Abstract
Decorin is a member of the small leucine-rich proteoglycan gene family and plays an important role in suppressing cancer cell growth and metastasis. To elucidate the importance of decorin in intestinal carcinogenesis, a decorin-deficient (Dcn−/−) mouse model was employed. We found that targeted inactivation of decorin was sufficient to cause intestinal tumor formation with 30% of the Dcn−/− mice developing intestinal tumors with no other chemical or genetic initiation. Moreover, a high-risk diet amplified and accelerated the tumors initiated by decorin deficiency. Further, tumorigenesis in Dcn−/− mice was associated with disruption of intestinal maturation, including decreased cell differentiation and increased proliferation, which were linked to the downregulation of p21WAF1/cip1, p27kip1, intestinal trefoil factor and E-cadherin and to the upregulation of β-catenin signaling. In addition, we found that decorin was highly expressed in the differentiated area of human normal colonic mucosa, but was dramatically reduced in paired colorectal cancer tissues. Taken together, our data demonstrate that decorin acts as a tumor suppressor gene and plays an important role in the maintenance of cell maturation and therefore homeostasis in the intestinal tract.
Introduction
Decorin is a member of the small leucine-rich proteoglycan gene family and has multiple biological functions, including the regulation of matrix assembly and fibrillogenesis and control of cell proliferation (1–3). Decorin is known to cause rapid phosphorylation of epidermal growth factor receptor thereby leading to an activation of the mitogen-activated protein kinase and upregulation of p21WAF1/cip1, a cyclin-dependent kinase inhibitor (4,5), and to growth arrest. In endothelial cells, induction of decorin upregulated p21WAF1/cip1 as well as p27kip1, another inhibitor of cyclin-dependent kinases, inhibiting G1 to S transition in the cell cycle and inducing cell differentiation (6). Most notably, decorin modulates transforming growth factor β activity (1) and plays an important role in the process of tumor growth and progression (7–9). De novo decorin gene expression suppresses the malignant phenotype of human colorectal cancer cells (10), which was associated with the induction of p21WAF1/cip1 (11). Moreover, transient transgene expression of a replication-deficient adenovirus-containing decorin causes a significant growth inhibition of colon and squamous cell carcinoma xenografts via inactivation of the epidermal growth factor receptor tyrosine kinase (8). Recent studies have shown that decorin prevents metastasis of breast cancer through decorin's long-term downregulation of the ErbB2 tyrosine kinase cascade (12).
To pursue the role of the decorin gene (Dcn) in intestinal maturation and carcinogenesis, we utilized a mouse model with targeted decorin gene inactivation and found that lack of decorin gene expression was associated with intestinal tumorigenesis and that a western-style high-risk diet, high in fat, low in calcium and vitamin D, amplified and accelerated intestinal tumorigenesis in response Dcn−/− mice. Further, the tumorigenesis in Dcn−/− mice was linked to the downregulation of p21, p27, intestinal trefoil factor (ITF) and E-cadherin and to the upregulation of β-catenin signaling, leading to decreased cell differentiation and increased cell proliferation.
Materials and methods
Decorin (Dcn) knockout mouse model
The original Dcn−/− mice (13) (mixed background of 129/Sv and Bl/Swiss) were backcrossed with C57BL/6J mice for 14 generations to generate Dcn heterozygous (+/−) mouse on a C57BL/6J background. Dcn+/− mice were mated with each other to generate Dcn+/+ or Dcn−/− mice, with genotyping done by polymerase chain reaction (PCR), as described (13). After weaning, mice with appropriate genotypes were randomized to either a defined AIN-76A control diet or a western-style diet for 36 weeks.
At the end point of 36 weeks, tumor incidence, frequency and histopathology were recorded. Proliferative cell nuclear antigen staining was used to evaluate cell proliferation. Alcian blue staining was performed to evaluate goblet cell differentiation. All procedures were standardized in our laboratory, as described previously (14,15).
Intestinal epithelial cell isolation and gene expression assay
Mouse intestinal epithelial cells were isolated by incubating the dissected, washed intestinal tissues in 15 mM ethylenediaminetetraacetic acid buffer at 37°C for 30 min as described previously (14). Total RNA was extracted from the epithelial cell pellet, and the quality and quantity of RNA was measured using a BioAnalyzer. As described previously, 5 μg of total RNA was used for Affymetrix microarray analysis using Mouse Genome 430-2.0 Arrayer chips. cDNA was synthesized from DNase-treated total RNA using TaqMan Multiscribe reverse transcriptase (Applied Biosystems, Foster City, CA). Quantitative real-time PCR analysis was performed using the ABI Prism 7900-HT sequence detection system (96 well, Applied Biosystems). The primers for p21, p27, Muc2, β-catenin and β-actin, the amplification conditions for the quantitative real-time PCR and methods of data analysis were reported previously (16).
