Abstract
This report presents a case of osteopetrosis in a 25-year-old male, which was complicated by the development of osteomyelitis in the maxilla and mandible following traumatic injury and tooth extractions. The osteomyelitis in the mandible was refractory to marginal resection and antibiotic therapy. Partial resection with mandible reconstruction was then carried out. Light and backscattered electron scanning microscopy revealed sclerosis of spongy bone and variations in mineral density of the bone matrix. There was also a prominent periosteal bone formation in regions affected by osteomyelitis. An 18-month follow-up showed absence of active infections in the face and oral structures, with a focal area of bone exposure in the right parasymphysis. However, development of anemia and bone marrow deficiency will likely affect prognosis. The importance of preventive oral health care and dental/periodontal managements in osteopetrosis is emphasized.
Keywords: Osteopetrosis, Osteomyelitis, Maxilla, Mandible, Plate, Light microscopy, Scanning electron microscopy
Introduction
Osteopetrosis, also know as Albers-Schönberg disease, osteopetrosis generalisata, or “marble bone disease”, is a rare genetic disease characterized by a generalized sclerosis of bone with a significant reduction in bone marrow spaces, due to an impairment of osteoclast activity that results in imbalance in bone remodeling [1–4]. The prevalence of the disease is estimated to be about 0.005% of the population [2]. Classical osteopetrosis exhibits a vast spectrum of clinical, physiologic, and genotypic expressions and has been classified into three clinically distinct forms: (1) an infantile malignant autosomal recessive form, (2) an intermediate mild autosomal recessive form, and (3) an adult benign autosomal dominant form [5]. Autosomal dominant osteopetrosis (ADO) results from ineffective osteoclast-mediated bone resorption caused by inactivating mutations in the chloride channel 7 (ClCN7) gene, likely disrupting acidification of the osteoclast resorption lacunae [6]. Clinical manifestations in ADO include mostly pathological fractures and osteomyelitis in addition to cranial nerve palsies, anemia, developmental abnormalities of the teeth, and other complications [4, 5]. The diagnosis of ADO is often made at the time of the diagnosis of other disease processes or during routine or specific radiological examinations [1].
In patients with osteopetrosis, osteomyelitis may take place in jaw bones, almost exclusively in the mandible; the rare occurrence of osteomyelitis in maxilla has been attributed to its thin cortical bone and rich collateral bloody supply [7]. Tooth extraction and mild trauma are known factors that contribute to the development of osteomyelitic lesions in facial bones affected by osteopetrosis [1, 5]. These have been treated using several therapies, including pharmacologic agents, hyperbaric oxygen, local wound care, and surgical procedures [3].
The present case report describes the management of severe osteomyelitis in the maxilla and mandible in a patient with adult ADO, which was refractory to antibiotic therapy and marginal resection. A detailed description of the osteopetrotic bone at the light and scanning electron microscope levels is presented. In addition, the importance of preventive oral health care and dental/periodontal managements in ADO is emphasized.
Case Report
Clinical Findings
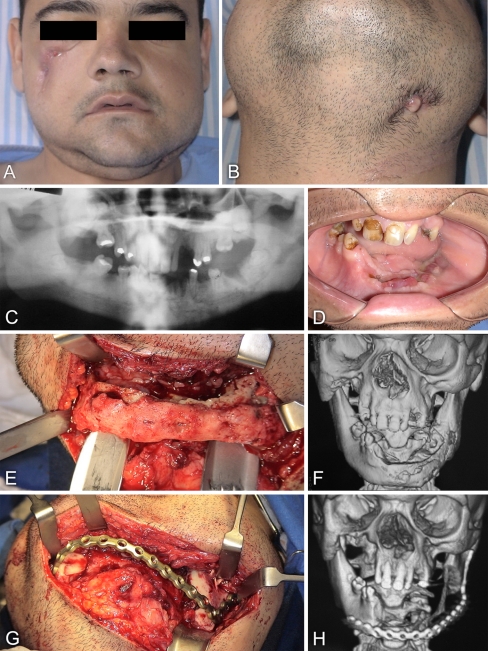
A 25-year-old man presented himself to the oral and maxillofacial surgery service at Santa Casa Hospital in Ribeirao Preto (SP, Brazil) with the major complaints of two draining cutaneous fistulae in the face, one in the right zygomatic area (Fig. 1a) and the other one in the left submandibular region (Fig. 1b), in addition to the presence of focal areas of maxillary and mandibular bone exposure in the oral cavity.
