Abstract
Based on the prognostic role of Her-2 amplification and protein overexpression in breast cancer, various studies have been performed in oral squamous cell carcinomas (OSCC) with inconsistent results. As in invasive breast carcinomas Her-2 overexpression has been related to an increased number of chromosome 17 copies, a common chromosomal alteration in OSCC, we evaluated the association between polysomy 17 and Her-2 protein expression in a series of primary OSCC. Forty-one incisional biopsies of primary OSCC were included in the study. Protein expression was evaluated immunochistochemically with CB11 mouse monoclonal anti-human antibody. The reaction was arbitrarily characterized as absent, faint, moderate, and strong, and staining pattern as cytoplasmic and membranous. Positive cases were analyzed by chromogenic in situ hybridisation (CISH) to access Her-2 status. The association between polysomy 17 and Her-2 expression was checked by Fisher’s exact test. Four cases were negative and 37 cases were positive for Her-2. Staining was faint in 15 cases and moderate in 22 cases. CISH showed that all cases with faint staining were diploid, while from the cases with moderate staining 10 were diploid and 12 polysomic for chromosome 17. Thirteen cases showed purely cytoplasmic staining, while in 24 there were areas of both cytoplasmic and membranous staining. There was a statistically significant correlation between intensity of the reaction and polysomy 17 (P = 0.0036), in particular for cases with both cytoplasmic and membranous staining (P = 0.0128). In some OSCC Her-2 immunohistochemical expression may be associated with chromosome 17 polysomy and not Her-2 amplification.
Keywords: Her-2 Gene, Genetic abnormalities, Oral squamous cell carcinoma, Immunohistochemistry, Hybridization in situ
Introduction
Human epidermal growth factor Receptor 2 proto-oncogene (HER-2, also known as c-erbB2, ERBB2) is the human homologue of the rat neuroglioblastoma oncogene (neu). Her-2/neu belongs to the HER gene family that regulates cell growth, survival, differentiation and migration [1, 2]. It is located on chromosome 17 (q12–q21) and encodes an 185 kD transmembrane protein with intrinsic tyrosine kinase activity that mediates the signal transduction pathway [1]. Her-2 is an orphan receptor, as no specific, high-affinity ligand to the extracellular domain has been identified [3]. It is hypothesized that its extracellular domain dimerises with other Her receptors upon ligand binding to them, probably resulting in inter-receptor activation and synergetic signal transduction [4].
Her-2/neu gene amplification and protein overexpression is one of the most common genetic alterations in invasive breast carcinomas, associated with poor prognosis and response of the tumor to the Her-2 monoclonal antibody trastuzumab [5]. Correlations of Her-2 to unfavorable prognosis have been found in a diverse array of human malignancies, including gliomas, and carcinomas of the ovaries, lung, colon, bladder, endometrium, pancreas, stomach, and salivary glands [3].
Her-2 expression in oral squamous cell carcinoma (OSCC) has been usually studied within the heterogenous group of head and neck carcinomas (HNSCC). Numerous immunohistochemical studies have shown protein expression from 2.5 to 88% of the cases examined [6–24], a variation attributed to differences in the methodology utilized, i.e. tissue fixation procedure, antibodies sensitivity, and scoring criteria [24–26]. Correlation of Her-2 expression with prognostic clinicopathologic parameters remains inconclusive. Positive correlations have been found with variables, such as stage, metastasis, or overall survival in some [8–10, 14, 20, 22, 27–29], but not all of the studies [6, 11, 13, 14, 17, 19, 24], while simultaneous expression of multiple ERBB receptors has been suggested as a better indicator of decreased survival [8, 10]. Loss of Her-2 immunostaining has been considered as an indicator of neoplastic transformation potential in premalignant lesions [18, 30–32], although there are reports to the contrary [32, 33].
Studies of Her-2 amplification in OSCC/HNSCC are limited and usually show Her-2 expression in the absence of Her-2 amplification [7, 15, 17, 24, 34–38]. In invasive breast carcinomas, where in approximately 95% of the cases overexpression of Her-2 protein results from Her-2 amplification [39], overexpression in non-amplified tumors has been related to an increased number of chromosome 17 copies, i.e. polysomy 17 [40–42]. It has been suggested that this genetic aberration may result in a significant increase of Her-2 gene copies in the tumor cells and an increased Her-2 protein production to the level that could be demonstrated by immunohistochemistry as overexpressed [5, 42].
