatic epithelia to become refractile to androgen ablation. Together, the data provides a mechanism for androgen independent prostatic epithelial survival and is suggestive of how prostate cancer can progress to become unresponsive to hormone ablation by hijacking pathways found in benign proximal ducts.
Materials and Methods
Transgenic Mice
Tgfbr2FloxE2/FloxE2 and Tgfbr2fspKO mice of C57-Bl/6 background were generated as previously described (23). The NKX3.1-Cre mice, also in the C57-Bl/6 background, were crossed with Tgfbr2FloxE2/FloxE2 mice to generate the Tgfbr2NKX3.1KO mouse model. The Tgfbr2NKX3.1KO mice were crossed with Rosa26 mice to enable visualization of cells undergoing Cre-mediated recombination. TOPGal mice (25) were purchased from Jackson Laboratories and crossed with Tgfbr2FloxE2/FloxE2 and the Tgfbr2FloxE2/wt-CreFSP mice to generate the Tgfbr2FloxE2/FloxE2/TOPGal and Tgfbr2fspKO/TOPGal mouse models. Prostatic epithelial organoids were generated from 15 week-old 12T7f LADY mice, at which time they have developed high-grade hyperplastic lesions and foci of adenocarcinoma (26, 27). All mice were genotyped from ear punch biopsies. Harlan Sprague Dawley (Indianapolis, IN) SCID CB17/ICR hsd and C57-Bl/6 mice were used for tissue recombination and tissue rescue allografting techniques, respectively. All animal procedures were approved by the Vanderbilt Institutional Animal Care and Use Committee.
Cell Culture
Tgfbr2FloxE2/FloxE2 and Tgfbr2fspKO mouse primary prostate stromal cell cultures were made from 6-8 week old mouse prostates. The prostates were dissected, cut to 2mm3, and plated in 6-well plates with high-glucose Dulbecco’s Modified Eagle’s Medium supplemented with 5% fetal bovine serum (HyClone, Fisher Scientific, Pittsburgh, PA), 5% NuSerum (BD Biosciences, Franklin Lakes, NJ), 5 μg/mL insulin, testosterone 10-8 M dissolved in ethanol (added fresh), and 0.1% penicillin/streptomycin (28). Stromal cell media was changed every 3-4 days. Cultures between passage ten and thirty were used for experiments.
LNCaP cells were purchased from ATCC and grown according to the protocol in RPMI medium supplemented with 10% fetal bovine serum (HyClone, Fisher Scientific) and 0.1% penicillin/streptomycin. This human cell line, derived from a prostate cancer lymph node metastatic lesion, lacks a functional TGF-ß type II receptor, yet has a functional androgen receptor (29).
Conditioned media experiments and 3H-thymidine Incorporation
Conditioned stromal media was generated by plating 750,000 Tgfbr2FloxE2/FloxE2 or Tgfbr2fspKO cells or for control 3,000,000 LNCaP cells on a 100mm dish. Tgfbr2FloxE2/FloxE2 and Tgfbr2fspKO stromal cells were transduced with either GFP (control) or SFRP-2 adenovirus at 105-virus particles/mL for 24 hours prior to replacing standard stromal cell culture media with the lower serum media to allow virus production in stromal cells. The stromal cells were incubated for 72 hours in the stromal cell media described above containing 10-8 M testosterone or if indicated, bicalutamide (10-5 M bicalutamide and no testosterone). The stromal conditioned media was centrifuged prior to treatment of LNCaP cells (10,000 cells per well) in 24 well plates. The conditioned media was replaced after 72 hours of incubation with fresh conditioned media. In select conditions fresh bicalutamide was also included as part of the conditioned media. Following 120 hours of incubation of LNCaP cells with stromal conditioned media, 3H-thymidine incorporation assays were performed. Three hours prior to assaying for proliferation, cells were given 2 μCi 3H-thymidine (PerkinElmer, Waltham, MA) in serum free RPMI per well. Cells were washed in 1 mL 10% TCA for 10 min, three times then lysed with 300 μl 200 mM NaOH for 30 min. The cell lysates (100 μl) were measured for 3H-thymidine activity using a scintillation counter. All treatment conditions were performed in triplicate.
Immunohistochemistry
Tissues were fixed with 4% paraformaldehyde, embedded in paraffin, and sectioned for histological analysis. Ki67 staining was performed to stain proliferating cells (by the Vanderbilt Immunohistochemistry Core facility). TUNEL staining was performed using the ApopTag Peroxidase In Situ Apoptosis Detection Kit (Chemicon, Temecula, CA). Double immunohistochemical staining was performed for TUNEL followed by staining for ß-galactosidase (1:5000, Abcam, Cambridge MA) developed with TrueBlue Peroxidase Substrate (KPL, Gaithersburg, MD). Immunohistochemical staining was systematically quantitated by taking a ratio of positively stained cells per epithelial nuclei per field (200x). Statistical significance was determined by two tailed student T test.
Immunofluorescence
50,000 cells were treated with 5 ng/ mL TGF-ß (Cell Sciences, Canton, MA) on glass coverslips for 6 hours. The cells were fixed with 4% paraformaldehyde for 10 min. at 4°C. Smad2 localization was visualized using primary (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA) and secondary anti-goat Alexa Fluor 594 antibody (1:500, Invitrogen. Carlsbad, CA) on a Nikon epifluorescence microscope.
ß-galactosidase tissue staining
Whole mouse prostates were dissected and fixed in 4% PFA for one to two hours, washed in PBS, incubated with X-gal for 3 hr at 30°C, washed in PBS, and fixed in 4% PFA overnight. Processed paraffin embedded tissues were sectioned at 8 μm and counterstained with Nuclear Fast Red (Electron Microscopy Sciences, Hatfield, PA).
Tissue recombination allografting
The tissue recombinations were performed as previously described (30). Epithelial organoids were derived by digesting prostates of 6-12 week old wild type C57/Bl6 mice or 15 week- old LADY 12T7f mice in 675 units/mL collagenase with 0.04% DNAse type I at 37°C for 2-3 hours as previously described (30). The organoids were washed and pipetted into 50 μl collagen with stromal cells from Tgfbr2FloxE2/FloxE2 or Tgfbr2fspKO mouse prostates and allowed to incubate overnight at 37°C. The tissue recombinants were then allografted under the renal capsule of syngenic C57-Bl/6 for 5 to 7 weeks or SCID mice for 6 weeks. If needed, castration was performed 3-7 days prior to sacrifice.
