Abstract
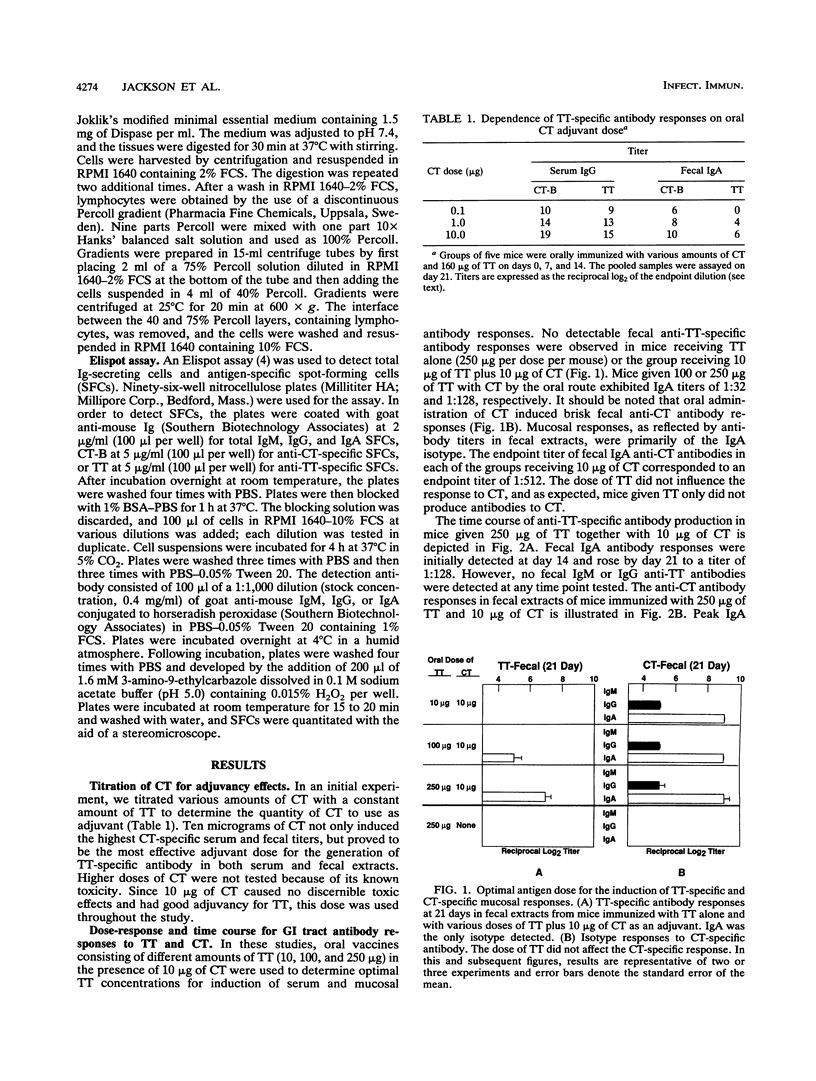
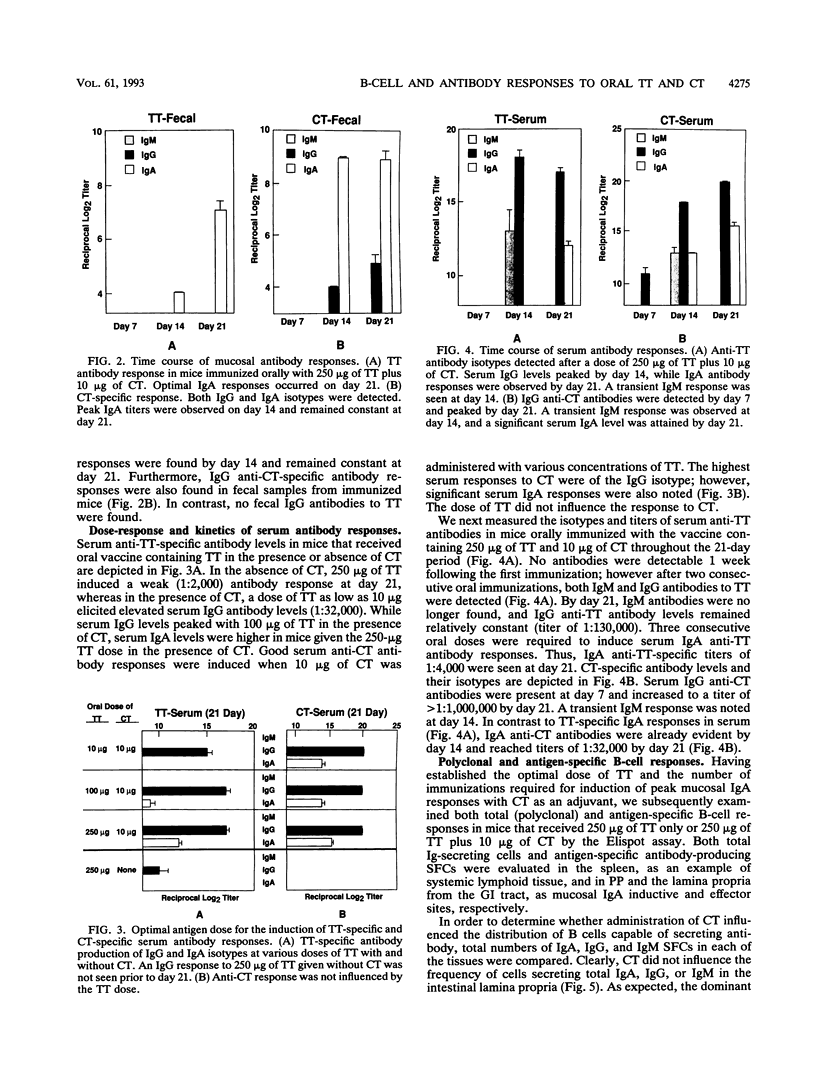
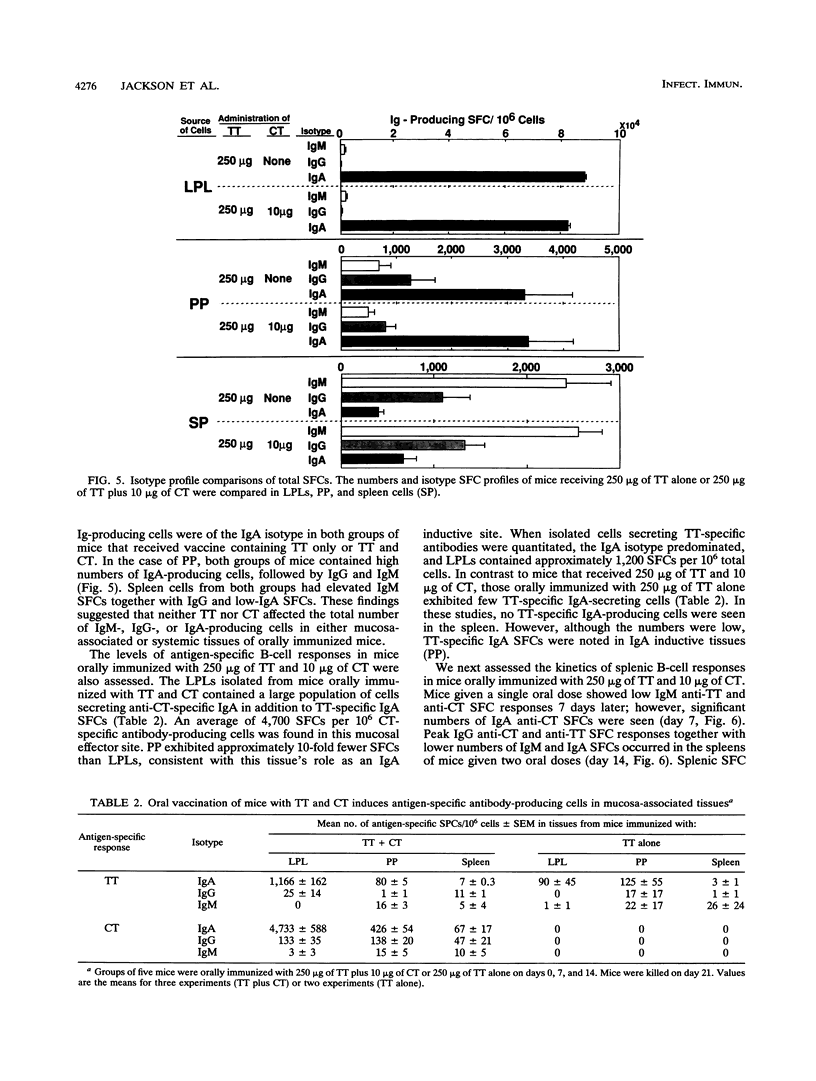
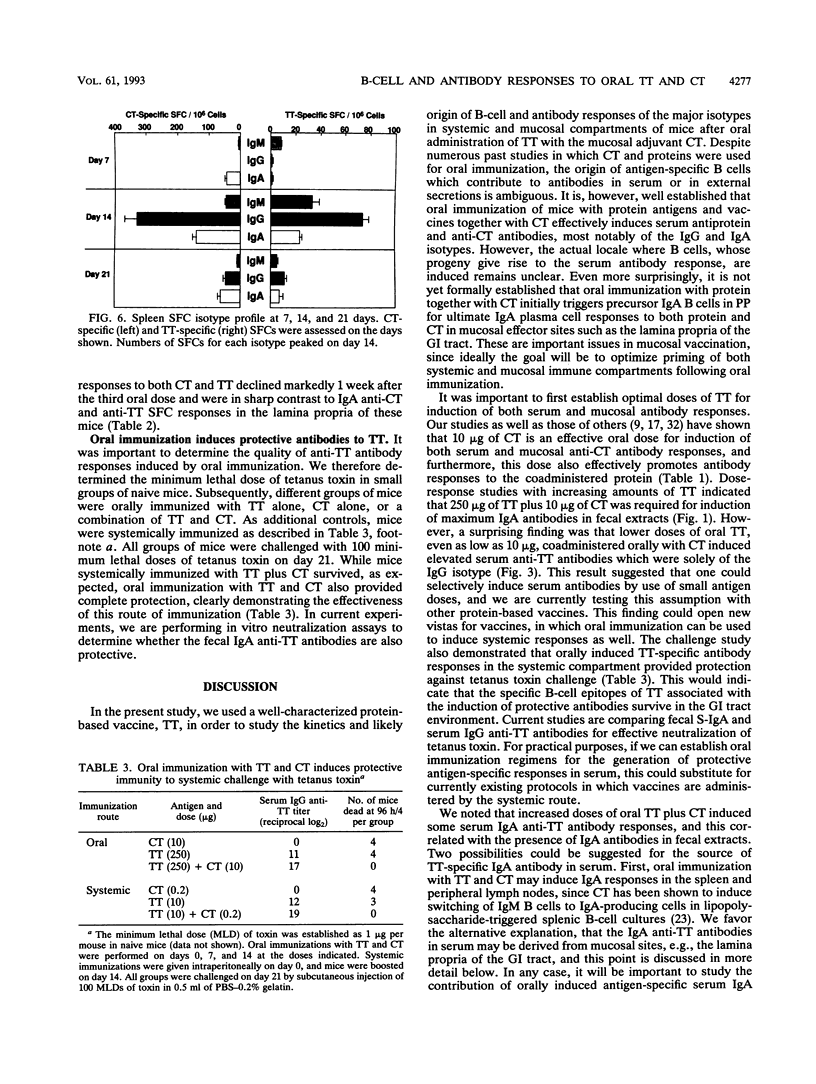
Cholera toxin (CT) is an effective mucosal antigen and acts as an adjuvant when given orally with various antigens; however, few studies have compared the levels of antibody responses to CT and coadministered protein in systemic and mucosal tissues. In this study, we used tetanus toxoid (TT) for assessment of immune responses. Time course and dose-response studies established that 250 micrograms of TT given orally with 10 micrograms of CT three times at weekly intervals induced high serum and gastrointestinal tract anti-TT and anti-CT antibody responses. Oral immunization with TT alone induced no detectable mucosal immunoglobulin A (IgA) antibodies in fecal extracts and only weak serum IgG anti-TT responses. The coadministration of CT and TT induced peak serum IgG anti-TT responses following two oral doses that remained constant after the third oral immunization, while optimal mucosal IgA responses were seen after the third oral immunization. The serum anti-TT response obtained with CT and TT proved protective against TT challenge (100 minimum lethal doses), whereas mice orally given CT or TT alone died. Antigen-specific B-cell responses were assessed with an isotype-specific Elispot assay of isolated lymphoid cells from the spleen, Peyer's patches, and the small intestinal lamina propria. Interestingly, approximately fourfold-higher numbers of IgA anti-CT than of anti-TT antibody-producing (spot-forming) cells occurred in lymphocytes from the lamina propria of mice orally immunized with both TT and CT. The adjuvant CT did not induce polyclonal B-cell responses in mice given CT by the oral route, since no significant differences in total numbers of B cells producing IgA, IgG, or IgM were found compared with the numbers in mice given TT alone. The results clearly indicate that serum and mucosal antibody responses develop with different kinetics and that protective TT-specific antibody responses are generated in the systemic compartment when TT is administered with CT via the oral route.