The role of chromosome 17 polysomy in Her-2 expression, in the absence of Her-2 amplification, has not been specifically addressed in OSCC, although chromosome 17 polysomy is a common chromosomal alteration in those tumors [43, 44]. In the present study we evaluated the association between polysomy 17 and Her-2 protein expression in a series of primary OSCC, utilizing immunohistochemistry and chromogenic in situ hybridization (CISH), the most commonly applied methods for evaluating Her-2 status in diagnostic pathology.
Materials and Methods
Specimens
This is a retrospective analysis of 41 non-consecutive patients diagnosed with primary OSCC at the Department of Oral Pathology and Surgery. All cases represent incisional biopsies that were fixed in 10% buffered formalin and embedded in paraffin wax (FFPE). Age, sex and site of the tumor were obtained from patients’ files and pathologic reports. Twenty patients were male (age range 43–91 years, average 61.72 years) and 21 female (age range 40–88 years, average 62.56 years). Most lesions were located on the tongue (n = 18), followed by the buccal mucosa (n = 7), gingiva (n = 7), lower lip mucosa (n = 4), floor of mouth (n = 3), and palate (n = 1). History of smoking and alcohol consumption were not available. Tumors were graded by the WHO classification of histological differentiation into well (n = 20), moderately (n = 16), and poorly (n = 5) differentiated. Due to the nature of our material (incisional biopsies) stage and survival data were not available.
Immunohistochemistry
Immunohistochemistry was performed on 4–5 μm-thick FFPE tissue sections with the mouse monoclonal anti-human antibody CB11 (NCL-L-CB11, Novocastra Laboratories Ltd., Newcastle Upon Tyne, UK), diluted at 1:80 in TBS, following the EnVision protocol (DAKO, Glostrup, Denmark) in the Ventana BenchmarkXT automated slide staining system (Ventana Medical Systems, AZ, USA). CB11 reacts with the internal domain of HER2 oncoprotein. Reaction was assessed as follows: absent (no staining is observed or focal staining is observed in less than 10% of the tumor cells), faint (a faint/barely perceptible staining is detected in more than 10% of the tumor cells), moderate (a weak to moderate complete staining is observed in more than 10% of the tumor cells), and strong (a strong complete staining is observed in more than 10% of the tumor cells), and staining pattern as cytoplasmic and membranous. The scoring system follows the rational of the HercepTestTM (Dako A/S, Glostrup, Denmark) that is routinely applied for the diagnostic assessment of breast carcinomas, but evaluates only membrane staining. In accordance to this system, only cases with strong reaction were considered as overexpressing Her-2. Omission of the primary antibody served as the negative control and a Her-2 3+ breast carcinoma as the positive control.
CISH Analysis
CISH analysis was performed with the Zymed SPoT-Light® HER-2 CISH Kit (Zymed Laboratories, CA, USA) on FFPE tissue sections of CB11 positive cases, according to the manufacturer’s instructions. The kit contains a digoxigenin labeled probe that specifically binds to the Her-2 gene locus on chromosome 17q12–21. FFPE positive control slides were included in the kit. Briefly, the slides were deparaffinized in xylene, soaked in 100% ethanol, and washed in dH2O. Then they were heated at 98°C for 15 min in Heat Pretreatment Solution (Zymed Laboratories), enzyme digested for 15 min and dehydrated in graded ethanol series. 15 μl of HER-2 probe were applied to each slide that was covered with coverslips. Denaturation and hybridisation were performed using a PCR machine, set at 95°C for 5 min for denaturation and at 37°C for 12 h for hybridization. The slides were subsequently washed in SCC RT/75°C for 5 min. After immunodetection, the slides were counterstained with hematoxylin.
CISH staining was evaluated according to Zymed SPoT-Light® HER-2 CISH Kit manufacturer’s instructions. Specifically, specimens with 1–2 HER-2 signals per nucleus in >50% of tumor cells, in the chosen area for enumeration, were considered to be normal diploid; 3–5 signals in >50% of tumor cells were indicative of polysomy for chromosome 17; and ≥6 signals in >50% of tumor cells were scored as Her-2 amplification.
Statistical Analysis
The association between polysomy 17 and Her-2 overexpression was checked by Fisher’s exact test.