RNA purification and RT-PCR
RNA from cell lysates was purified using the RNeasy mini kit (Qiagen, Valencia, CA) according to the manufacturer’s directions. RT-PCR was performed for 32 cycles with the following primer sets: Wnt-2 forward 5′GTTTGCCCGTGCCTTTGTAGATG reverse 5′CCGGGTGACGTGGATGTG, Wnt-3a forward 5′TCTGCAGGAACTACGTGGAGATCA reverse 5′TCCCGAGAGACCATTCCTCCAAAT, Wnt-5a forward 5′TCGCCATGAAGAAGCCCATTGGAA reverse 5′TGTCCTTGAGAAAGTCCTGCCAGT, Wnt-9a forward 5′ACTGCTTTCCTCTACGCCATCTCT reverse 5′TTTGCAAGTGGTTTCCACTCCAGC, Wnt-11 forward 5′CTGACATGCGCTGGAACTGCTC reverse 5′AGGCCCGGGCGATGGTGT, and 18S forward 5′ CAAGAACGAAAGTCGGAGGTTC reverse 5′ GGACATCTAAGGGCATCACAG.
Results
Stromal TGF-ß responsiveness enables prostatic regression after androgen depletion
Androgen ablation causes prostate regression associated with a transient elevation of TGF-ß expression (14). Yet, the role of TGF-ß on the prostate following androgen ablation is not clear. We first developed a conditional Tgfbr2 knockout targeted to the epithelia by crossing NKX3.1-Cre mice with Tgfbr2FloxE2/FloxE2 mice, termed Tgfbr2NKX3.1KO. Further crossing the Tgfbr2NKX3.1KO mice into the Rosa26 line enabled the immunohistochemical localization of ß-galactosidase expression associated with Cre-mediated recombination in the prostatic epithelia (Figure 1A). Hematoxylin and eosin (H&E) staining revealed little difference in the ductal structures of the Tgfbr2NKX3.1KO prostates compared to Tgfbr2FloxE2/FloxE2 controls (Figure 1B). However, the Tgfbr2NKX3.1KO prostates seemed to have greater epithelial turn-over, based on the apoptotic TUNEL staining pattern compared to Tgfbr2FloxE2/FloxE2 prostates. Following castration there was a further elevation of epithelial TUNEL staining in the Tgfbr2NKX3.1KO prostates as was observed in Tgfbr2FloxE2/FloxE2 allografts (Figure 1B). Taken together, there was no significant difference in apoptosis observed between Tgfbr2FloxE2/FloxE2 and Tgfbr2NKX3.1KO prostates following castration.
Figure 1. Conditional knockout of Tgfbr2 in the prostatic epithelia (Tgfbr2NKX3.1KO) did not significantly affect the response to androgen ablation compared to control Tgfbr2FloxE2/FloxE2 prostates.
(A) Immunohistochemistry for ß-galactosidase expression (brown) in tissue rescued Tgfbr2NKX3.1KO/Rosa26 prostates indicates Cre-recombination in the prostatic epithelia compared to control, Tgfbr2FloxE2/FloxE2/Rosa26 prostates with no detectible staining. Sections were counterstained with hematoxylin (blue). The epithelial (E) and stromal (S) compartments are indicated. (B) (Upper Panels) H&E staining of Tgfbr2NKX3.1KO and Tgfbr2FloxE2/FloxE2 tissue rescue allografts suggest similar prostatic development (n=4). (Lower Panels) TUNEL staining indicates differential apoptosis of the prostatic epithelia of Tgfbr2NKX3.1KO and Tgfbr2FloxE2/FloxE2 allografts in hosts that were not castrated (+Androgen) compared to hosts that were castrated (-Androgen).
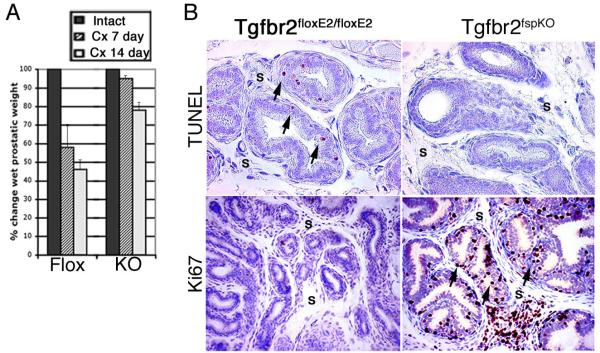
As the epithelial TGF-ß signaling did not seem to affect androgen responsiveness, the role of stromal TGF-ß signaling was studied using the Tgfbr2fspKO mouse model. The castration of Tgfbr2FloxE2/FloxE2 mice resulted in 42% and 55% decrease in the prostate wet weights, in seven and fourteen days, respectively (Figure 2A). In contrast, Tgfbr2fspKO prostates decreased in wet weight very little seven days following castration with approximately 20% regression by fourteen days. Histologic analysis revealed that seven days following castration, the Tgfbr2FloxE2/FloxE2 prostates, with otherwise low cellular turnover, had an expected increase in TUNEL-positive apoptotic cells in both the epithelial and stromal compartments and little evidence of proliferation observed by Ki67 staining (Figure 2B). Prior to castration, Tgfbr2fspKO mice exhibited slightly higher rates of apoptosis than Tgfbr2FloxE2/FloxE2 mice (1.4-fold), accompanied by significant proliferation consistent with the described PIN phenotype (23). Seven days following castration, prostates from Tgfbr2fspKO mice had little apparent epithelial apoptosis, yet remained highly proliferative based on TUNEL and Ki67 staining, respectively. Together, these data suggest that normal prostatic epithelial regression occurs in response to paracrine signals derived from stromal TGF-ß signaling.
Figure 2. Tgfbr2fspKO prostates lose androgen responsiveness after androgen ablation.
