Abstract
Treatment with asparaginase for acute lymphoblastic leukemia (ALL) can cause acute pancreatitis. Complication of pancreatitis by pancreatic pseudocyst formation can prolong the hospital stay, delay chemotherapy, and necessitate long-term parenteral nutrition. We report five children with ALL who developed acute pancreatitis complicated by pancreatic pseudocysts. They required modifications to their chemotherapy regimen and prolonged parenteral nutrition but no surgical intervention. All five patients survive in first remission and their pseudocysts resolved after 3 to 37 months or continued to decrease in size at last follow-up. These cases illustrate that non-surgical management of pancreatic pseudocyst is safe, though pseudocyst resolution may require many months. In addition, these patients demonstrate that oral feeding can be initiated after the acute episode of pancreatitis resolves even if a pseudocyst is present.
Keywords: Pancreatitis, pancreatitis pseudocyst, L-asparaginase, pediatric oncology, acute lymphoblastic leukemia
Introduction
More than 80% of children with acute lymphoblastic leukemia (ALL) are cured (1;2), but successful treatment requires many chemotherapeutic agents, each with potential side effects. L-asparaginase improves event-free survival in ALL, but can cause pancreatitis, which can lead to hospitalization, delays in chemotherapy, and formation of pancreatic pseudocysts. The management of pancreatic pseudocyst is controversial and can include surgery, percutaneous drainage, or close observation. Some patients receive prolonged parenteral nutrition in an attempt to hasten pseudocyst resolution. We describe resolution of pancreatic pseudocysts without surgical intervention and with early initiation of oral intake in five children with ALL who developed this complication after L-asparaginase therapy.
Case Presentation
Patient 1, a 17 year old male with T-cell ALL, was treated on the higher-risk arm of the Total XV protocol (3), which includes L-asparaginase during remission induction and weekly L-asparaginase for 20 weeks with rotating combinations of dexamethasone, vincristine, cytarabine, cyclophosphamide, methotrexate, and mercaptopurine during continuation therapy. Two courses of delayed intensification also contain L-asparaginase and dexamethasone.
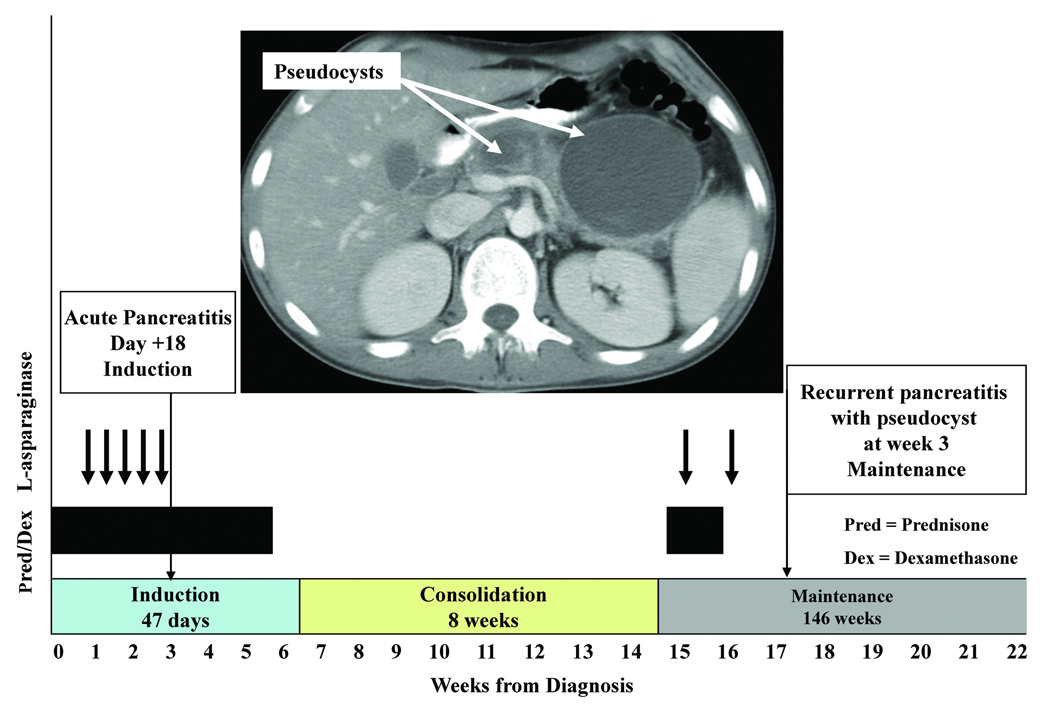
On day 18 of remission induction, two days after his fifth dose of L-asparaginase, the patient developed acute pancreatitis (Figure 1). His amylase and lipase peaked at 500 units/L [U/L; normal <77 U/L] and 413 units/L [normal <61 U/L], respectively. He was discharged after six days of hospitalization without residual symptoms and with normal pancreatic enzymes.