Results
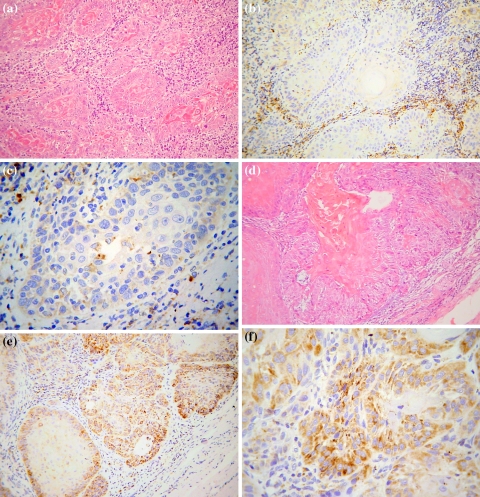
In 4 out of 41 tumors (9.75%) Her-2 immunostaining was characterized as absent. Of the 37 positive cases, 15 (40.54%) showed faint staining (Fig. 1a–c) and 22 (59.45%) moderate staining (Fig. 1d–f). No case with strong staining (Her-2 overexpression) was seen. Cases with moderate staining usually showed more positive cells than cases with faint staining. CISH showed that all cases with faint staining were diploid, while from the cases with moderate staining 10 were diploid and 12 polysomic for chromosome 17. No case with Her-2 amplification was found.
Fig. 1.
Representative immunohistochemical staining for the expression of HER-2 with CB-11 in a well-differentiated OSCCs: a H&E, b, c Her-2 faint staining, d H&E, e, f Her-2 moderate staining (a, b, d, e: original magnification ×200; c, f: original magnification ×400)
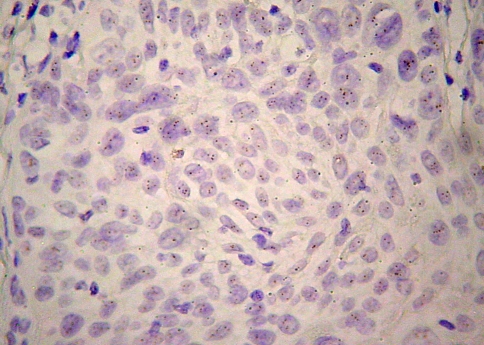
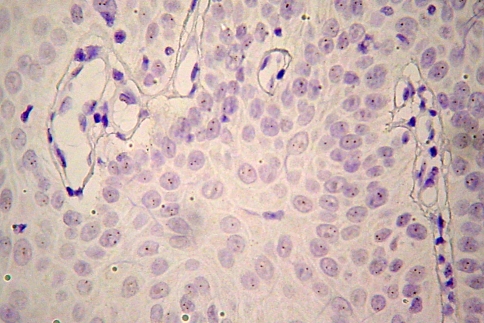
Considering the staining pattern, 13 (31.13%) showed purely cytoplasmic staining (Fig. 2), while in 24 (64.86%) there were areas of both cytoplasmic and membranous staining (Fig. 3). From the 13 cases with purely cytoplasmic expression, three with faint staining were normal diploid, 2 with moderate staining were polysomic and 8 with moderate staining were normal diploid. Regarding the 24 cases with both cytoplasmic and membranous staining, 8 with faint staining were normal diploid, 10 with moderate staining were polysomic for chromosome 17 (Fig. 4), and 6 with moderate staining were normal diploid (Fig. 5).
Fig. 2.
Moderate cytoplasmic staining pattern in a well-differentiated OSCC: a H&E and b HER-2 staining with CB-11 (original magnification ×400)
Fig. 3.
Membranous staining pattern in a well-differentiated OSCC: a H&E and b HER-2 staining with CB-11 (original magnification ×400)
Fig. 4.
CISH polysomic nuclei in a moderately stained Her-2 OSCC (Zymed SPoT-Light® HER-2 CISH Kit, original magnification ×400)
Fig. 5.
CISH diploid nuclei in a faintly stained Her-2 OSCC (Zymed SPoT-Light® HER-2 CISH Kit, original magnification ×400)
In the adjacent epithelium, usually showing varying degrees of epithelial dysplasia, faint and diffuse cytoplasmic reaction was seen in the prickle layer of positive cases.
As is shown in Table 1, there is a statistically significant correlation between intensity of the reaction and polysomy 17 (P = 0.0036), in particular for cases with both cytoplasmic and membranous staining (P = 0.0064). No statistical significant correlation was found between the sex and age of the patient, or the location and grade of the tumor, and the immunohistochemical score or CISH ploidy status.
Table 1.