(A) Percentage change in wet prostate weight is shown seven and fourteen days following castration (Cx). Average Tgfbr2FloxE2/FloxE2 and Tgfbr2fspKO prostatic wet weights are shown as a percentage of the respective prostates from intact Tgfbr2FloxE2/FloxE2 (Flox) and Tgfbr2fspKO (KO) mice (n=5). (B) Three days following castration, prostates of Tgfbr2FloxE2/FloxE2 and Tgfbr2fspKO mice were subjected to TUNEL staining (brown) indicating apoptotic cells and Ki67 staining (brown) indicating proliferative cells. Hematoxylin (blue) was used as a nuclear counter stain. The stromal (S) compartment is indicated and black arrowheads indicate positively stained epithelial cells. Prostates were dissected from 5-7 week old male mice. Dorsolateral lobes are shown (n=8).
Before further studying the androgen independence of the prostates in Tgfbr2fspKO mice, we needed to establish that the epithelial response resulted from prostatic stroma and not other systemic factors in these mice. The FSP-1 Cre promoter targets a subset of fibroblasts throughout the body, not specifically prostatic fibroblasts (23, 31). We verified the loss of TGF-ß responsiveness in Tgfbr2fspKO cultures by immunolocalization of Smad2 (Figure 3A). A tissue recombination allograft approach was then utilized to identify the role of the prostatic stromal cells in the phenotype observed. The tissue recombination technique consisted of combining cultured stromal cells derived from Tgfbr2FloxE2/FloxE2 or Tgfbr2fspKO prostates with epithelial organoids isolated by digesting mature wild type C57-Bl/6 mouse prostates. The tissue recombinants were allografted to the sub-renal capsules of syngeneic male mice (32). Five weeks following grafting, the Tgfbr2FloxE2/FloxE2 and Tgfbr2fspKO stromal cells organized themselves around the epithelial organoids to form prostatic glands (Figure 3B). Seven days following castration of the hosts, a 6-fold increase in TUNEL-positive epithelia in the Tgfbr2FloxE2/FloxE2 stroma-associated glands was observed in comparison to Tgfbr2fspKO stroma-associated glands (Figure 3C). Ki67 staining suggested minimal proliferation in recombinants associated with Tgfbr2FloxE2/FloxE2 stroma before and after castration. In contrast, the recombinants associated with Tgfbr2fspKO stroma had 2.5-fold greater epithelial proliferation compared to Tgfbr2FloxE2/FloxE2, even following castration. Collectively, castration of the host mice resulted in regression of the allografted tissue recombinants associated with Tgfbr2FloxE2/FloxE2, but not Tgfbr2fspKO stromal cells. This confirmed our observation that the androgen refractile phenotype of our Tgfbr2fspKO mice resulted from interactions within the prostate microenvironment.
Figure 3. Loss of TGF-ß responsiveness in prostatic stromal cells causes the prostate to become refractory to androgen ablation.
(A) To assess the competency of Tgfbr2FloxE2/FloxE2 and Tgfbr2fspKO prostatic stromal cells for TGF-ß signaling, Smad2 was localized by Alexa Fluor 594 (red) immunofluorescence following TGF-ß treatment. Hoechst nuclear counter stain was used (blue). (B) H&E staining of prostatic glandular structures in tissue recombination grafts of Tgfbr2FloxE2/FloxE2 and Tgfbr2fspKO stromal cells with epithelial organoids that were allografted for five weeks in intact syngenic host male mice (+ Androgen) (n=8), (C) H&E staining of prostatic glandular structures in tissue recombination grafts of Tgfbr2FloxE2/FloxE2 and Tgfbr2fspKO stromal cells with epithelial organoids that were allografted for five weeks and castrated for the last week of grafting in syngenic host male mice (- Androgen) (n=8). “Kd” indicates kidney tissue. TUNEL staining (indicated with black arrowheads) in Tgfbr2FloxE2/FloxE2 and Tgfbr2fspKO tissue recombination grafts from castrated mice (- Androgen) (n=4). Ki67 staining (black arrowheads) indicate proliferative cells of Tgfbr2FloxE2/FloxE2 and Tgfbr2fspKO allografts from castrated mice (n=4). Open arrowheads indicate background staining of likely dead cells and “Kd” indicates kidney tissue.
Proximal ductal epithelial activation of Wnt signaling following androgen ablation mediates stromal-epithelial crosstalk
The next step was to determine how TGF-ß signaling within the stroma was responsible for the observed regression in the Tgfbr2FloxE2/FloxE2, or lack thereof in the Tgfbr2fspKO, mouse prostates. The proximal ducts of the prostate remain intact as the distal ducts regress in the absence of androgen signaling. Up-regulation of Wnt ligands and activating mutations of ß-catenin in prostate cancer epithelium is a potential mechanism for androgen refractory prostatic epithelial proliferation (33-35). To address the potential of a parallel Wnt signaling mechanism to support androgen independent prostate survival phenotype observed, we developed a Tgfbr2fspKO/TOPGal mouse model. Intact and castrated male Tgfbr2FloxE2/FloxE2/TOPGal and Tgfbr2fspKO/TOPGal prostates were subjected to whole mount ß-galactosidase staining to visualize canonical Wnt activity. In whole mount staining of prostates from intact Tgfbr2FloxE2/FloxE2/TOPGal mice, ß-galactosidase expression was not detected (Figure 4A, B). However, three days after castration ß-galactosidase activity was detected exclusively in the proximal ducts of the prostate indicating activated ß-catenin signaling (Figure 4C). By the seventh day following castration, little ß-galactosidase activity was detected in Tgfbr2FloxE2/FloxE2/TOPGal prostates (data not shown). In contrast to that observed in Tgfbr2FloxE2/FloxE2/TOPGal prostates, the Tgfbr2fspKO/TOPGal prostates had ß-galactosidase expression in the entire gland before castration with further elevated expression following castration (Figure 4A, B, C). In both Tgfbr2FloxE2/FloxE2/TOPGal and Tgfbr2fspKO/TOPGal prostates, only the epithelial compartment showed strong positive ß-galactosidase activity (Figure 4B, C). Co-staining for TUNEL and ß-galactosidase in Tgfbr2FloxE2/FloxE2/TOPGal prostate glands four days following castration illustrated that the epithelia expressing ß-galactosidase was different from that undergoing apoptosis (Figure 4D). This finding suggested that the epithelia in the proximal glands supported canonical Wnt activity during normal prostatic regression following androgen ablation. Thus, the hormone refractory Tgfbr2fspKO prostates supported constitutive paracrine canonical Wnt signaling. The coincident localization of canonical Wnt signaling and the reported region surviving androgen ablation supported the possibility of a causal relationship between the two events.