Figure 1. Time course of leukemia treatment and pancreatitis.
This chart shows the occurrence of pancreatitis and pancreatic pseudocyst for Patient 1 in relation to chemotherapy administration.
Three months later, on week 2 of continuation therapy, after two weekly doses of L-asparaginase, the patient developed severe recurrent pancreatitis. His amylase rose to 562 U/L and lipase to 2821 U/L. Abdominal imaging showed pancreatitis complicated by multiple pancreatic pseudocysts, of which the largest measured 7.4×8.1×8.3 cm3. The patient was placed on gut rest with parenteral nutrition support for 6 weeks; chemotherapy was omitted for 14 days during recovery. After this episode, dexamethasone and L-asparaginase were permanently discontinued, and low-dose methotrexate was substituted for dexamethasone. Figure 1 shows the time course of treatment. Serial imaging documented the gradual decrease in the number and size of pseudocysts over a period of 37 months. A CT scan performed at the time of completion of all chemotherapy showed resolution of all pseudocysts with evidence of pancreatic atrophy. The patient remains asymptomatic and has no evidence of pancreatic insufficiency 39 months after the episode of acute pancreatitis.
Patient 2, a 15 year old male with T-cell ALL, was treated on the higher-risk arm of Total XV(3) and developed acute pancreatitis on day 12 of his second course of delayed intensification, 4 days after his 24th dose of L-asparaginase. His amylase and lipase values peaked at 193 U/L and 1049 U/L, respectively. Abdominal ultrasound showed a pancreatic pseudocyst which measured 4.1×3.1×5.6 cm3. He was placed on gut rest with parenteral nutrition for 15 days and chemotherapy was delayed for 15 days, with resolution of the pseudocyst 11 weeks later. His subsequent chemotherapy included dexamethasone, but his final dose of L-asparaginase was omitted.
Patient 3, a 4 year old female with B-cell precursor ALL, was treated on the lower-risk arm of Total XV (3) which includes less intensive L-asparaginase treatment. She developed hyperglycemia on day 9 of induction therapy and was admitted the following day with pancreatitis, the day that she received her third dose of L-asparaginase. Her amylase and lipase peaked at 74 U/L and 725 U/L, respectively. After recovery, chemotherapy was resumed, including glucocorticoids and L-asparaginase.
After her second course of delayed intensification, she again developed symptomatic pancreatitis, this time with multiple pancreatic pseudocysts, the largest measuring 7.8 × 7.4×5.2 cm3. She was placed on gut rest with parenteral nutrition for 7 weeks and her abdominal imaging showed resolution of pseudocysts after 7 months. Chemotherapy was held for 17 days during her acute illness, and dexamethasone was added back to her regimen 11 weeks after development of symptoms but no further L-asparaginase was given. She had no further episodes of pancreatitis.
Patient 4 is a 10 year old male with B lineage ALL who was treated on the standard risk arm of Total XVI, our current institutional ALL protocol, which includes PEG-asparaginase, prednisone, vincristine, daunorubicin, cyclophosphamide and 6-thioguanine during induction therapy. PEG-asparaginase is also given every other week during continuation therapy for 15 doses for standard risk patients.
During week 4 of continuation, he developed abdominal pain and vomiting seven days after administration of his 3rd dose of PEG-asparaginase. His amylase and lipase peaked at 235 U/L and 2916 U/L, respectively. A CT scan at the time of admission showed severe pancreatitis. He subsequently developed ascites and bilateral pleural effusions, and was transferred to the intensive care unit. He remained inpatient for 13 days, without solid or liquids by mouth (NPO) for 7 days and on parenteral nutrition for 14 days. A repeat CT scan five days after admission showed the interval development of a pancreatic pseudocyst measuring 10.9×10.5×7.7 cm3. Chemotherapy was held for 21 days, and all further doses of PEG-asparaginase were replaced by low dose methotrexate. A CT scan one month after the incident showed that the pseudocyst had decreased in size, but was still present. At this time, the patient remains asymptomatic.