Staining intensity by immunohistochemistry and CISH chromosome 17 ploidy status in 37 Her-2 positive cases of oral squamous cell carcinomas
| Cytoplasmic and membarnous | Purely cytoplasmic | Both staining patterns | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Faint | Moderate | Total | Faint | Moderate | Total | Faint | Moderate | Total | |
| Diploid | 8 | 6 | 14 | 7 | 4 | 11 | 15 | 10 | 25 |
| Polysomic | 0 | 10 | 10 | 0 | 2 | 2 | 0 | 12 | 12 |
| Total | 8 | 16 | 24 | 7 | 6 | 13 | 15 | 22 | 37 |
| p = 0.006429 | p = 0.192308 | p = 0.0036 | |||||||
Discussion
In the present study Her-2 expression was assessed with the CB11 mouse monoclonal antibody that is highly sensitive [25], but produces significant cytoplasmic staining [24]. The importance of cytoplasmic staining and whether it may be evaluated when determining Her-2 expression in OSCC/HNSCC is controversial [24]. In various studies purely membranous [6, 12–14] or cytoplasmic [6, 11, 13, 24, 45], and mixed membranous-cytoplasmic [6, 8–10, 21, 23, 46] expression have been reported, while mixed positivity has been shown in adult oral epithelium and dysplastic lesions [23]. Some authors suggest that cytoplasmic staining is an artifact or non-specific finding due to antibody cross-reactivity [6, 47], possibly with keratin [46]. Others, however, propose that it may represent true protein overexpression [8, 48], probably due to incomplete receptor degradation [9]. The staining pattern and intensity considered as positive may account for discrepancies in the percentage of Her-2 positive cases reported in various studies [24]. In our study, membranous staining was always associated with cytoplasmic staining. Obviously, as long as the biological basis and prognostic significance of cytoplasmic staining in OSCC has not been settled, discrimination among the different staining patterns, as applied in this study, would be useful.
This study is the first to apply CISH on OSCC. CISH is a reliable alternative to FISH for assessing Her-2 gene amplification when immunohistochemistry shows Her-2 protein overexpression, especially in cases with weak expression [49–51]. Studies have shown that there is a 96–100% concordance between CISH and FISH, the gold standard for the assessment of ERBB2 status in breast cancer [49, 52]. Apart from being faster and cheaper than FISH, CISH achieves stable signal intensity over time, and tissue and cellular features can be directly correlated with gene status.
Her-2 amplification was reported by Beckhardt et al. [7] in 2 out of 11 cell lines of HNSCC with blotting techniques, and by O-Charoenrat et al. [53] in none of 15 cell lines of OSCC with PCR. No Her-2 amplification in HNSCC was found by Kearsley et al. [46] in 46 cases (24 OSCC) by Southern blot, and Leonard et al. [36] in 66 cases and Rodrigo et al. [35] in 59 cases by PCR. Willmore-Payne et al. [37] did not find Her-2 activating mutations in 24 cases by high-resolution melting amplicon analysis (HRMAA) and direct DNA sequencing. Utilizing FISH, Her-2 amplification was found by Khan et al. [15] in 4 (5.2%) out of 77 cases of oropharyngeal SCC; by Freier et al. [54] in 8 (3.8%) out of 213 OSCC; and by Angiero et al. [24] in 1(2.5%) out of 40 tongue SCC. In contrast, Scheer et al. [17] reported Her-2 amplification in 14 (33.3%) out of 42 OSCC; 3 cases showed amplification without overexpression. We did not find Her-2 amplification in 37 OSCC, but it should be noticed that CISH was applied only in CB11 positive cases, when this antibody may not select all Her-2 CISH amplified tumors [25].
Thus, in the present study, a significant percentage of OSCC showed weak or moderate Her-2 expression without CISH Her-2 amplification. In breast carcinomas, lack of amplification has been found in 3–15% of cases overexpressing Her-2, and this has been attributed to transcriptional or posttranslational activation of Her-2, artifactual high sensitivity of immunohistochemical assays, single copy overexpression of the HER-2 gene at the messenger RNA (mRNA) transcription level, or gene amplification below the level of detection of FISH assays [26]. A role for chromosome 17 polysomy has also been investigated. Lal et al. [42], Bose et al. [55], and Salido et al. [5] found that in non-amplified breast tumors polysomy 17 was associated with immunohistochemical expression of HER-2 protein, in particular borderline to weak expression. In contrast, Varshney et al. [26] reported that in cases without gene amplification chromosome 17 polysomy is not associated with weak expression of HER-2, but has a role in moderate (weakly positive or equivocal) protein expression without amplification. Sauer et al. [52] concluded that an abnormal number of chromosome 17 copies has a low impact on Her-2 and its expression. The discordant results may be partly explained by the different criteria applied for the definition of chromosome 17 polysomy [26]. However, it seems than in none-amplified breast carcinomas cases, expression of Her-2 may be associated with polysomy 17 and those cases seem to behave more similarly to Her-2 negative tumors [5, 42].