Figure 4. Inhibiting TGF-ß signaling in the prostatic stroma results in constitutive Wnt signaling through out the prostatic epithelia associated with survival following androgen ablation.

Anterior prostate lobes from intact and three day castrated six week old TOPGal mice were stained for ß-galactosidase activity. (A) Tgfbr2FloxE2/FloxE2/TOPGal and Tgfbr2fspKO/TOPGal prostates from intact and three days following castration were stained for ß-galactosidase activity (blue) and imaged as whole mounts (n=8) to show areas of canonical Wnt signaling activity. (B) Paraffin sections of the ß-galactosidase stained intact Tgfbr2FloxE2/FloxE2/TOPGal and Tgfbr2fspKO/TOPGal prostates were counter stained with Nuclear Fast Red. Asterisks indicate the stromal compartment. (Prostates from intact and castrated Tgfbr2fspKO/TOPGal mice were similar. Only the intact Tgfbr2fspKO/TOPGal tissue section is shown.) (C) Three days after castration, paraffin sections of the ß-galactosidase stained Tgfbr2FloxE2/FloxE2/TOPGal prostate was counter stained with Nuclear Fast Red. Asterisks indicate the stromal compartment. (D) Following castration of control Tgfbr2FloxE2/FloxE2/TOPGal mice, the ß-galactosidase activity stained prostate sections were co-stained for TUNEL (brown) indicated with black arrows and ß-galactosidase expression (blue) (n=4). Asterisks indicate the stromal compartment and black arrows indicate the epithelia with no ß-galactosidase activity following castration (n = 8).
To determine if the observed epithelial Wnt activity corresponded with stromal regulation of Wnt ligand production, Tgfbr2FloxE2/FloxE2 and Tgfbr2fspKO cultured prostatic stromal cells were treated with an androgen receptor antagonist, bicalutamide. The expression of Wnt ligands was measured following androgen ablation over a time course of five days by semi-quantitative RT-PCR. There was a transient increase in Wnt-2, Wnt-3a and Wnt-5a one and three days following bicalutamide treatment of Tgfbr2FloxE2/FloxE2 stromal cells, with a decrease in expression by five days (Figure 5A). A similar trend was seen with Tgfbr2fspKO stromal cells, but with elevated basal expression for Wnt-2, Wnt-3a and Wnt-5a. Wnt-9a and Wnt-11 expression were unchanged in both stromal cell types under the same conditions. Addition of TGF-ß to the bicalutamide treatment of the Tgfbr2FloxE2/FloxE2 stromal cultures resulted in a loss of Wnt expression seen with bicalutamide alone (data not shown). Thus, inhibiting either the androgen or the TGF-ß signaling pathway could induce specific Wnt gene expression in prostatic stromal cells.
Figure 5. Wnt signaling induced by androgen ablation of stromal cells affects epithelial proliferation.
(A) Tgfbr2FloxE2/FloxE2 and Tgfbr2fspKO stromal cells were treated with bicalutamide over five days to analyze Wnt gene expression by semi-quantitative RT-PCR. The expression of 18S ribosomal RNA was used as a loading control. (B) 3H-thymidine incorporation assays were performed on Tgfbr2FloxE2/FloxE2 and Tgfbr2fspKO stromal cells transduced with GFP-adenovirus and grown either in media containing testosterone or bicalutamide (Bic) in the absence of testosterone. 3H-thymidine incorporation assays were performed on LNCaP epithelial cells also treated with or without bicalutamide for five days. Each bar represents the average of three or more replicates and error bars indicate standard deviation. Data is representative of three or more individual experiments. (C) Androgen responsive proliferation of LNCaP cells in response to a 120 hour treatment with conditioned stromal cell media from Tgfbr2FloxE2/FloxE2 or Tgfbr2fspKO for 120 hours as measured by 3H-thymidine incorporation assays. Tgfbr2FloxE2/FloxE2 and Tgfbr2fspKO stromal cells were transduced either with GFP adenovirus as a control or Wnt antagonist SFRP-2 adenovirus prior to starting collection of conditioned media. ‘Bic on Stro’ indicates that bicalutamide was added to stromal cell cultures during the generation of conditioned media. ‘Bic on Epi’ indicates that bicalutamide was added to epithelial LNCaP cells simultaneously with stromal conditioned media treatment. Each bar represents the average of three or more replicates and error bars indicate standard deviation. Compared to the GFP control, p-values for SFRP-2-Tgfbr2FloxE2/FloxE2 conditions are 0.207, 0.339, and 0.011, and for SFRP-2-Tgfbr2fspKO conditions 0.002, 0.005, and 0.004, respectively. Each asterisk indicates statistical significance between the control and knockout data points for a given condition with a p-value less than 0.05.