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beagley K. W., Eldridge J. H., Kiyono H., Everson M. P., Koopman W. J., Honjo T., McGhee J. R. Recombinant murine IL-5 induces high rate IgA synthesis in cycling IgA-positive Peyer's patch B cells. J Immunol. 1988 Sep 15;141(6):2035–2042. [PubMed] [Google Scholar]
- Bessen D., Fischetti V. A. Influence of intranasal immunization with synthetic peptides corresponding to conserved epitopes of M protein on mucosal colonization by group A streptococci. Infect Immun. 1988 Oct;56(10):2666–2672. doi: 10.1128/iai.56.10.2666-2672.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig S. W., Cebra J. J. Peyer's patches: an enriched source of precursors for IgA-producing immunocytes in the rabbit. J Exp Med. 1971 Jul 1;134(1):188–200. doi: 10.1084/jem.134.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerkinsky C. C., Nilsson L. A., Nygren H., Ouchterlony O., Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983 Dec 16;65(1-2):109–121. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- Czerkinsky C., Russell M. W., Lycke N., Lindblad M., Holmgren J. Oral administration of a streptococcal antigen coupled to cholera toxin B subunit evokes strong antibody responses in salivary glands and extramucosal tissues. Infect Immun. 1989 Apr;57(4):1072–1077. doi: 10.1128/iai.57.4.1072-1077.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson C. O., Ealding W. Cholera toxin feeding did not induce oral tolerance in mice and abrogated oral tolerance to an unrelated protein antigen. J Immunol. 1984 Dec;133(6):2892–2897. [PubMed] [Google Scholar]
- Elson C. O., Ealding W. Ir gene control of the murine secretory IgA response to cholera toxin. Eur J Immunol. 1987 Mar;17(3):425–428. doi: 10.1002/eji.1830170320. [DOI] [PubMed] [Google Scholar]