Alterations of protein assayed by western blot and immunohistochemical staining
Protein was also extracted from the isolated intestinal epithelial cells. In brief, mouse intestinal epithelial cells were washed twice with ice-cold phosphate-buffered saline, and then cell lysis buffer (Cell Signaling, Beverly, MA) was added with incubation on ice for 15 min. After brief sonication, total cell lysate was centrifuged at 10, 000 r.p.m. for 10 min at 4°C. The supernatant was fractionated by electrophoresis in a 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The following primary antibodies were utilized as probe: anti-p21, anti-p27, anti-β-catenin, anti-cdk4 and anti-cyclin D1 (Santa Cruz Biotechnology, Santa Cruz, CA); anti-β-actin (Sigma, St Louis, MO) and anti-ITF rabbit polyclonal antibody (kindly provided by Dr Podolsky, Massachusetts General Hospital, MA). Signal was detected by the enhanced chemiluminescence technique (Amersham Life Science, Piscataway, NJ). Signal was quantified and normalized to β-actin.
Protein expression in the intestine was also analyzed by immunohistochemistry, as we described previously (14). Briefly, formalin-fixed and paraffin-embedded mouse intestinal tissues were sectioned, deparaffinated and dehydrated. To block endogenous peroxidase, the sections were incubated in H2O2 (0.3%)/methanol for 20 min. The sections were then incubated with 10% normal goat serum to block non-specific antibody binding. To expose epitopes for immunodetection, sections were treated in a steamer for 20 min in citrate buffer (pH 6.1). The sections were incubated with the following primary antibodies at 4°C overnight: anti-β-catenin (1:50) (Santa Cruz Biotechnology) and anti-ITF (1:2000). Detection was with biotinylated anti-rabbit IgG (Santa Cruz Biotechnology), followed by incubation with avidin–biotin complex (Vector Laboratories, Burlingame, CA) and the substrate 3′5′-diaminobenzidine. Finally, the sections were counterstained with hematoxylin. Images were captured by the Aperio Image Scope system (Aperio Technologies, Vista, CA).
Human colorectal cancer tissues for immunohistochemical staining
Twenty-four pairs of colorectal cancer tissues and their adjacent normal mucosa were obtained from the archived paraffin-embedded blocks that were collected from surgically resected colorectal cancer patients at University of Illinois Medical Center under a protocol approved by the Institutional Review Board. Similar procedures as those used for mouse intestine were employed for the immunohistochemical staining of decorin in human colorectal tissues. The tissues were probed with anti-human decorin polyclonal antibody (1:2000) (kindly provided by Dr Fisher, National Institutes of Health, Bethesda, MD) and counterstained with hematoxylin.
Results
Targeted inactivation of the decorin gene caused intestinal tumor formation
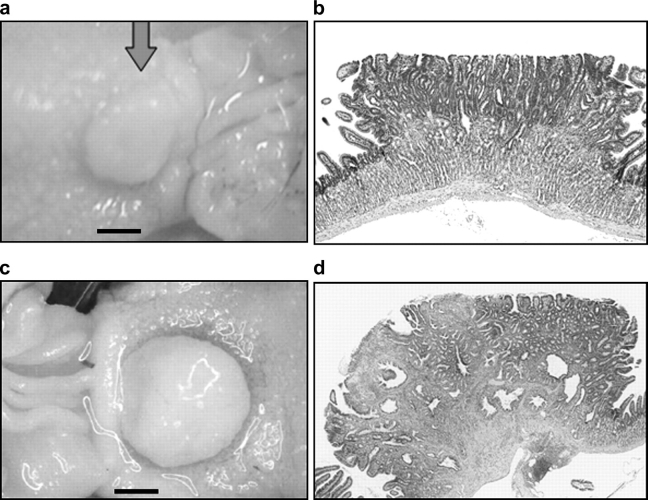
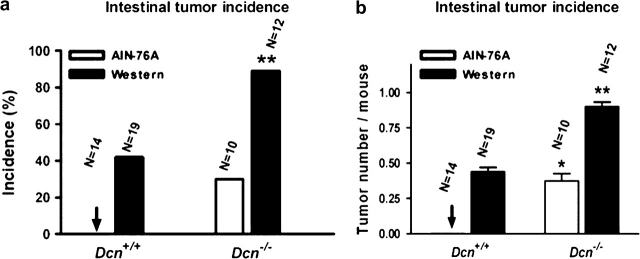
Mutant mice with targeted deficiency of the decorin gene spontaneously developed tumors in the intestine when the mice were fed a defined control AIN-76A diet for 36 weeks (Figure 1a). Similar as in other mouse models (e.g. Apc+/−, p27−/−, etc.) of intestinal tumor (16,17), tumors in Dcn−/− mice were also located in small intestine, but not in large intestine. Histological examination of a sessile lesion showed adenomatous features (Figure 1b), considered to be premalignant lesion. Approximately 30% (3/10) of the Dcn−/− mice fed with the AIN-76A diet had intestinal tumors, at an average frequency of 0.40 tumors per mouse (Figure 2). No tumors (0/14) were detected in decorin wild-type mice (Dcn+/+). Statistical differences of tumor incidence and frequency were significant (P < 0.05). This finding suggests that decorin behaves as a classical tumor suppressor gene.