To determine the role of paracrine Wnt signaling on the epithelial response to androgen ablation, the proliferation of prostatic epithelial cells was measured in the presence of conditioned media collected from either Tgfbr2FloxE2/FloxE2 or Tgfbr2fspKO stromal cell cultures. While bicalutamide treatment had little proliferative effect on either stromal cell type grown in culture, as measured by 3H-thymidine incorporation assays, the Tgfbr2fspKO stromal cells were notably more proliferative than the Tgfbr2FloxE2/FloxE2 stromal cells (Figure 5B). Bicalutamide treatment decreased the proliferation of the androgen responsive prostate cancer cell line, LNCaP epithelial cells, as expected. Therefore, LNCaP cells were subsequently used as the target epithelia to assess proliferative responsiveness to conditioned stromal media treatment. Control green fluorescent protein (GFP)-transduced Tgfbr2FloxE2/FloxE2-conditioned media resulted in a low proliferative response on LNCaP cells compared to the enhanced proliferation using the GFP-transduced Tgfbr2fspKO-conditioned media (Figure 5C). Wnt expression by the stroma was antagonized through adenoviral expression of secreted frizzled related protein-2 (SFRP-2) to stromal cell cultures. The SFRP-2 transduced Tgfbr2fspKO-conditioned media resulted in significantly decreased proliferation of LNCaP cells, compared to GFP-transduced Tgfbr2fspKO-conditioned media. Bicalutamide treatment to the Tgfbr2fspKO stroma during generation of conditioned media (Bic on Stro) further decreased LNCaP proliferation, demonstrating the paracrine effect of androgen ablation on epithelial cells. However, most dramatic was the direct effect of androgen ablation on the epithelia with SFRP-2 transduced Tgfbr2fspKO-conditioned stromal media (Bic on Epi), which resulted in a 50% decrease in LNCaP proliferation compared to the GFP-transduced Tgfbr2fspKO-conditioned stromal media control. This suggested that Wnt ligands expressed by both Tgfbr2FloxE2/FloxE2 and Tgfbr2fspKO stromal cells support epithelial survival in response to androgen antagonism.
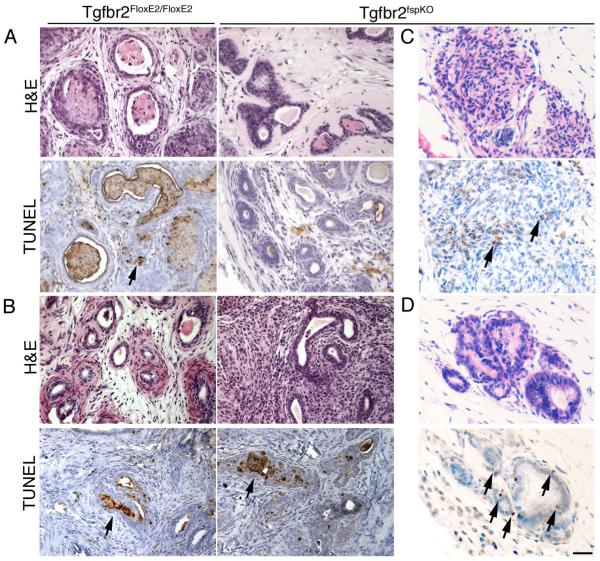
Next, the role of TGF-ß in Wnt expression during androgen ablation in mice was determined. Based on SFRP-2 expression-mediated inhibition of the proliferation of prostatic epithelial cells in vitro, we hypothesized that it may restore androgen dependence to the Tgfbr2fspKO prostate. The fragile condition of Tgfbr2fspKO mice did not allow expression of SFRP-2 in these mice directly. Therefore, we transduced control GFP or SFRP-2 adenovirus in intact Tgfbr2FloxE2/FloxE2 and Tgfbr2fspKO prostate lobes and subsequently allografted the tissues under the renal capsules of SCID mice in tissue rescue experiments. Three days following allografting the host mice were either left intact or castrated for an additional three days. There were no observable differences in apoptotic rate resulting from the expression of GFP or SFRP-2 on Tgfbr2FloxE2/FloxE2 and Tgfbr2fspKO rescued prostates in the intact host mice, based on TUNEL staining (data not shown). Following castration of the host mice, the Tgfbr2FloxE2/FloxE2 prostates had the expected elevation of TUNEL staining in both SFRP-2- and GFP-expressing prostates (Figure 6A, B). The expression of GFP by Tgfbr2fspKO prostates recapitulated the original observation of refractivity to androgen ablation. Quantitation of TUNEL-positive epithelial cells associated with GFP-Tgfbr2FloxE2/FloxE2 stromal cells three days following castration was in the range of 2-3.2% (SD +/- 0.6%) compared to the statistically different 1% (SD +/- 0.4%) GFP-Tgfbr2fspKO stromal cells under similar conditions. In comparison, the expression of SFRP-2 resulted in a three-fold increase in the apoptosis of Tgfbr2fspKO-associated epithelia following castration. The restoration of prostate regression in SFRP-2-Tgfbr2fspKO prostates following castration indicated cooperation among the TGF-ß, Wnt, and androgen signaling pathways.
Figure 6. Inhibition of Wnt signaling restores Tgfbr2fspKO prostate responsiveness to androgen ablation.
(A) Tgfbr2FloxE2/FloxE2 or Tgfbr2fspKO mature mouse prostates were transduced with GFP or (B) SFRP-2 adenovirus and grafted in male SCID mice. Host mice were castrated for three days. Tissues were harvested on day six (n=12) and subjected to H&E staining as well as TUNEL staining for apoptotic cells (brown). (C) Tissue recombinations of 12T7f LADY epithelial organoids and Tgfbr2fspKO prostatic stromal cells were allografted in SCID mice for six weeks. The host mice were given GFP or (D) SFRP-2 adenovirus through out the grafting period. Host mice were castrated seven days prior to harvesting the prostatic grafts. Tissue recombinants were harvested at week six (n=4) and subjected to H&E staining as well as TUNEL staining for apoptotic cells (brown). Scale bar indicates 50 μm.
Stromal TGF-ß signaling allows prostatic ductal regression after androgen ablation in prostate cancer
Next, the role of stromal TGF-ß signaling in a prostate cancer model system was examined. We allografted tissue recombinants consisting of Tgfbr2FloxE2/FloxE2 or Tgfbr2fspKO stromal cells with epithelial organoids from 12T7f LADY mouse prostates. Host mice were treated weekly with either SFRP-2 or GFP control adenovirus. Five weeks following xenografting half of the host male SCID mice were castrated. All grafts were analyzed six weeks following grafting. The GFP-Tgfbr2FloxE2/FloxE2 stroma-associated grafts formed prostatic ductal structures with minimal hyperplasia. In contrast, GFP-Tgfbr2fspKO stroma-associated grafts developed adenocarcinoma. Following castration, the GFP-Tgfbr2FloxE2/FloxE2 stroma-associated grafts had elevated apoptosis indicated by TUNEL-positive epithelia, while GFP-Tgfbr2fspKO stroma-associated grafts had lower levels of apoptosis (Figure 6C, D). SFRP-2-Tgfbr2FloxE2/FloxE2 stroma-associated grafts were similar to GFP controls with regard to apoptotic response to androgen ablation. However, after castration, SFRP-2-Tgfbr2fspKO stroma-associated grafts had elevated levels of TUNEL-positive epithelia typical of normal prostate regression. Thus, stromal androgen and TGF-ß signaling cooperates with epithelial Wnt signaling to regulate responsiveness to androgen ablation in benign, pre-neoplastic, and adenocarcinoma of the prostate.