Fig. 1.
a Facial view of a 25-year-old man with osteopetrosis complicated with mandibular osteomyelitis, showing a fistula in the right zygomatic area. b The fistula of the left submandibular region drained purulent secretion. c Panoramic radiograph taken prior to tooth extractions, showing areas of increased bone density and empty tooth sockets (lower left incisor and molar areas). d Intraoral view prior to the first surgical procedure, revealing edentulous areas on the maxilla and mandible with bone exposure at the sites of tooth extractions in the left hemimandible. e Appearance of the affected mandible during surgical exposure. f Three-dimensional computerized tomography (CT) scan reconstruction reveals an irregular contour of the buccal surface of the left hemimandible, suggestive of periosteal bone formation. g Photograph of the titanium plate implanted to reconstruct the mandible without any bone grafting. h Three-dimensional CT scan reconstruction taken after surgery and showing the implanted titanium plate
The patient reported that in the year before he had experienced a fracture of the left femur and a facial abscess in the right zigomatic region as a result of a motorcycle accident. The orthopedic fracture treatment consisted of immobilization and the facial abscess was drained; despite that a cutaneous fistula persisted. The patient also reported recent extractions of the teeth 13, 32, 34, and 36 (Fig. 1c). A previous physician investigation had already established the diagnosis of ADO. There was no family history of osteopetrosis.
The intraoral examination revealed a poor hygiene, edentulous areas on the maxilla and mandible with asymptomatic bone exposure at the sites of recent tooth extractions, and the remaining teeth exhibiting poor conditions (Fig. 1d). The fistula of the zygomatic area was restricted to the skin, while the submandibular one was associated with an infectious process in the mandible (osteomyelitis).
A conservative approach was initially opted to treat mandibular osteomyelitis. As the patient had been treated by antibiotic therapy for 30 days, we chose to maintain the patient on 600 mg clindamycin every 8 h, administered orally, for 30 days. In addition mouthwashes and routine professional dental prophylaxis on a weekly basis were prescribed. This treatment protocol proved unsuccessful and therefore surgical treatment under general anesthesia was undertaken.
The routine pre-operative blood tests showed that two parameters related to the red cells were altered (hemoglobin (Hb): 8.16 g/dl; hematocrit (Ht): 26.2%; leucocytes: 7,400/mm3; platelets: 242,000/mm3), although they were within acceptable standards for the surgical procedure. A marginal resection in the right maxilla and in the mandible extending from the right parasymphysis to the left angle, and fistulectomies in the right zygomatic and left submandibular areas were then carried out. During the surgery, exposed bone exhibited an unusual aspect, with no intraosseous bleeding. Occlusive sutures were performed in both areas, and a Bichat’s fat pad graft was used to close an oroantral communication in the right maxilla. Histopathologic analysis revealed the diagnosis of osteopetrosis and osteomyelitis in the maxilla. Antibiotic therapy with clindamycin was applied 48 h before and during surgery, and maintained post-operatively (600 mg every 8 h) for 30 days. Recommendations on adequate oral hygiene, the use of mouthwashes, and guidelines for diet were given to the patient.
Post-operative monitoring revealed a significant improvement in the healing process of maxilla and no cutaneous fistula in the zygomatic area. In contrast, the mandible exhibited areas of dehiscence with substantial intraoral bone exposure, which was associated with the recurrence of the left submandibular fistula. After 3 months of monitoring, a more aggressive intervention was decided on. Based on the results of the blood tests (Hb: 6.2 g/dl; Ht: 21.1%; leucocytes: 4,500/mm3; platelets: 240,000/mm3), bone marrow transplantation was considered and a medical evaluation was therefore requested. The medical team carried out compatibility testing and concluded that the patient’s relatives were not suitable donors for bone marrow transplantation. Considering that white blood cell numbers were within normal ranges, red blood cell transfusion was indicated preoperatively, which resulted in increased levels for Hb and Ht (8.9 g/dl and 29%, respectively).
The second surgical procedure consisted of partial resection of the mandible, extending from the right parasymphysis to the left mandibular ramus (Fig. 1e, f), and reconstruction of the mandible with a 2.4 mm titanium plate (Neoortho, Curitiba, Brazil), to restore facial harmony (Fig. 1g, h). No bone grafts were used. The histopathologic analysis of block biopsy confirmed the diagnosis of osteopetrosis and osteomyelitis (described in the text given later). Antibiotic therapy with clindamycin was used 48 h before and during the operation, and maintained post-operatively, as specified for the first intervention. The patient was given again recommendations on adequate oral hygiene and guidelines for diet (semi-liquid foods). An 18-month follow-up after the second surgical intervention revealed absence of active infections in the face and oral structures, with a focal area of bone exposure in the right parasymphysis.