Fig. 1.
Intestinal tumors formed in Dcn−/− mice. (a) One adenoma was seen in the intestine of a Dcn−/− mouse on AIN-76 diet (scale, 2 mm); (b) histopathology of the tumor in (a) is shown in (b) (original magnification, ×20); (c) a large adenocarcinoma was seen in a Dcn−/− mouse fed western-style diet (scale, 2 mm). Histopathological features of the tumor in (c) are shown in (d) (×20). Note that the layers of the intestinal wall were destroyed with infiltration of the carcinoma.
Fig. 2.
The incidence and frequency of intestinal tumors in Dcn+/+ or Dcn−/− mice fed AIN-76A or a western-style diet for 36 weeks. (*P < 0.05; **P < 0.01, compared with Dcn+/+ mice, by Fisher's exact or Student’s t-test, respectively). N, the number of animals studied in each group.
The western-style high-risk diet is formulated based on nutrient density to mimic the intake of major risk factors (high fat and phosphate, low calcium and vitamin D) for colorectal cancer in the USA and other developed countries. It has been shown to be highly effective in augmenting intestinal tumorigenesis in rodent models (15,16,18). Similarly, in this study, the same western-style diet significantly increased tumor incidence and tumor frequency, regardless of the decorin genotype. When the mice were fed the western-style diet, the incidence of tumors dramatically increased to 83% (10/12) and the number of tumors per mouse increased to 0.91 in Dcn−/− mice, respectively (Figure 2, black column). Tumor incidence and frequency were more significant (P < 0.01) in comparison with Dcn+/+ mice, even though tumorigenesis was observed in the wild-type mice (Dcn+/+) by the western-style diet, which was consistent with the previous report (19). More impressively, a few intestinal malignant tumors were seen in Dcn−/− mice fed with the western-style diet. Figure 1c is an example of a large tumor in a Dcn−/− mouse fed the western-style diet, which was an adenocarcinoma that invaded the connective tissues and muscular layer of the intestinal wall (Figure 1d). Moreover, tumor sizes were larger in Dcn−/− mice (6.9 ± 0.9 mm2) than those in Dcn+/+ mice (5.0 ± 0.9 mm2), although the difference is not significant (P = 0.12). Thus, the western-style diet was synergistic with the loss of the decorin gene in intestinal tumorigenesis, indicating that interaction of genetic and dietary factors plays an important role in intestinal tumor formation.
Intestinal tumorigenesis in Dcn−/− mouse was linked to the decrease of cell differentiation and increase of cell proliferation
To investigate whether tumorigenesis in Dcn−/− mice was due to the disruption of intestinal maturation and homeostasis, we analyzed cell differentiation and proliferation in the intestinal tract. The appearance of the intestinal crypt–villus was normal, but goblet cell formation in Dcn−/− mice was significantly reduced as assessed by Alcian blue staining, in comparison with Dcn+/+ mice (Figure 3a). Goblet cell is one of intestinal secretory cell lineages that synthesize and secret the mucin Muc2, the principal component of the intestinal mucus. Alteration of Muc2 expression has been identified in intestinal pathologies such as inflammatory bowel disease and colorectal cancer (20). Genetic inactivation of mouse Muc2 gene resulted in alterations of cell proliferation, migration, apoptosis and tumor formation (21). The reduction of cell differentiation was validated by the staining for ITF, another marker of intestinal goblet cell differentiation (Figure 3b). In contrast, cell proliferation at the bottom of the crypt, assessed by proliferative cell nuclear antigen staining, was markedly increased in Dcn−/− mice compared with Dcn+/+ mice (Figure 3c). Thus, inactivation of the decorin gene could cause perturbation of intestinal cell differentiation and proliferation, resulting in intestinal tumor formation.
Fig. 3.