Discussion
Our study demonstrated that stromal responsiveness to TGF-ß allows androgen sensitivity in the prostate epithelia through paracrine Wnt signaling. The direct role of TGF-ß signaling on the prostate epithelia following castration was minimal based on histologic and apoptoic differences between the Tgfbr2NKX3.1KO and Tgfbr2FloxE2/FloxE2 prostates. It is possible that compensatory signaling by Activin and its cognate receptors may provide Smad protein activity similar to TGF-ß. However, the conditional knockout of TGF-ß type II receptor in fibroblasts, in the Tgfbr2fspKO mice and tissue recombinants, indicated the importance TGF-ß responsiveness in the stromal compartment following castration. Further crossing of the Tgfbr2fspKO mice to the TOPGal reporter gene mouse model demonstrated the regulatory role of stromal TGF-ß signaling on Wnt signaling in epithelial cells refractory to androgen ablation. The Tgfbr2fspKO stromal cells themselves acquired a more proliferative phenotype (presumably by the loss of the growth inhibitory signal from TGF-ß itself) and promoted nearby epithelia to increase their rate of proliferation and overcome hormonal dependence. The mechanisms behind the observed phenomena highlight a stromal derived paracrine Wnt signaling axis that is triggered upon androgen ablation. The stark proximal ductal localization of canonical Wnt signaling activity in control prostates following castration indicated duplicity of responses to the androgen ablation, one of survival and another for cell death. Thus, the data indicate that the role for TGF-ß signaling following androgen ablation is to suppress Wnt signals in much of the distal prostate to enable regression.
Previous studies have shown that Wnt genes or proteins in the Wnt signaling pathway are up-regulated or mutated in androgen independent prostate cancers (11, 12). Recently, preosteoblasts were reported to induce Wnt signaling as a result of androgen stimulation, and through paracrine signaling the preosteoblasts subsequently increased the proliferation of prostate cancer cells in a coculture system in vitro (36). As preosteoblasts and prostatic stromal cells are both of mesenchymal origin, together with the results presented, this suggests a directional regulation of paracrine Wnt signaling by androgens. Castration provided a transient elevation of canonical Wnt signaling in Tgfbr2FloxE2/FloxE2 prostates (Figure 4). In parallel, Tgfbr2FloxE2/FloxE2 prostatic stromal cells transiently express specific Wnt genes in response to an androgen antagonist, bicalutamide (Figure 5). In contrast, Tgfbr2fspKO stromal cells had elevated basal expression of Wnt-2, Wnt-3a, and Wnt-5. Yet, antagonizing Wnt signaling in the absence of androgen ablation in Tgfbr2FloxE2/FloxE2 and Tgfbr2FSPKO prostates did not induce apoptosis. Thus, although TGF-ß and androgen signaling converge in the stroma to affect Wnt signaling they act through distinct pathways. This showed that TGF-ß and androgens intersect to regulate Wnt signaling within the stroma.
Here we demonstrate that activated canonical Wnt signaling helps to limit prostatic regression. It is known that Wnt ligands bind Frizzled receptors on the epithelial surface and transmit signals through the canonical pathway that activate ß-catenin/TCF in the nucleus (37). Activating mutations in ß-catenin have been identified to affect androgen receptor transcriptional activity and ligand specificity (33, 34). Based on the specific TCF/ß-catenin activity in the proximal prostatic ducts of the castrated Tgfbr2FloxE2/FloxE2/TOPGal mice, the mechanism for the survival of the proximal ducts may be through an initial activation of epithelial canonical Wnt signaling. Comparing the Tgfbr2FloxE2/FloxE2/TOPGal and Tgfbr2fspKO/TOPGal mice it was evident that TGF-ß signaling is important to regulate the spatial localization of Wnt signaling to enable regression of the distal ducts in a temporal coordination with androgen signaling. This is consistent with studies showing androgen-induced regeneration occurring at the distal tips of the prostate (38). One mechanism by which the localization of Wnt signaling is limited could be due to secreted Wnt inhibitors (e.g. SFRP-2, DKK-1) in the distal glands. Long term androgen ablation responses were not studied, as the focus of the study was to determine the role of the immediate upregulation of TGF-ß following androgen ablation. However, as the prostate continues to regress after day seven, Wnt signaling apparently is replaced by another mechanism to prevent continued regression of the proximal ducts. The latter events and maintenance of the regressed prostate is through a separate mechanism that is more dependent on the availability of androgens since some prostate regression was observed in Tgfbr2fspKO mice 14 days following castration (Figure 2).
Together, the upregulation of TGF-ß expression in the prostate, coincident with androgen ablation, would support the regression of the prostate by antagonizing stromal Wnt expression brought on from an androgen regulated mechanism. In normal proximal prostatic tissue the epithelial Frizzled receptor is activated resulting in canonical, and possibly non-canonical, Wnt signaling which may contribute to the phenotypes seen in our mouse models. However, based on previous reports that canonical Wnt signaling supports survival and proliferation (22, 39), this is the most likely mechanism that maintains the viability of proximal prostate tissues in the context of androgen ablation. The entire prostate does not involute in the absence of androgens, since subsequent replacement of testosterone results in the re-growth of prostatic tissue to original size (40). Intriguingly, in other mammals such as deer, antler regeneration is reported to demonstrate a site-specific Wnt signaling activation during regeneration (13). The fact that activating mutations of ß-catenin are found in androgen-independent prostate cancers (33) suggests that cancer cells can hijack the same Wnt signaling pathways used to support proximal prostate survival to aid in tumor survival. Understanding paracrine interactions of TGF-ß, androgen, and Wnt signaling in regulating prostate regression may be linked to its regeneration following androgen supplementation.