Polysomy 17 seems to be a common chromosomal aberration in oral carcinogenesis [44], but the association of Her-2 expression with polysomy 17 has not been previously studied in OSCC. Khan et al. [15] reported 7 cases with polysomy 17 by FISH in 77 oral and oropharyngeal SCC. Four cases were strongly positive, 2 faintly positive, and 1 negative, but with intense cytoplasmic staining. We found a statistically significant correlation between polysomy for chromosome 17 and moderate Her-2 expression in cases with both cytoplasmic and membranous staining pattern, but not in those with purely cytoplasmic pattern. On the other hand, it has been shown that in OSCC polysomy 17 correlates with p53 mutation [43] or overexpression [44], while loss of normal p53 function play a potential role in multistep tumorigenesis in OSCC [43, 56, 57] Thus, the association between Her-2 expression and p53 alterations would be interesting to be investigated.
We conclude that in some OSCC Her-2 immunohistochemical expression, in particular moderate expression, may be associated with chromosome 17 polysomy and not Her-2 amplification. As polysomy 17 seems to correlate with oral carcinogenesis [43, 44] and Her-2 amplification is rather uncommon in OSCC, Her-2 expression in previous studies may not reflect true amplification and this may account for the discrepant results considering prognosis. Utilization of more strict and consistent methodology in studies of OSCC, e.g. definition of positive staining, scoring according to the ASCO/CAP system [58] and application of FISH/CISH, is necessary for determining the role of Her-2 expression in OSCC and its association with polysomy 17.
Contributor Information
Dimitrios Papavasileiou, Email: dimipapavasileiou@yahoo.com.
Konstantinos Tosios, Phone: +30-210-7461003, FAX: +30-210-7461220, Email: ktosios@dent.uoa.gr.
Panos Christopoulos, Email: panosch@otenet.gr.
Dimitrios Vlachodimitropoulos, Email: dvlacho@med.uoa.gr.
References
- 1.Schechter AL, Stern DF, Vaidyanathan L, et al. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature. 1984;312:513–516. doi: 10.1038/312513a0. [DOI] [PubMed] [Google Scholar]
- 2.Rubin I, Yarden Y. The basic biology of HER2. Ann Oncol. 2001;12(Suppl 1):S3–S8. doi: 10.1023/A:1011195320446. [DOI] [PubMed] [Google Scholar]
- 3.Casalini P, Iorio MV, Galmozzi E, et al. Role of HER receptors family in development and differentiation. J Cell Physiol. 2004;200:343–350. doi: 10.1002/jcp.20007. [DOI] [PubMed] [Google Scholar]
- 4.O-Charoenrat P, Rhys-Evans PH, Modjtahedi H, et al. The role of c-erbB receptors and ligands in head and neck squamous cell carcinoma. Oral Oncol. 2002;38:627–640. doi: 10.1016/S1368-8375(02)00029-5. [DOI] [PubMed] [Google Scholar]
- 5.Salido M, Tusquets I, Corominas JM, et al. Polysomy of chromosome 17 in breast cancer tumors showing an overexpression of ERBB2: a study of 175 cases using fluorescence in situ hybridization and immunohistochemistry. Breast Cancer Res. 2005;7:R267–R273. doi: 10.1186/bcr996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craven JM, Pavelic ZP, Stambrook PJ, et al. Expression of c-erbB-2 gene in human head and neck carcinoma. Anticancer Res. 1992;12:2273–2276. [PubMed] [Google Scholar]
- 7.Beckhardt RN, Kiyokawa N, Xi L, et al. HER-2/neu oncogene characterization in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 1995;121:1265–1270. doi: 10.1001/archotol.1995.01890110041008. [DOI] [PubMed] [Google Scholar]
- 8.Xia W, Lau YK, Zhang HZ, et al. Strong correlation between c-erbB-2 overexpression and overall survival of patients with oral squamous cell carcinoma. Clin Cancer Res. 1997;3:3–9. [PubMed] [Google Scholar]
- 9.Ibrahim SO, Vasstrand EN, Liavaag PG, et al. Expression of c-erbB proto-oncogene family members in squamous cell carcinoma of the head and neck. Anticancer Res. 1997;17:4539–4546. [PubMed] [Google Scholar]
- 10.Xia W, Lau YK, Zhang HZ, et al. Combination of EGFR, HER-2/neu, and HER-3 is a stronger predictor for the outcome of oral squamous cell carcinoma than any individual family members. Clin Cancer Res. 1999;5:4164–4174. [PubMed] [Google Scholar]