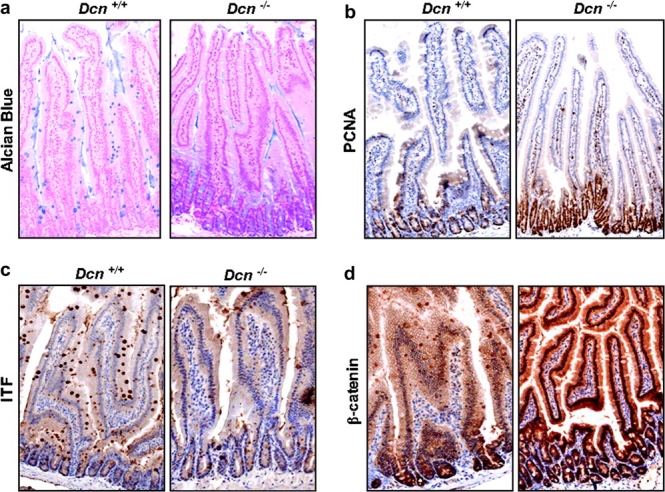
Intestinal cell differentiation [assayed by Alcian blue and ITF staining (a and b)] was decreased, but cell proliferation [proliferative cell nuclear antigen (PCNA) staining (c)] and β-catenin expression (d) were increased in Dcn−/− mice fed AIN-76A control diet (original magnification, ×20).
Molecular mechanisms of intestinal homeostasis perturbation in Dcn−/− mice
To gain further insight into the mechanisms by which tumors form in the Dcn−/− mice, we screened for differential gene expression in isolated intestinal epithelial cells of Dcn−/− mice on AIN-76A control diet using Affymetrix gene arrays. Among the alterations in Dcn−/− intestinal mucosa compared with those of wild-type mice were downregulation of the cyclin-dependent kinase inhibitors p27kip1 and p21WAF1/cip1 and differentiation marker ITF, which were downregulated by 1.3-, 1.5- and 7.7-fold, respectively, in the intestinal epithelial cells from the Dcn−/− mice; in contrast, β-catenin, a key mediator in Wnt-signaling pathway, was upregulated by 1.6-fold.
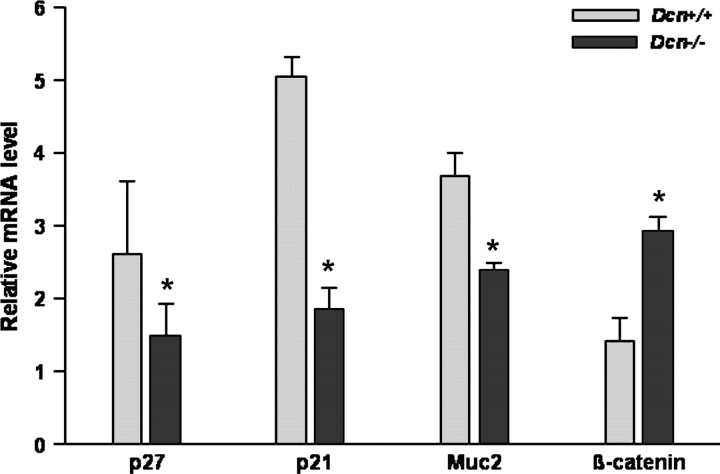
The gene expression changes detected by microarray analysis were validated by quantitative real-time PCR that showed that p27, p21 and Muc2 were significantly reduced by 1.8-, 2.7- and 1.6-fold, but, β-catenin was increased by 2.1-fold in messenger RNA (mRNA) level, respectively (Figure 4). Therefore, perturbation of intestinal mucosa by decorin deficiency was linked to the downregulation of p21, p27 and ITF/Muc2 and the upregulation of β-catenin.
Fig. 4.
Gene expression was validated by quantitative real-time reverse transcription–PCR. The relative mRNA levels of p21, p27, Muc2 and β-catenin were analyzed from intestinal epithelial cells of Dcn+/+ or Dcn−/− mice fed AIN-76A diet (five animals per group). (*P < 0.05, compared with Dcn+/+ mice by Mann–Whitney test.).