- Fuhrman J. A., Cebra J. J. Special features of the priming process for a secretory IgA response. B cell priming with cholera toxin. J Exp Med. 1981 Mar 1;153(3):534–544. doi: 10.1084/jem.153.3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Lindholm L., Lönnroth I. Interaction of cholera toxin and toxin derivatives with lymphocytes. I. Binding properties and interference with lectin-induced cellular stimulation. J Exp Med. 1974 Apr 1;139(4):801–819. doi: 10.1084/jem.139.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyono H., McGhee J. R., Wannemuehler M. J., Frangakis M. V., Spalding D. M., Michalek S. M., Koopman W. J. In vivo immune response to a T-cell-dependent antigen by cultures of disassociated murine Peyer's patch. Proc Natl Acad Sci U S A. 1982 Jan;79(2):596–600. doi: 10.1073/pnas.79.2.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebman D. A., Griffin P. M., Cebra J. J. Relationship between expression of IgA by Peyer's patch cells and functional IgA memory cells. J Exp Med. 1987 Nov 1;166(5):1405–1418. doi: 10.1084/jem.166.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X. P., Lamm M. E., Nedrud J. G. Oral administration of cholera toxin-Sendai virus conjugate potentiates gut and respiratory immunity against Sendai virus. J Immunol. 1988 Sep 1;141(5):1495–1501. [PubMed] [Google Scholar]
- Lycke N., Bromander A. K., Ekman L., Karlsson U., Holmgren J. Cellular basis of immunomodulation by cholera toxin in vitro with possible association to the adjuvant function in vivo. J Immunol. 1989 Jan 1;142(1):20–27. [PubMed] [Google Scholar]
- Lycke N., Hellström U., Holmgren J. Circulating cholera antitoxin memory cells in the blood one year after oral cholera vaccination in humans. Scand J Immunol. 1987 Aug;26(2):207–211. doi: 10.1111/j.1365-3083.1987.tb02253.x. [DOI] [PubMed] [Google Scholar]