- 11.Giatromanolaki A, Koukourakis MI, Sivridis E, et al. c-erbB-2 oncoprotein is overexpressed in poorly vascularised squamous cell carcinomas of the head and neck, but is not associated with response to cytotoxic therapy or survival. Anticancer Res. 2000;20:997–1004. [PubMed] [Google Scholar]
- 12.Hoffmann TK, Ballo H, Braunstein S, et al. Serum level and tissue expression of c-erbB-1 and c-erbB-2 proto-oncogene products in patients with squamous cell carcinoma of the head and neck. Oral Oncol. 2001;37:50–56. doi: 10.1016/S1368-8375(00)00056-7. [DOI] [PubMed] [Google Scholar]
- 13.Khademi B, Shirazi FM, Vasei M, et al. The expression of p53, c-erbB-1 and c-erbB-2 molecules and their correlation with prognostic markers in patients with head and neck tumors. Cancer Lett. 2002;184:223–230. doi: 10.1016/S0304-3835(02)00242-2. [DOI] [PubMed] [Google Scholar]
- 14.Kuropkat C, Venkatesan TK, Caldarelli DD, et al. Abnormalities of molecular regulators of proliferation and apoptosis in carcinoma of the oral cavity and oropharynx. Auris Nasus Larynx. 2002;29:165–174. doi: 10.1016/S0385-8146(01)00129-8. [DOI] [PubMed] [Google Scholar]
- 15.Khan AJ, King BL, Smith BD, et al. Characterization of the HER-2/neu oncogene by immunohistochemical and fluorescence in situ hybridization analysis in oral and oropharyngeal squamous cell carcinoma. Clin Cancer Res. 2002;8:540–548. [PubMed] [Google Scholar]
- 16.Nagler RM, Kerner H, Laufer D, et al. Squamous cell carcinoma of the tongue: the prevalence and prognostic roles of p53, Bcl-2, c-erbB-2 and apoptotic rate as related to clinical and pathological characteristics in a retrospective study. Cancer Lett. 2002;186:137–150. doi: 10.1016/S0304-3835(02)00265-3. [DOI] [PubMed] [Google Scholar]
- 17.Scheer M, Prange W, Petmecky K, et al. Evaluation of her-2/neu amplification/overexpression in OSCC with fluorescence in situ hybridization (FISH) and immunohistochemistry. Mund Kiefer Gesichtschir. 2003;7:138–145. doi: 10.1007/s10006-003-0460-5. [DOI] [PubMed] [Google Scholar]
- 18.Parise Junior O, Carvalho LV, Miguel RE, et al. Prognostic impact of p53, c-erbB-2 and epidermal growth factor receptor on head and neck carcinoma. Sao Paulo Med J. 2004;122:264–268. doi: 10.1590/S1516-31802004000600007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schartinger VH, Kacani L, Andrle J, et al. Pharmacodiagnostic value of the HER family in head and neck squamous cell carcinoma. ORL J Otorhinolaryngol Relat Spec. 2004;66:21–26. doi: 10.1159/000077229. [DOI] [PubMed] [Google Scholar]
- 20.Ulanovski D, Stern Y, Roizman P, et al. Expression of EGFR and Cerb-B2 as prognostic factors in cancer of the tongue. Oral Oncol. 2004;40:532–537. doi: 10.1016/j.oraloncology.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Bei R, Budillon A, Masuelli L, et al. Frequent overexpression of multiple ErbB receptors by head and neck squamous cell carcinoma contrasts with rare antibody immunity in patients. J Pathol. 2004;204:317–325. doi: 10.1002/path.1642. [DOI] [PubMed] [Google Scholar]
- 22.Cavalot A, Martone T, Roggero N, et al. Prognostic impact of HER-2/neu expression on squamous head and neck carcinomas. Head Neck. 2007;29:655–664. doi: 10.1002/hed.20574. [DOI] [PubMed] [Google Scholar]
- 23.Rautava J, Jee KJ, Miettinen PJ, et al. ERBB receptors in developing, dysplastic and malignant oral epithelia. Oral Oncol. 2008;44:227–235. doi: 10.1016/j.oraloncology.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Angiero F, Sordo RD, Dessy E, et al. Comparative analysis of c-erbB-2 (HER-2/neu) in squamous cell carcinoma of the tongue: does over-expression exist? And what is its correlation with traditional diagnostic parameters? J Oral Pathol Med. 2008;37:145–150. doi: 10.1111/j.1600-0714.2007.00603.x. [DOI] [PubMed] [Google Scholar]