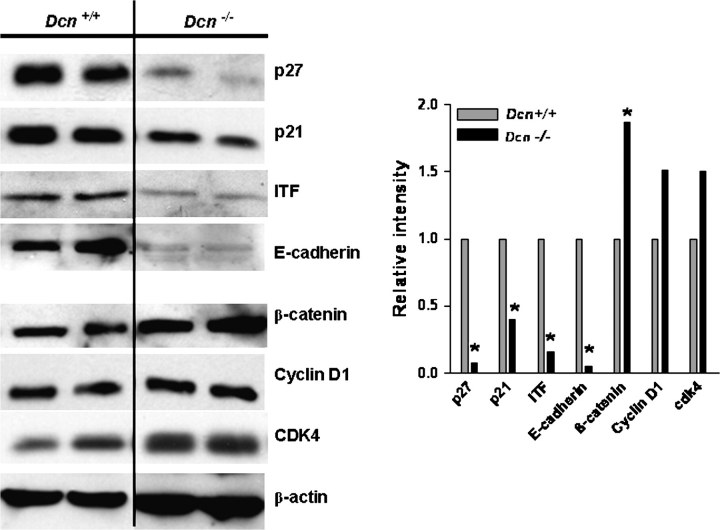
To elucidate protein alterations, protein extracted from mouse intestinal epithelial cells was evaluated by western blot. In consistent with the alterations of mRNA and as shown in Figure 5, the protein levels of p27 decreased 12.9-fold, p21 decreased 2.5-fold and ITF decreased 6.2-fold, respectively, in Dcn−/− mouse intestinal epithelial cells in comparison with the wild-type mice. In contrast, the level of β-catenin was significantly increased in the Dcn−/− mouse intestine, which was confirmed by in situ immunohistochemical staining (Figure 3d). Intriguingly, the β-catenin–Tcf targets, cyclin D1 and cdk4, were also modestly increased. In addition, E-cadherin, which can form a complex with β-catenin, was significantly downregulated by 20-fold in Dcn−/− mouse intestinal epithelial cells (Figure 5).
Fig. 5.
Alterations of protein in Dcn mice fed with AIN-76A diet assayed by western blots. p21, p27, ITF and E-cadherin were significantly reduced and β-catenin, cdk4 and cyclin D1 were increased in Dcn−/− mouse intestinal epithelial cells. Proteins were extracted from two individual mice of each genetic group. Signal quantification was normalized to β-actin and Dcn+/+ mouse, and the average fold changes were shown in the columns.
Decorin was highly expressed in normal colonic mucosa, but was reduced in human colorectal cancer
To assess the potential role of decorin in human colorectal cancer, we stained resected colorectal tissues from patients with colorectal cancer, including adenocarcinomas and their adjacent normal tissues, by in situ immunohistochemistry to determine the expression and tissue distribution of decorin in these tissues. In all 24 pairs of cancer/normal samples, decorin was expressed in the cytoplasm of cells of the normal colonic mucosa, especially in cells in the differentiation compartment encompassing the upper crypts and lumenal surface. However, decorin expression was significantly reduced in the cancer tissues (Figure 6). This finding is consistent with the tumor suppressor activity of decorin we documented in the mouse, and that decorin expression suppressed the malignant phenotype in human colon cancer cells (10). The distribution of decorin expression along the crypt–villus axis also suggests that decorin is involved in maintaining intestinal tissue homeostasis.
Fig. 6.
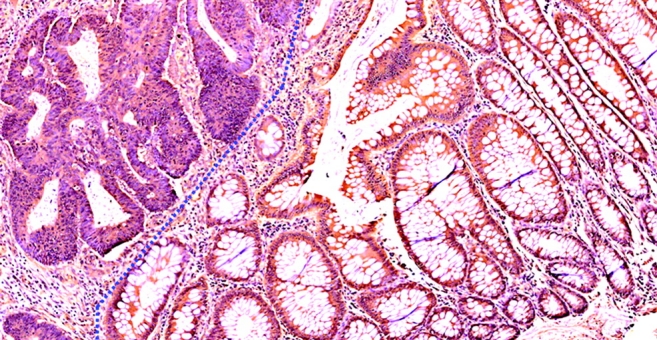
Decorin is highly expressed in human normal colon mucosa (right side) and decreased in colon cancer tissues (left side). (original magnification, ×20).
Discussion
Our study demonstrates for the first time that targeted inactivation of the decorin gene is sufficient to cause intestinal tumorigenesis and that this process is associated with downregulation of p21, p27, ITF/Muc2 and E-cadherin and upregulation of β-catenin signaling. The end result of decorin deficiency is a marked decrease in cell differentiation and a concurrent increase in cell proliferation in the intestine of the mouse. Moreover, we provide evidence that interactions of this genetic factor with dietary factors synergize to modulate intestinal tumor formation.
These findings are consistent with a previous study which demonstrated that inactivation of both decorin and p53 gene, a classical tumor suppressor gene, cooperated to accelerate lymphoma tumorigenesis (22). However, this observation contrasted with a previous study in which disruption of the decorin gene did not cause the spontaneous generation of intestinal neoplasia (13). There are several reasons for this discrepancy. First, the genetic background is different. The currently used Dcn−/− mice were on a constant C57BL/6J background, compared with the mixed background in the previous report (13). Second, the mice here were fed with a defined AIN-76A diet rather than standard rodent chow diet, and tumor formation is probably suppressed by chow diet by phytochemicals in such plant-based diets (18). Therefore, genetic background and environmental factors (e.g. diet) may have contributed to tumor initiation and localization in the gastrointestinal tract by the decreased decorin function. Even though extensive combinational experiments will be necessary to sort out the influence of multiple dietary factors, the fundamental conclusion is that inactivation of decorin is capable of initiating intestinal tumor formation.