Histopathologic Analysis
The block biopsy was fixed in 10% neutral buffered formalin for 48 h. A 5-mm thick cross section of the lower left premolar area was then obtained, dehydrated in increasing concentrations of ethanol, and processed for embedding in methylmethacrylate. The hardened block was cut along the buccolingual plane with an annular blade using a Microslice 2 precision saw (Ultra Tec Manufacturing Inc., Santa Ana, CA), and sections were ground and polished to a thickness of about 20 μm. Sections were stained either with Stevenel’s blue and Alizarin red or with toluidine blue for light microscopic analysis. Unstained sections were examined in a JEOL JSM-6460LV (JEOL, Tokyo, Japan) variable pressure scanning electron microscope operated at 30 kV and 50–70 Pa. The acquired digital images were processed with Adobe Photoshop for sizing and brightness and contrast adjustments.
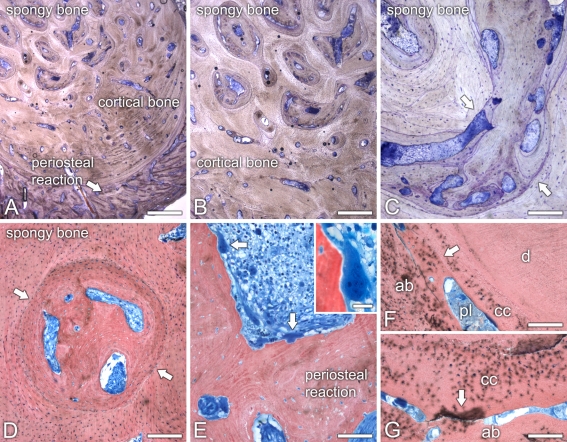
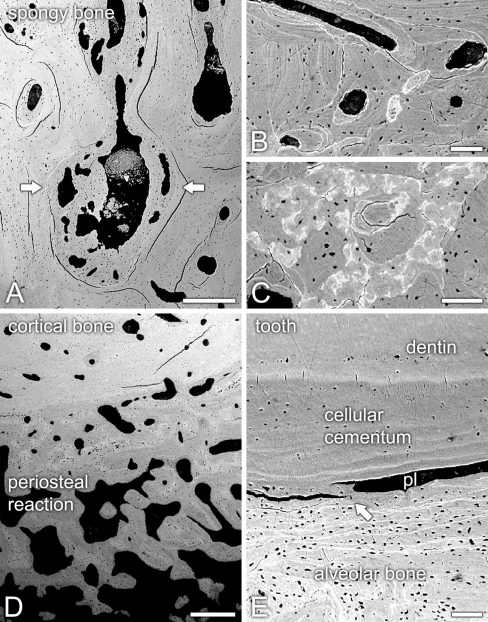
Light and backscattered electron (BSE) scanning microscopy revealed that the architecture of the mandibular bone was divided into three distinct areas, i.e., sclerotic spongy bone, cortical bone, and periosteal reaction (Figs. 2a; 3a–d). Apposition of lamellar bone resulted in a dramatic reduction of the bone marrow cavities in the spongy bone region of the mandible (Figs. 2b–d; 3a). Indeed, both light and BSE scanning microscopy revealed numerous resting, cement lines (Figs. 2c; 3b). In addition, BSE imaging showed variations in mineral density of the bone matrix (Fig. 3c). Some areas of the lamellar bone exhibited large amounts of osteocytic lacunae with osteocytes (Fig. 2d). The remaining marrow spaces were filled either with poorly vascularized, loose connective tissue or necrotic debris. Cortical bone was characterized by Haversian systems with narrowed canals, some of them also containing necrotic tissue. Both spongy and cortical bones exhibited areas with thin osteoid lined by flattened osteoblastic cells. Only rarely were osteoclasts observed, as judged by a paucity of multinucleated cells apposed to bone surfaces. The outer layer of cortical bone exhibited partial circumferential lamellae and was covered by a conspicuous periosteal reaction (Figs. 2a; 3d). The bone in this surface region consisted of thick interconnecting trabeculae of lamellar bone and a well-vascularized, intervening loose connective tissue moderately infiltrated by chronic inflammatory cells (Fig. 2e), with only focal aggregates of neutrophils. Large multinucleated osteoclastic cells, which stained deeply with Stevenel’s blue, could be noticed adjacent to, or in direct contact with the calcified matrix of bone trabeculae (Fig. 2e).