- 25.Nunes CB, Rocha RM, Reis-Filho JS, et al. Comparative analysis of six different antibodies against Her2 including the novel rabbit monoclonal antibody (SP3) and chromogenic in situ hybridisation in breast carcinomas. J Clin Pathol. 2008;61:934–938. doi: 10.1136/jcp.2007.053892. [DOI] [PubMed] [Google Scholar]
- 26.Varshney D, Zhou YY, Geller SA, et al. Determination of HER-2 status and chromosome 17 polysomy in breast carcinomas comparing HercepTest and PathVysion FISH assay. Am J Clin Pathol. 2004;121:70–77. doi: 10.1309/FUQH92B039025LHG. [DOI] [PubMed] [Google Scholar]
- 27.Ekberg T, Nestor M, Engstrom M, et al. Expression of EGFR, HER2, HER3, and HER4 in metastatic squamous cell carcinomas of the oral cavity and base of tongue. Int J Oncol. 2005;26:1177–1185. doi: 10.3892/ijo.26.5.1177. [DOI] [PubMed] [Google Scholar]
- 28.Albuquerque RL, Jr, Miguel MC, Costa AL, et al. Correlation of c-erbB-2 and S-100 expression with the malignancy grading and anatomical site in oral squamous cell carcinoma. Int J Exp Pathol. 2003;84:259–265. doi: 10.1111/j.0959-9673.2004.00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Do NY, Lim SC, Im TS. Expression of c-erbB receptors, MMPs and VEGF in squamous cell carcinoma of the head and neck. Oncol Rep. 2004;12:229–237. [PubMed] [Google Scholar]
- 30.Kilpi A, Rich AM, Konttinen YT, et al. The expression of c-erbB-2 protein in the keratinocytes of oral mucosal lichen planus. Br J Dermatol. 1995;133:847–852. doi: 10.1111/j.1365-2133.1995.tb06915.x. [DOI] [PubMed] [Google Scholar]
- 31.Kilpi A, Rich AM, Konttinen YT, et al. Expression of c-erbB-2 protein in keratinocytes of oral mucosal lichen planus and subsequent squamous cell carcinoma. Eur J Oral Sci. 1996;104:278–284. doi: 10.1111/j.1600-0722.1996.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 32.Wilkman TS, Hietanen JH, Malmstrom MJ, et al. Immunohistochemical analysis of the oncoprotein c-erbB-2 expression in oral benign and malignant lesions. Int J Oral Maxillofac Surg. 1998;27:209–212. doi: 10.1016/S0901-5027(98)80012-X. [DOI] [PubMed] [Google Scholar]
- 33.Hou L, Shi D, Tu SM, et al. Oral cancer progression and c-erbB-2/neu proto-oncogene expression. Cancer Lett. 1992;65:215–220. doi: 10.1016/0304-3835(92)90234-M. [DOI] [PubMed] [Google Scholar]
- 34.Freier K, Bosch FX, Flechtenmacher C, et al. Distinct site-specific oncoprotein overexpression in head and neck squamous cell carcinoma: a tissue microarray analysis. Anticancer Res. 2003;23:3971–3977. [PubMed] [Google Scholar]
- 35.Rodrigo JP, Ramos S, Lazo PS, et al. Amplification of ERBB oncogenes in squamous cell carcinomas of the head and neck. Eur J Cancer. 1996;32A:2004–2010. doi: 10.1016/0959-8049(96)00223-7. [DOI] [PubMed] [Google Scholar]
- 36.Leonard JH, Kearsley JH, Chenevix-Trench G, et al. Analysis of gene amplification in head-and-neck squamous cell carcinoma. Int J Cancer. 1991;48:511–515. doi: 10.1002/ijc.2910480406. [DOI] [PubMed] [Google Scholar]
- 37.Willmore-Payne C, Holden JA, Layfield LJ. Detection of EGFR- and HER2-activating mutations in squamous cell carcinoma involving the head and neck. Mod Pathol. 2006;19:634–640. doi: 10.1038/modpathol.3800552. [DOI] [PubMed] [Google Scholar]
- 38.O-Charoenrat P, Rhys-Evans P, Eccles S. Characterization of ten newly-derived human head and neck squamous carcinoma cell lines with special reference to c-erbB proto-oncogene expression. Anticancer Res. 2001;21:1953–63. [PubMed] [Google Scholar]
- 39.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 40.Rashid-Kolvear F, Pintilie M, Done SJ. Telomere length on chromosome 17q shortens more than global telomere length in the development of breast cancer. Neoplasia. 2007;9:265–270. doi: 10.1593/neo.07106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hyun CL, Lee HE, Kim KS, et al. The effect of chromosome 17 polysomy on HER-2/neu status in breast cancer. J Clin Pathol. 2008;61:317–321. doi: 10.1136/jcp.2007.050336. [DOI] [PubMed] [Google Scholar]