We used microarray analysis to screen for the expression of the genes involved in tumor formation in the Dcn−/− mice, which provided important information for selection of genes for further study of the molecular mechanisms relating to the development of intestinal carcinogenesis. Among these genes, the cyclin-dependent kinase inhibitors p21 and p27, the cell differentiation markers Muc2 and ITF and Wnt/β-catenin-signaling pathway are particular interest.
The p21WAF1 gene plays a critical role in regulating intestinal cell proliferation, differentiation and apoptosis (23). Loss of both expression and topological regulation is detected early in colon tumor formation (24,25). In vitro studies have shown that decorin induces expression of the endogenous cyclin-dependent kinase inhibitor p21WAF1 (11,26) and a subsequent arrest of the cells in the G1 phase of the cell cycle (10). These cytostatic effects occur in a wide variety of the tumor cell lines (26,27) and also can affect murine tumor cells (27) and normal human cells, such as endothelial cells (6) and macrophages (28). Consistent with the in vitro studies, in this report, p21 was significantly decreased in Dcn−/− mice at both mRNA and protein levels. It is of interest that we have previously demonstrated that inactivation of p21 could enhance intestinal tumorigenesis in both Apc+/− and Muc2−/− mouse models (15,29), in which tumorigenesis is either dependent or independent, respectively, on elevations in β-catenin signaling (17,21). Importantly, targeted inactivation of the Muc2 gene, which encodes the major colonic mucin (21,30,31), lead to an absence of recognizable goblet cells and to tumor formation in the small and large intestine (21), and the enhancement of intestinal tumorigenesis in Muc2−/− mice by loss of p21 was linked to the downregulation of p27 (29), another cyclin-dependent kinase inhibitor (32). But unlike p21, abnormally low levels of p27 protein are frequently found in human carcinomas, and these low levels are directly correlated with histological aggressiveness, lymph node metastasis and poor prognosis of esophagus, gastric, breast and colorectal carcinomas (33–39). Targeted inactivation of p27 is sufficient to initiate intestinal tumor formation (16) and accelerates Apc-initiated intestinal tumorigenesis (18). Therefore, the downregulation of p21, p27 and Muc2 caused by decorin deficiency in the mucosa was likely key to the perturbation of intestinal homeostasis (i.e. decreased cell differentiation and increased cell proliferation) and to the eventual development of intestinal tumor. However, whether loss of decorin plays a direct role in regulating cell differentiation and mucin synthesis or an indirect role through failure of cells to arrest in the cell cycle as they migrate up the crypt is not yet known.
β-Catenin is also a crucial mediator in Wnt-signaling pathway and plays an important role in intestinal cancer development. Indeed, β-catenin–Tcf4 signaling directly regulates differentiation of intestinal epithelial cells as well as intestinal cell proliferation (40). Increased expression of β-catenin–Tcf4 target genes, such as cyclin D1 and cdk4, all regulators of the cell cycle is associated with tumor development or progression (40). β-Catenin stability is tightly controlled by cytoplasmic complex GSK3β–Axin–Apc via phosphorylation and subsequent degradation via the proteasome pathway (41). In addition, β-catenin level and localization throughout cell are controlled by E-cadherin, a protein that mediates cell–cell adhesion through calcium-dependent homophilic interactions of extracellular domain. E-cadherin binds β-catenin through its cytoplasmic tail and the latter binds β-catenin, which in turn links these complexes to the actin cytoskeleton (42). Previous studies have provided evidence that perturbation of E-cadherin-mediated cell adhesion is involved in tumor progression and metastasis, and that loss of E-cadherin expression or function in vitro has been associated with decreased differentiation and increased invasive capacity of cancer cell lines (reviewed in ref. 42). In colorectal cancer, both Apc mutation and E-cadherin downregulation are linked to enhanced β-catenin-mediated transcriptional activities (41). In this study, deregulation of E-cadherin expression occurred frequently in the Dcn−/− mouse, which might be the main cause of increases of β-catenin mRNA and protein levels in mouse intestinal epithelial cells, which in turn disrupted intestinal cell proliferation and differentiation and facilitated intestinal tumor formation.
In conclusion, our present study demonstrates that decorin plays an important role in the maintenance of intestinal maturation and in the suppression of intestinal tumorigenesis. The potential that induction of decorin by pharmacological or dietary means could increase efficacy of chemotherapy, and/or prevent intestinal tumor formation, is under further investigation.
Funding
National Cancer Institute (R01 CA112081 to W.Y.), (U54 CA100926 to L.H.A.), (P30-13330 to Albert Einstein Cancer Center), (R01 CA39481 to R.V.I.); American Institute for Cancer Research (05A121 to W.Y.).