Stromal-epithelial interactions have proven to be important in embryonic development and tumorigenesis. Based on the Knudson multi-hit hypothesis of tumor development we attempted to further the progression of the PIN lesions associated with the LADY 12T-7f epithelia, expressing the large T antigen, by recombining them with Tgfbr2fspKO prostatic stromal cells, as the second mutagenic hit (41). Figure 6 illustrated that the Wnt signaling associated with the Tgfbr2fspKO cells not only enabled the PIN lesions to progress to adenocarcinoma, but also enabled the epithelia to become resistant to androgen ablation. Inhibition of Wnt signaling with SFRP-2 seemed to partially reverse both the tumorigenic and androgen sensitivity phenotypes. Future prostate cancer therapies would most likely benefit by not only antagonizing the traditional androgen-signaling pathway, but acting on Wnt signaling as well. This would allow therapies to target both the epithelial and stromal compartments, as well as androgen-dependent and independent tumor cells.
Acknowledgments
We thank Dr. Elaine Fuchs (Rockefeller Institute, NY) for the use of TOPGal mice, Dr. Sanjoy Das (Vanderbilt University, TN) for the SFRP-2 adenovirus. The work was supported by NIH through CA108646, CA126505, and the DOD through DAMD 17-02-1-0063, W81XWH-04-1-0046. Institutional training grant supported V.R.P. from GM062459, CA09592 and NIH grant FGM079879A. M.M.S. NIH grants (CA115985 and DK076602) supported the generation of Nkx3.1 Cre mice.
Abbreviations
- TGF-ß
Transforming growth factor beta
- Tgfbr2
TGF-ß type II receptor gene
- Tgfbr2FloxE2/FloxE2
TGF-ß type II receptor conditional floxed mouse
- Tgfbr2fspKO
TGF-ß type II receptor conditional fibroblast knockout mouse
- Tgfbr2NKX3.1KO
TGF-ß type II receptor conditional epithelial knockout mouse
- SFRP-2
secreted frizzled related protein-2
- Tgfbr2FloxE2/FloxE2/TOPGal
ß-catenin/TCF inducible ß-galactosidase expressing mouse crossed with the TGF-ß type II receptor floxed mouse
- Tgfbr2fspKO/TOPGal
ß-catenin/TCF inducible ß-galactosidase expressing mouse crossed with the TGF-ß type II receptor conditional fibroblast knockout mouse
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
References
- 1.Jemal A, Tiwari RC, Murray T, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Cunha GR, Donjacour AA. Mesenchymal-epithelial interactions in the growth and development of the prostate. Cancer Treat Res. 1989;46:159–75. doi: 10.1007/978-1-4613-1595-7_9. [DOI] [PubMed] [Google Scholar]
- 3.Cunha GR, Chung LW. Stromal-epithelial interactions--I. Induction of prostatic phenotype in urothelium of testicular feminized (Tfm/y) mice. J Steroid Biochem. 1981;14:1317–24. doi: 10.1016/0022-4731(81)90338-1. [DOI] [PubMed] [Google Scholar]
- 4.Donjacour AA, Cunha GR. Assessment of prostatic protein secretion in tissue recombinants made of urogenital sinus mesenchyme and urothelium from normal or androgen-insensitive mice. Endocrinology. 1993;132:2342–50. doi: 10.1210/endo.132.6.7684975. [DOI] [PubMed] [Google Scholar]
- 5.Kurita T, Wang YZ, Donjacour AA, et al. Paracrine regulation of apoptosis by steroid hormones in the male and female reproductive system. Cell Death Differ. 2001;8:192–200. doi: 10.1038/sj.cdd.4400797. [DOI] [PubMed] [Google Scholar]
- 6.Otnes B, Harvei S, Fossa SD. The burden of prostate cancer from diagnosis until death. British journal of urology. 1995;76:587–94. doi: 10.1111/j.1464-410x.1995.tb07783.x. [DOI] [PubMed] [Google Scholar]
- 7.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22:232–40. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 8.Prins GS, Cooke PS, Birch L, et al. Androgen receptor expression and 5 alpha-reductase activity along the proximal-distal axis of the rat prostatic duct. Endocrinology. 1992;130:3066–73. doi: 10.1210/endo.130.5.1572313. [DOI] [PubMed] [Google Scholar]
- 9.Lee C, Sensibar JA, Dudek SM, Hiipakka RA, Liao ST. Prostatic ductal system in rats: regional variation in morphological and functional activities. Biology of reproduction. 1990;43:1079–86. doi: 10.1095/biolreprod43.6.1079. [DOI] [PubMed] [Google Scholar]
- 10.Rouleau M, Leger J, Tenniswood M. Ductal heterogeneity of cytokeratins, gene expression, and cell death in the rat ventral prostate. Molecular endocrinology (Baltimore, Md. 1990;4:2003–13. doi: 10.1210/mend-4-12-2003. [DOI] [PubMed] [Google Scholar]
- 11.Verras M, Sun Z. Roles and regulation of Wnt signaling and beta-catenin in prostate cancer. Cancer Lett. 2006;237:22–32. doi: 10.1016/j.canlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Terry S, Yang X, Chen MW, Vacherot F, Buttyan R. Multifaceted interaction between the androgen and Wnt signaling pathways and the implication for prostate cancer. J Cell Biochem. 2006;99:402–10. doi: 10.1002/jcb.20983. [DOI] [PubMed] [Google Scholar]
- 13.Mount JG, Muzylak M, Allen S, Althnaian T, McGonnell IM, Price JS. Evidence that the canonical Wnt signalling pathway regulates deer antler regeneration. Dev Dyn. 2006;235:1390–9. doi: 10.1002/dvdy.20742. [DOI] [PubMed] [Google Scholar]
- 14.Kyprianou N, Isaacs JT. Expression of transforming growth factor-beta in the rat ventral prostate during castration-induced programmed cell death. Molecular endocrinology (Baltimore, Md. 1989;3:1515–22. doi: 10.1210/mend-3-10-1515. [DOI] [PubMed] [Google Scholar]
- 15.Hsing AY, Kadomatsu K, Bonham MJ, Danielpour D. Regulation of apoptosis induced by transforming growth factor-beta1 in nontumorigenic rat prostatic epithelial cell lines. Cancer Res. 1996;56:5146–9. [PubMed] [Google Scholar]