- Lycke N., Holmgren J. Adoptive transfer of gut mucosal antitoxin memory by isolated B cells 1 year after oral immunization with cholera toxin. Infect Immun. 1989 Apr;57(4):1137–1141. doi: 10.1128/iai.57.4.1137-1141.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycke N., Holmgren J. Intestinal mucosal memory and presence of memory cells in lamina propria and Peyer's patches in mice 2 years after oral immunization with cholera toxin. Scand J Immunol. 1986 May;23(5):611–616. doi: 10.1111/j.1365-3083.1986.tb01995.x. [DOI] [PubMed] [Google Scholar]
- Lycke N., Holmgren J. Long-term cholera antitoxin memory in the gut can be triggered to antibody formation associated with protection within hours of an oral challenge immunization. Scand J Immunol. 1987 Apr;25(4):407–412. doi: 10.1111/j.1365-3083.1987.tb02207.x. [DOI] [PubMed] [Google Scholar]
- Lycke N., Holmgren J. Strong adjuvant properties of cholera toxin on gut mucosal immune responses to orally presented antigens. Immunology. 1986 Oct;59(2):301–308. [PMC free article] [PubMed] [Google Scholar]
- Lycke N., Karlsson U., Sjölander A., Magnusson K. E. The adjuvant action of cholera toxin is associated with an increased intestinal permeability for luminal antigens. Scand J Immunol. 1991 Jun;33(6):691–698. doi: 10.1111/j.1365-3083.1991.tb02542.x. [DOI] [PubMed] [Google Scholar]
- Lycke N., Lindholm L., Holmgren J. Cholera antibody production in vitro by peripheral blood lymphocytes following oral immunization of humans and mice. Clin Exp Immunol. 1985 Oct;62(1):39–47. [PMC free article] [PubMed] [Google Scholar]
- Lycke N., Strober W. Cholera toxin promotes B cell isotype differentiation. J Immunol. 1989 Jun 1;142(11):3781–3787. [PubMed] [Google Scholar]
- McGhee J. R., Mestecky J., Dertzbaugh M. T., Eldridge J. H., Hirasawa M., Kiyono H. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine. 1992;10(2):75–88. doi: 10.1016/0264-410x(92)90021-b. [DOI] [PubMed] [Google Scholar]
- McGhee J. R., Mestecky J., Elson C. O., Kiyono H. Regulation of IgA synthesis and immune response by T cells and interleukins. J Clin Immunol. 1989 May;9(3):175–199. doi: 10.1007/BF00916814. [DOI] [PubMed] [Google Scholar]