Acknowledgments
We would like to thank Dr L.Fisher (National Institutes of Health, Bethesda, MD) for providing the decorin antibody, Dr D.K.Podolsky (Massachusetts General Hospital, Boston, MA) for providing ITF antibody, Dr Y.Xu (University of Minnesota, MN) for microarray data analysis, Ms C.Nicolas (Albert Einstein Cancer Center, New York, NY) and Ms R.Poon (University of Illinois at Chicago, Chicago, IL) for technical assistance.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- ITF
intestinal trefoil factor
- mRNA
messenger RNA
- PCR
polymerase chain reaction
References
- 1.Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu. Rev. Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- 2.Iozzo RV, et al. Decorin is a biological ligand for the epidermal growth factor receptor. J. Biol. Chem. 1999;274:4489–4492. doi: 10.1074/jbc.274.8.4489. [DOI] [PubMed] [Google Scholar]
- 3.Reed CC, et al. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj. J. 2002;19:249–255. doi: 10.1023/A:1025383913444. [DOI] [PubMed] [Google Scholar]
- 4.Csordas G, et al. Sustained down-regulation of the epidermal growth factor receptor by decorin. A mechanism for controlling tumor growth in vivo. J. Biol. Chem. 2000;275:32879–32887. doi: 10.1074/jbc.M005609200. [DOI] [PubMed] [Google Scholar]
- 5.Moscatello DK, et al. Decorin suppresses tumor cell growth by activating the epidermal growth factor receptor. J. Clin. Invest. 1998;101:406–412. doi: 10.1172/JCI846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schonherr E, et al. Decorin-mediated signal transduction in endothelial cells. Involvement of Akt/protein kinase B in up-regulation of p21(WAF1/CIP1) but not p27(KIP1) J. Biol. Chem. 2001;276:40687–40692. doi: 10.1074/jbc.M105426200. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee AG, et al. Aberrant expression and localization of decorin in human oral dysplasia and squamous cell carcinoma. Cancer Res. 2003;63:7769–7776. [PubMed] [Google Scholar]
- 8.Reed CC, et al. Suppression of tumorigenicity by adenovirus-mediated gene transfer of decorin. Oncogene. 2002;21:3688–3695. doi: 10.1038/sj.onc.1205470. [DOI] [PubMed] [Google Scholar]
- 9.Wick W, et al. Transforming growth factor-beta: a molecular target for the future therapy of glioblastoma. Curr. Pharm. Des. 2006;12:341–349. doi: 10.2174/138161206775201901. [DOI] [PubMed] [Google Scholar]
- 10.Santra M, et al. De novo decorin gene expression suppresses the malignant phenotype in human colon cancer cells. Proc. Natl Acad. Sci. USA. 1995;92:7016–7020. doi: 10.1073/pnas.92.15.7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Luca A, et al. Decorin-induced growth suppression is associated with up-regulation of p21, an inhibitor of cyclin-dependent kinases. J. Biol. Chem. 1996;271:18961–18965. doi: 10.1074/jbc.271.31.18961. [DOI] [PubMed] [Google Scholar]
- 12.Reed CC, et al. Decorin prevents metastatic spreading of breast cancer. Oncogene. 2005;24:1104–1110. doi: 10.1038/sj.onc.1208329. [DOI] [PubMed] [Google Scholar]
- 13.Danielson KG, et al. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J. Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tong C, et al. c-Jun NH2-terminal kinase 1 plays a critical role in intestinal homeostasis and tumor suppression. Am. J. Pathol. 2007;171:297–303. doi: 10.2353/ajpath.2007.061036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang W, et al. Targeted inactivation of the p21(WAF1/cip1) gene enhances Apc-initiated tumor formation and the tumor-promoting activity of a Western-style high-risk diet by altering cell maturation in the intestinal mucosal. Cancer Res. 2001;61:565–569. [PubMed] [Google Scholar]
- 16.Yang W, et al. Targeted inactivation of p27kip1 is sufficient for large and small intestinal tumorigenesis in the mouse, which can be augmented by a Western-style high-risk diet. Cancer Res. 2003;63:4990–4996. [PubMed] [Google Scholar]
- 17.Fodde R, et al. A targeted chain-termination mutation in the mouse Apc gene results in multiple intestinal tumors. Proc. Natl Acad. Sci. USA. 1994;91:8969–8973. doi: 10.1073/pnas.91.19.8969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang W, et al. p27kip1 in intestinal tumorigenesis and chemoprevention in the mouse. Cancer Res. 2005;65:9363–9368. doi: 10.1158/0008-5472.CAN-05-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newmark HL, et al. A Western-style diet induces benign and malignant neoplasms in the colon of normal C57Bl/6 mice. Carcinogenesis. 2001;22:1871–1875. doi: 10.1093/carcin/22.11.1871. [DOI] [PubMed] [Google Scholar]