Fig. 2.
Light microscopy of ground sections of the osteopetrotic mandibular bone from the lower left premolar area. a The bone exhibited three distinct regions, i.e., sclerotic spongy bone, cortical bone, and periosteal reaction. b–d Sclerosis of the spongy bone resulted from the apposition of lamellar bone into the bone marrow cavities (c, d, arrows). The remaining bone marrow tissue was partially necrotic. e The periosteal reaction consisted of thick lamellar bone trabeculae surrounded by a loose connective with chronic inflammatory infiltrate. Osteoclastic cells were conspicuously observed (arrows and inset) in this area. f, g Ankylosis (arrows) of a residual tooth root (d, dentin; cc, cellular cementum; ab, alveolar bone) was associated with narrowing and degenerescence of the periodontal ligament (pl). a–cToluidine blue. d–gStevenel’s blue and Alizarin red. Scale bars: A = 1.25 mm; B = 800 μm; C–G = 200 μm; E, inset = 20 μm
Fig. 3.
Backscattered electron scanning microscopy of unstained sections of the osteopetrotic mandible. a Sclerotic spongy bone, with lamellar bone (arrows) partially obliterating the bone marrow cavities. b Cortical bone exhibited clearly evident cement lines (brighter white lines). c Transition between cortical bone and periosteal reaction showed varying compositional contrasts, indicating different degrees of lamellar bone mineralization. d Thick interconnecting periosteal bone trabeculae overlaid the outer cortical surface. e In the area of tooth ankylosis (arrow), cellular cementum exhibited no signs of hyperplasia, whereas periodontal ligament space (pl) was narrowed. Scale bars: A, D = 500 μm; B, C, E = 100 μm
Part of the apical third of a residual tooth root could be observed in the sections. The root exhibited areas of ankylosis likely due to a continuous alveolar bone apposition toward cellular cementum (Figs. 2f, g; 3e). Indeed, no signs of hyperplastic cementum were observed. The remaining narrowed periodontal ligament space was filled with degenerating and necrotic tissue (Fig. 2f, g).
Discussion
This report presents a case of ADO complicated by the occurrence of osteomyelitis in the maxilla and mandible, likely as a result of traumatic injuries. Indeed, there was a direct correlation between the facial trauma and tooth extractions and the subsequent development of osteomyelitis and fistulae. Despite the initial surgical approach (marginal resection) and antibiotic therapy, the osteomyelitic process in the mandible remained refractory and therefore partial resection was carried out. Histopathologic analyses confirmed the diagnosis of osteopetrosis and osteomyelitis.
Osteomyelitis is a well-described complication of osteopetrosis [1, 2, 4, 7] taking place in approximately 10% of cases, mostly in the mandible [4]. Impaired blood white cell function and reduced vascular supply have been considered key factors associated with its development [2]. In addition, the decreased blood supply limits the availability of antibiotics at the sites of infection [2]. In the present case, the remarkable reduction of bone marrow cavities, osteonal canals, and periodontal ligament space unquestionably affected blood circulation likely leading to enhanced susceptibility of the patient to infection. The poor vascularization also prevented the use of free bone grafting or myo-osseous flap during reconstruction with the titanium plate. Although white blood cell count was within normal ranges, the possibility that these cells were defective was not evaluated and therefore cannot be ruled out as contributing factor to osteomyelitis.
The histopathologic analyses remarkably showed sclerosis of spongy bone and variations in mineral density of the bone matrix. Interestingly, the periosteal reaction appeared to be exclusively associated to the osteomyelitic mandible (left hemimandible), as judged by the CT scan reconstruction. The stroma surrounding periosteal trabeculae exhibited numerous osteoclastic cells, a finding that has also been described for endosteal bone deposition in osteopetrosis [8]. The abundance of these cells, however, does not necessarily reflect higher degrees of bone resorption since osteoclasts in osteopetrosis may lack the ruffled border and exhibit impaired functions [9]. It has also been recently reported that targeted disruption of the Cl−/HCO3− exchanger Ae2 in osteoclasts leads to osteopetrosis [10]. The imbalance between bone formation and bone resorption also alters the periodontal ligament unit, leading to ankylosis of the tooth [8].