- McKenzie S. J., Halsey J. F. Cholera toxin B subunit as a carrier protein to stimulate a mucosal immune response. J Immunol. 1984 Oct;133(4):1818–1824. [PubMed] [Google Scholar]
- Mestecky J., McGhee J. R. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- Neutra M. R., Kraehenbuhl J. P. M cell-mediated antigen transport and monoclonal IgA antibodies for mucosal immune protection. Adv Exp Med Biol. 1992;327:143–150. doi: 10.1007/978-1-4615-3410-5_16. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Gowans J. L. Cellular kinetics of the intestinal immune response to cholera toxoid in rats. J Exp Med. 1975 Dec 1;142(6):1550–1563. doi: 10.1084/jem.142.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi T., McGhee J. R., Coffman R. L., Beagley K. W., Eldridge J. H., Takatsu K., Kiyono H. Analysis of Th1 and Th2 cells in murine gut-associated tissues. Frequencies of CD4+ and CD8+ T cells that secrete IFN-gamma and IL-5. J Immunol. 1990 Jul 1;145(1):68–77. [PubMed] [Google Scholar]
- Tamura S., Samegai Y., Kurata H., Nagamine T., Aizawa C., Kurata T. Protection against influenza virus infection by vaccine inoculated intranasally with cholera toxin B subunit. Vaccine. 1988 Oct;6(5):409–413. doi: 10.1016/0264-410x(88)90140-5. [DOI] [PubMed] [Google Scholar]
- Tomasi T. B., Jr Oral tolerance. Transplantation. 1980 May;29(5):353–356. doi: 10.1097/00007890-198005000-00001. [DOI] [PubMed] [Google Scholar]
- Vajdy M., Lycke N. Y. Cholera toxin adjuvant promotes long-term immunological memory in the gut mucosa to unrelated immunogens after oral immunization. Immunology. 1992 Mar;75(3):488–492. [PMC free article] [PubMed] [Google Scholar]
- Woogen S. D., Ealding W., Elson C. O. Inhibition of murine lymphocyte proliferation by the B subunit of cholera toxin. J Immunol. 1987 Dec 1;139(11):3764–3770. [PubMed] [Google Scholar]
- deVos T., Dick T. A. A rapid method to determine the isotype and specificity of coproantibodies in mice infected with Trichinella or fed cholera toxin. J Immunol Methods. 1991 Aug 9;141(2):285–288. doi: 10.1016/0022-1759(91)90155-9. [DOI] [PubMed] [Google Scholar]