Patient 5 is an 18 year old male with T cell ALL treated on the standard risk arm of Total XVI who presented with abdominal pain and vomiting during week 2 of continuation therapy, 13 days after his 3rd dose of PEG-asparaginase. His amylase and lipase rose to 480 U/L and 1691 U/L, respectively. An ultrasound performed at admission showed a normal pancreas, but a repeat ultrasound two days later showed evidence of pancreatitis. A CT scan performed on hospital day 7 showed an 11.1×9.3 X 4 cm3 pseudocyst. He was hospitalized for 10 days, during which he developed ascites, decreased urine output, hyponatremia and bilateral pleural effusions. He was NPO for five days and on parenteral nutrition for five days. His chemotherapy was suspended for 9 days and no further asparaginase was given. A repeat CT scan two months after this episode showed a persistent pseudocyst that had decreased in size, and he remains asymptomatic.
All five patients remain in first complete remission 37, 14, 56, 7, and 7 months from diagnosis.
Discussion
L-asparaginase-induced pancreatitis occurs in 1-18% of children with ALL, and is usually mild and self-limited (4). However, it can lead to multiorgan failure, pseudocyst formation, and even death (5). Our five cases illustrate that pancreatic pseudocyst can lead to hospitalization, delays in chemotherapy, prolonged parenteral nutrition, significant morbidity, and discontinuation of asparaginase or dexamethasone treatment, potentially compromising long-term leukemia control. Although this is not a new complication, there are no published guidelines for management of pancreatic pseudocyst in children with ALL. Reported practices include delaying or suspending certain chemotherapeutic agents, gut rest with parenteral nutrition, surgical intervention, percutaneous drainage, and/or observation.
An important management decision is whether re-challenge with asparaginase is warranted, especially in patients who develop pancreatitis early in their leukemia therapy. Asparaginase is usually withheld after a serious episode of pancreatitis, yet the relative risks and benefits of omitting subsequent asparaginase depend on the ALL risk group and immunophenotype. Silverman et al. (2) showed that patients receiving 25 or fewer weeks of L-asparaginase have significantly lower event-free survival than those receiving at least 26 weeks of L-asparaginase. Therefore, discontinuing L-asparaginase early may increase the risk of leukemia relapse. Knoderer et al. (4) reported that of 26 patients re-challenged with L-asparaginase after pancreatitis, only two developed recurrent pancreatitis. These observations may support the practice of administering asparaginase after a first episode of pancreatitis in patients at high risk of relapse. Patients 1 and 3 were re-challenged with L-asparaginase after their initial episode of pancreatitis. Patient 1 was re-challenged because he was in the higher risk category and because L-asparaginase is especially effective in T-cell ALL (6). Patient 3 was re-challenged because her pancreatitis developed early in therapy and discontinuing further asparaginase could lead to decreased leukemic control. Although both developed recurrent pancreatitis and pancreatic pseudocyst, this outcome may not be the norm for all patients re-challenged with asparaginase after pancreatitis. None of the other patients have been re-challenged with asparaginase.
Indications for immediate or delayed surgical intervention or percutaneous drainage are not well defined, but many advocate intervention if symptoms such as nausea, vomiting, or abdominal pain do not resolve (7–10). Spontaneous resolution of pancreatic cyst is well recognized (7;8;11). One study reported that pseudocysts that persist for more than six weeks after an episode of pancreatitis do not resolve spontaneously (12), but other studies and the patients in this series do not support this contention (7;8;13). Spontaneous resolution occurs in 8% to 70% of adult patients with pseudocyst due to various causes (11), and longer observation times may lead to higher rates of resolution. In children with ALL, complications after surgical intervention may be increased because of the need for continued chemotherapy treatment.
When and how to initiate oral feeding in patients with pancreatic pseudocyst remains controversial. Some clinicians permit oral intake when vomiting stops and abdominal pain improves, others only when amylase and lipase values normalize, and still others when imaging demonstrates resolution of the pseudocyst. In patient 1, the pseudocyst did not decrease in size after 6 weeks, so oral feeding was initiated since the time to resolution was predicted to be very long. In the subsequent two patients, oral intake was permitted after 2 and 7 weeks, respectively, when symptoms had resolved and pancreatic enzymes normalized. In patients 4 and 5, oral feedings were started when the patient was hungry and was advanced slowly as long as abdominal symptoms did not return. For this reason, the need for parenteral nutrition and NPO time was decreased in these final two patients, likely contributing significantly to their quality of life.
Final Remarks
These cases indicate that non-surgical management of asymptomatic patients with pancreatic pseudocyst is safe and effective in children with ALL. Resolution of the pseudocyst may require months or years, but surgical procedures need only be considered if symptoms develop or persist. Further research should focus on defining factors that predict asparaginase-related pancreatitis and determining when re-challenge with L-asparaginase is appropriate (Table I). Also, the need for gut rest in asymptomatic patients, the optimal duration of parenteral nutrition, and the best timing and method to reintroduce oral intake need to be determined (Table I). Until these issues are clarified in pediatric ALL, we recommend initial non-surgical management of pancreatic pseudocyst, use of parenteral nutrition and suspension of chemotherapy until the episode of acute pancreatitis has resolved, initiation of oral feedings when abdominal pain subsides and advancement of the diet as long as pain does not return (even if the pseudocyst is present), and permanent discontinuation of asparaginase.