Whereas the diagnosis of osteopetrosis is straightforward, mostly based on typical radiographic appearance different bones can assume [3, 11] the management of osteopetrotic patients is complex and a major challenge for the clinician. Development of osteomyelitis in some of these patients requires prolonged and adequate antimicrobial treatment [2]. The desirable sequence of treatment includes incision and drainage, antibiotic therapy, sequestrectomy, tooth extraction, saucerization, decortication, bone resection, and hyperbaric oxygen [3, 7]. However, only bone resection and hyperbaric oxygen therapy have been clinically proven to successfully treat osteomyelitis in osteopetrotic patients [1–4, 7, 11].
Despite the better prognosis for the adult ADO compared to its recessive forms [1], the patient developed anemia and bone marrow deficiency. Unfortunately, the lack of suitable patient’s relative donors will likely affect prognosis. In conclusion, the present case shows the importance of aggressive surgical treatment and preventive oral health care and dental/periodontal managements in patients with osteopetrosis, especially when mandibular tooth extraction is under consideration, aiming to avoid aggravation of the clinical condition.
Acknowledgments
The authors thank Sebastião Carlos Bianco (University of São Paulo, Brazil) for the preparation and staining of histological sections, and Sylvia Francis Zalzal (Université de Montréal, Canada) for BSE imaging.
References
- 1.Bakeman RJ, Abdelsayed RA, Sutley SH, et al. Osteopetrosis: a review of the literature and report of a case complicated by osteomyelitis of the mandible. J Oral Maxillofac Surg. 1998;56:1209–1213. doi: 10.1016/S0278-2391(98)90774-1. [DOI] [PubMed] [Google Scholar]
- 2.Barbaglio A, Cortelazzi R, Martignoni G, et al. Osteopetrosis complicated by osteomyelitis of the mandible: a case report including gross and microscopic findings. J Oral Maxillofac Surg. 1998;56:393–398. doi: 10.1016/S0278-2391(98)90122-7. [DOI] [PubMed] [Google Scholar]
- 3.Barry CP, Ryan CD, Stassen LF. Osteomyelitis of the maxilla secondary to osteopetrosis: a report of 2 cases in sisters. J Oral Maxillofac Surg. 2007;65:144–147. doi: 10.1016/j.joms.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Satomura K, Kon M, Tokuyama R, et al. Osteopetrosis complicated by osteomyelitis of the mandible: a case report including characterization of the osteopetrotic bone. Int J Oral Maxillofac Surg. 2007;36:86–93. doi: 10.1016/j.ijom.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Batra P, Shah N. Recalcitrant osteomyelitis following tooth extraction in a case of malignant osteopetrosis. Int Dent J. 2004;54:418–423. doi: 10.1111/j.1875-595x.2004.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 6.Waguespack SG, Hui SL, Dimeglio LA, et al. Autosomal dominant osteopetrosis: clinical severity and natural history of 94 subjects with a chloride channel 7 gene mutation. J Clin Endocrinol Metab. 2007;92:771–778. doi: 10.1210/jc.2006-1986. [DOI] [PubMed] [Google Scholar]
- 7.Barry CP, Ryan CD. Osteomyelitis of the maxilla secondary to osteopetrosis: report of a case. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:12–15. doi: 10.1067/moe.2003.25. [DOI] [PubMed] [Google Scholar]
- 8.Younai F, Eisenbud L, Sciubba JJ. Osteopetrosis: a case report including gross and microscopic findings in the mandible at autopsy. Oral Surg Oral Med Oral Pathol. 1988;65:214–221. doi: 10.1016/0030-4220(88)90168-5. [DOI] [PubMed] [Google Scholar]
- 9.Silvestrini G, Ferraccioli GF, Quaini F, et al. Adult osteopetrosis: study of two brothers. Appl Pathol. 1987;5:184–189. [PubMed] [Google Scholar]
- 10.Josephsen K, Praetorius J, Frische S, et al. Targeted disruption of the Cl−/HCO3− exchanger Ae2 results in osteopetrosis in mice. Proc Natl Acad Sci USA. 2009;106:1638–1641. doi: 10.1073/pnas.0811682106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Er N, Kasaboğlu O, Atabek A, Oktemer K, Akkocaoğlu M. Topical phenytoin treatment in bimaxillary osteomyelitis secondary to infantile osteopetrosis: report of a case. J Oral Maxillofac Surg. 2006;64:1160–1164. doi: 10.1016/j.joms.2005.11.047. [DOI] [PubMed] [Google Scholar]