- 42.Lal P, Salazar PA, Ladanyi M, et al. Impact of polysomy 17 on HER-2/neu immunohistochemistry in breast carcinomas without HER-2/neu gene amplification. J Mol Diagn. 2003;5:155–159. doi: 10.1016/S1525-1578(10)60467-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanekawa A, Tsuji T, Oga A, et al. Chromosome 17 abnormalities in squamous cell carcinoma of the oral cavity, and its relationship with p53 and Bcl-2 expression. Anticancer Res. 1999;19:81–86. [PubMed] [Google Scholar]
- 44.Voravud N, Shin DM, Ro JY, et al. Increased polysomies of chromosomes 7 and 17 during head and neck multistage tumorigenesis. Cancer Res. 1993;53:2874–2883. [PubMed] [Google Scholar]
- 45.Field JK, Spandidos DA, Yiagnisis M, et al. C-erbB-2 expression in squamous cell carcinoma of the head and neck. Anticancer Res. 1992;12:613–619. [PubMed] [Google Scholar]
- 46.Kearsley JH, Leonard JH, Walsh MD, et al. A comparison of epidermal growth factor receptor (EGFR) and c-erbB-2 oncogene expression in head and neck squamous cell carcinomas. Pathology. 1991;23:189–194. doi: 10.3109/00313029109063564. [DOI] [PubMed] [Google Scholar]
- 47.Press MF, Hung G, Godolphin W, et al. Sensitivity of HER-2/neu antibodies in archival tissue samples: potential source of error in immunohistochemical studies of oncogene expression. Cancer Res. 1994;54:2771–2777. [PubMed] [Google Scholar]
- 48.Weinstein GS, Nuamah IF, Tucker J, et al. Evaluation of HER-2/neu (c-erbB-2) oncogene expression in whole organ sections of supraglottic squamous cell carcinoma. Ann Otol Rhinol Laryngol. 1996;105:275–279. doi: 10.1177/000348949610500406. [DOI] [PubMed] [Google Scholar]
- 49.Saez A, Andreu FJ, Segui MA, et al. HER-2 gene amplification by chromogenic in situ hybridisation (CISH) compared with fluorescence in situ hybridisation (FISH) in breast cancer-A study of two hundred cases. Breast. 2006;15:519–527. doi: 10.1016/j.breast.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Hanna WM, Kwok K. Chromogenic in situ hybridization: a viable alternative to fluorescence in situ hybridization in the HER2 testing algorithm. Mod Pathol. 2006;19:481–487. doi: 10.1038/modpathol.3800555. [DOI] [PubMed] [Google Scholar]
- 51.Bilous M, Morey A, Armes J, et al. Chromogenic in situ hybridisation testing for HER2 gene amplification in breast cancer produces highly reproducible results concordant with fluorescence in situ hybridisation and immunohistochemistry. Pathology. 2006;38:120–124. doi: 10.1080/00313020600561518. [DOI] [PubMed] [Google Scholar]
- 52.Sauer T, Wiedswang G, Boudjema G, et al. Assessment of HER-2/neu overexpression and/or gene amplification in breast carcinomas: should in situ hybridization be the method of choice? APMIS. 2003;111:444–450. doi: 10.1034/j.1600-0463.2003.t01-1-1110210.x. [DOI] [PubMed] [Google Scholar]
- 53.O-Charoenrat P, Rhys-Evans P, Eccles S. Characterization of ten newly-derived human head and neck squamous carcinoma cell lines with special reference to c-erbB proto-oncogene expression. Anticancer Res. 2001;21:1953–1963. [PubMed] [Google Scholar]
- 54.Freier K, Joos S, Flechtenmacher C, et al. Tissue microarray analysis reveals site-specific prevalence of oncogene amplifications in head and neck squamous cell carcinoma. Cancer Res. 2003;63:1179–1182. [PubMed] [Google Scholar]
- 55.Bose S, Mohammed M, Shintaku P, et al. Her-2/neu gene amplification in low to moderately expressing breast cancers: possible role of chromosome 17/Her-2/neu polysomy. Breast J. 2001;7:337–344. doi: 10.1046/j.1524-4741.2001.21018.x. [DOI] [PubMed] [Google Scholar]
- 56.Charuruks N, Shin DM, Voravud N, et al. Genetic instabilities of chromosome 9, 17 and accumulation of p53 overexpression during multistage tumorigesis in head and neck cancer. J Med Assoc Thai. 1996;79(Suppl 1):S104–S112. [PubMed] [Google Scholar]
- 57.Shin DM, Charuruks N, Lippman SM, et al. p53 protein accumulation and genomic instability in head and neck multistep tumorigenesis. Cancer Epidemiol Biomarkers Prev. 2001;10:603–609. [PubMed] [Google Scholar]
- 58.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131:18–43. doi: 10.5858/2007-131-18-ASOCCO. [DOI] [PubMed] [Google Scholar]