- 20.Andrianifahanana M, et al. Regulation of mucin expression: mechanistic aspects and implications for cancer and inflammatory diseases. Biochim. Biophys. Acta. 2006;1765:189–222. doi: 10.1016/j.bbcan.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Velcich A, et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 22.Iozzo RV, et al. Cooperative action of germ-line mutations in decorin and p53 accelerates lymphoma tumorigenesis. Proc. Natl Acad. Sci. USA. 1999;96:3092–3097. doi: 10.1073/pnas.96.6.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harper JW, et al. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 24.el-Deiry WS, et al. Topological control of p21WAF1/CIP1 expression in normal and neoplastic tissues. Cancer Res. 1995;55:2910–2919. [PubMed] [Google Scholar]
- 25.Polyak K, et al. Early alteration of cell-cycle-regulated gene expression in colorectal neoplasia. Am. J. Pathol. 1996;149:381–387. [PMC free article] [PubMed] [Google Scholar]
- 26.Nash MA, et al. In vitro growth inhibition of ovarian cancer cells by decorin: synergism of action between decorin and carboplatin. Cancer Res. 1999;59:6192–6196. [PubMed] [Google Scholar]
- 27.Santra M, et al. Ectopic expression of decorin protein core causes a generalized growth suppression in neoplastic cells of various histogenetic origin and requires endogenous p21, an inhibitor of cyclin-dependent kinases. J. Clin. Invest. 1997;100:149–157. doi: 10.1172/JCI119507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xaus J, et al. Decorin inhibits macrophage colony-stimulating factor proliferation of macrophages and enhances cell survival through induction of p27(Kip1) and p21(Waf1) Blood. 2001;98:2124–2133. doi: 10.1182/blood.v98.7.2124. [DOI] [PubMed] [Google Scholar]
- 29.Yang W, et al. Inactivation of p21WAF1/cip1 enhances intestinal tumor formation in Muc2−/− mice. Am. J. Pathol. 2005;166:1239–1246. doi: 10.1016/S0002-9440(10)62342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Velcich A, et al. Organization and regulatory aspects of the human intestinal mucin gene (MUC2) locus. J. Biol. Chem. 1997;272:7968–7976. doi: 10.1074/jbc.272.12.7968. [DOI] [PubMed] [Google Scholar]
- 31.van Klinken BJ, et al. Gastrointestinal expression and partial cDNA cloning of murine Muc2. Am. J. Physiol. 1999;276:G115–G124. doi: 10.1152/ajpgi.1999.276.1.G115. [DOI] [PubMed] [Google Scholar]
- 32.Sherr CJ, et al. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 33.Cariou S, et al. Prognostic implications of expression of the cell cycle inhibitor protein p27Kip1. Breast Cancer Res. Treat. 1998;52:29–41. doi: 10.1023/a:1006154900130. [DOI] [PubMed] [Google Scholar]
- 34.Deschenes C, et al. Role of p27(Kip1) in human intestinal cell differentiation. Gastroenterology. 2001;120:423–438. doi: 10.1053/gast.2001.21199. [DOI] [PubMed] [Google Scholar]
- 35.Lloyd RV, et al. p27kip1: a multifunctional cyclin-dependent kinase inhibitor with prognostic significance in human cancers. Am. J. Pathol. 1999;154:313–323. doi: 10.1016/S0002-9440(10)65277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas GV, et al. Down-regulation of p27 is associated with development of colorectal adenocarcinoma metastases. Am. J. Pathol. 1998;153:681–687. doi: 10.1016/S0002-9440(10)65610-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsihlias J, et al. The prognostic significance of altered cyclin-dependent kinase inhibitors in human cancer. Annu. Rev. Med. 1999;50:401–423. doi: 10.1146/annurev.med.50.1.401. [DOI] [PubMed] [Google Scholar]
- 38.Yao J, et al. Down-regulation of p27 is a significant predictor of poor overall survival and may facilitate metastasis in colorectal carcinomas. Int. J. Cancer. 2000;89:213–216. [PubMed] [Google Scholar]
- 39.Zhang H, et al. Loss of p27 expression predicts poor prognosis in patients with Dukes’ B stage or proximal colorectal cancer. Int. J. Oncol. 2001;19:49–52. [PubMed] [Google Scholar]
- 40.van de Wetering M, et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 41.Logan CY, et al. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 42.Wheelock MJ, et al. Cadherins as modulators of cellular phenotype. Annu. Rev. Cell Dev. Biol. 2003;19:207–235. doi: 10.1146/annurev.cellbio.19.011102.111135. [DOI] [PubMed] [Google Scholar]