- 16.Brodin G, ten Dijke P, Funa K, Heldin CH, Landstrom M. Increased smad expression and activation are associated with apoptosis in normal and malignant prostate after castration. Cancer Res. 1999;59:2731–8. [PubMed] [Google Scholar]
- 17.Massague J, Gomis RR. The logic of TGFbeta signaling. FEBS Lett. 2006;580:2811–20. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 18.Hayes SA, Zarnegar M, Sharma M, et al. SMAD3 represses androgen receptor-mediated transcription. Cancer Res. 2001;61:2112–8. [PubMed] [Google Scholar]
- 19.Chipuk JE, Cornelius SC, Pultz NJ, et al. The androgen receptor represses transforming growth factor-beta signaling through interaction with Smad3. J Biol Chem. 2002;277:1240–8. doi: 10.1074/jbc.M108855200. [DOI] [PubMed] [Google Scholar]
- 20.Kang HY, Lin HK, Hu YC, Yeh S, Huang KE, Chang C. From transforming growth factor-beta signaling to androgen action: identification of Smad3 as an androgen receptor coregulator in prostate cancer cells. Proc Natl Acad Sci U S A. 2001;98:3018–23. doi: 10.1073/pnas.061305498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang X, Chen MW, Terry S, et al. Complex regulation of human androgen receptor expression by Wnt signaling in prostate cancer cells. Oncogene. 2006;25:4256. doi: 10.1038/sj.onc.1209366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Widelitz R. Wnt signaling through canonical and non-canonical pathways: recent progress. Growth Factors. 2005;23:111–6. doi: 10.1080/08977190500125746. [DOI] [PubMed] [Google Scholar]
- 23.Bhowmick NA, Chytil A, Plieth D, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–51. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 24.Chytil A, Magnuson MA, Wright CV, Moses HL. Conditional inactivation of the TGF-beta type II receptor using Cre:Lox. Genesis. 2002;32:73–5. doi: 10.1002/gene.10046. [DOI] [PubMed] [Google Scholar]
- 25.DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–68. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- 26.Kasper S, Sheppard PC, Yan Y, et al. Development, progression, and androgen-dependence of prostate tumors in probasin-large T antigen transgenic mice: a model for prostate cancer. Lab Invest. 1998;78:i–xv. [PubMed] [Google Scholar]
- 27.Shappell SB, Thomas GV, Roberts RL, et al. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 2004;64:2270–305. doi: 10.1158/0008-5472.can-03-0946. [DOI] [PubMed] [Google Scholar]
- 28.Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res. 2002;8:2912–23. [PubMed] [Google Scholar]
- 29.Kim IY, Zelner DJ, Sensibar JA, et al. Modulation of sensitivity to transforming growth factor-beta 1 (TGF-beta 1) and the level of type II TGF-beta receptor in LNCaP cells by dihydrotestosterone. Exp Cell Res. 1996;222:103–10. doi: 10.1006/excr.1996.0013. [DOI] [PubMed] [Google Scholar]
- 30.Hayward SW, Haughney PC, Rosen MA, et al. Interactions between adult human prostatic epithelium and rat urogenital sinus mesenchyme in a tissue recombination model. Differentiation. 1998;63:131–40. doi: 10.1046/j.1432-0436.1998.6330131.x. [DOI] [PubMed] [Google Scholar]
- 31.Strutz F, Okada H, Lo CW, et al. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol. 1995;130:393–405. doi: 10.1083/jcb.130.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayward SW. Approaches to Modeling Stromal-Epithelial Interactions. J Urol. 2002;168:1165–72. doi: 10.1016/S0022-5347(05)64620-4. [DOI] [PubMed] [Google Scholar]
- 33.Voeller HJ, Truica CI, Gelmann EP. Beta-catenin mutations in human prostate cancer. Cancer Res. 1998;58:2520–3. [PubMed] [Google Scholar]
- 34.Truica CI, Byers S, Gelmann EP. Beta-catenin affects androgen receptor transcriptional activity and ligand specificity. Cancer Res. 2000;60:4709–13. [PubMed] [Google Scholar]
- 35.Chen G, Shukeir N, Potti A, et al. Up-regulation of Wnt-1 and beta-catenin production in patients with advanced metastatic prostate carcinoma: potential pathogenetic and prognostic implications. Cancer. 2004;101:1345–56. doi: 10.1002/cncr.20518. [DOI] [PubMed] [Google Scholar]
- 36.Liu XH, Kirschenbaum A, Yao S, Liu G, Aaronson SA, Levine AC. Androgen-induced Wnt signaling in preosteoblasts promotes the growth of MDA-PCa-2b human prostate cancer cells. Cancer Res. 2007;67:5747–53. doi: 10.1158/0008-5472.CAN-07-0478. [DOI] [PubMed] [Google Scholar]
- 37.Yardy GW, Brewster SF. Wnt signalling and prostate cancer. Prostate Cancer Prostatic Dis. 2005;8:119–26. doi: 10.1038/sj.pcan.4500794. [DOI] [PubMed] [Google Scholar]
- 38.Sugimura Y, Cunha GR, Donjacour AA, Bigsby RM, Brody JR. Whole-mount autoradiography study of DNA synthetic activity during postnatal development and androgen-induced regeneration in the mouse prostate. Biol Reprod. 1986;34:985–95. doi: 10.1095/biolreprod34.5.985. [DOI] [PubMed] [Google Scholar]
- 39.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–51. [PubMed] [Google Scholar]
- 40.Wright AS, Douglas RC, Thomas LN, Lazier CB, Rittmaster RS. Androgen-induced regrowth in the castrated rat ventral prostate: role of 5alpha-reductase. Endocrinology. 1999;140:4509–15. doi: 10.1210/endo.140.10.7039. [DOI] [PubMed] [Google Scholar]
- 41.Knudson AG, Jr., Hethcote HW, Brown BW. Mutation and childhood cancer: a probabilistic model for the incidence of retinoblastoma. Proc Natl Acad Sci U S A. 1975;72:5116–20. doi: 10.1073/pnas.72.12.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]