Table I.
Clinical questions and results in the patients in this series
| Clinical situation |
Decision | Action taken | Results in these cases |
|---|---|---|---|
| Acute pancreatitis during remission induction therapy |
Should asparaginase be discontinued or changed to a different type? |
L-asparaginase was restarted in patients 1 and 3 with weekly monitoring of amylase and lipase. No further asparaginase was used in patients 2, 4, and 5. |
Recurrent severe pancreatitis in patients 1 and 3 after L- asparaginase administered as part of continuation therapy |
| Large pancreatic pseudocyst |
Should asparaginase therapy be restarted after complete clinical recovery? |
L-asparaginase was discontinued permanently in patients 1 and 3 after the second episode of pancreatitis, in which a pseudocyst developed. Low dose methotrexate replaced the remaining asparaginase. Patient 2 had completed all but one dose of L-asparaginase at diagnosis of pseudocyst. Patients 4 & 5 received no further PEG asparaginase after pseudocyst development. |
The pancreatic pseudocyst in patients 1 & 3 resolved after 37 months & 11 weeks respectively. |
| When and how should oral feedings be reintroduced? |
Parenteral nutrition and gut rest were used for up to 6 weeks, and then oral feeding gradually introduced; amylase and lipase values were monitored regularly. |
Oral feeds were tolerated despite persistence of the pseudocyst in all patients. Pancreatitis did not recur. |
Acknowledgments
This work was supported by grants (CA-21765, CA-51001, CA-36401, CA-78224, CA-60419, GM-61393) from the National Institutes of Health, and by the American Lebanese Syrian Associated Charities. Dr. Pui is an American Cancer Society Professor.
Reference List
- 1.Pui CH, Sandlund JT, Pei D, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Total Therapy Study XIIIB at St Jude Children's Research Hospital. Blood. 2004;104:2690–2696. doi: 10.1182/blood-2004-04-1616. [DOI] [PubMed] [Google Scholar]
- 2.Silverman LB, Gelber RD, Dalton VK, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood. 2001;97:1211–1218. doi: 10.1182/blood.v97.5.1211. [DOI] [PubMed] [Google Scholar]
- 3.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 4.Knoderer HM, Robarge J, Flockhart DA. Predicting asparaginase-associated pancreatitis. Pediatr Blood Cancer. 2007;49:634–639. doi: 10.1002/pbc.21037. [DOI] [PubMed] [Google Scholar]
- 5.Steinberg W, Tenner S. Acute pancreatitis. N Engl J Med. 1994;330:1198–1210. doi: 10.1056/NEJM199404283301706. [DOI] [PubMed] [Google Scholar]
- 6.Amylon MD, Shuster J, Pullen J, et al. Intensive high-dose asparaginase consolidation improves survival for pediatric patients with T cell acute lymphoblastic leukemia and advanced stage lymphoblastic lymphoma: a Pediatric Oncology Group study. Leukemia. 1999;13:335–342. doi: 10.1038/sj.leu.2401310. [DOI] [PubMed] [Google Scholar]
- 7.Yeo CJ, Bastidas JA, Lynch-Nyhan A, et al. The natural history of pancreatic pseudocysts documented by computed tomography. Surg Gynecol Obstet. 1990;170:411–417. [PubMed] [Google Scholar]
- 8.Vitas GJ, Sarr MG. Selected management of pancreatic pseudocysts: operative versus expectant management. Surgery. 1992;111:123–130. [PubMed] [Google Scholar]
- 9.Cheruvu CV, Clarke MG, Prentice M, et al. Conservative treatment as an option in the management of pancreatic pseudocyst. Ann R Coll Surg Engl. 2003;85:313–316. doi: 10.1308/003588403769162413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singhal D, Kakodkar R, Sud R, et al. Issues in management of pancreatic pseudocysts. JOP. 2006;7:502–507. [PubMed] [Google Scholar]
- 11.Andren-Sandberg A, Dervenis C. Pancreatic pseudocysts in the 21st century. Part II: natural history. JOP. 2004;5:64–70. [PubMed] [Google Scholar]
- 12.Warshaw AL, Rattner DW. Timing of surgical drainage for pancreatic pseudocyst. Clinical and chemical criteria. Ann Surg. 1985;202:720–724. doi: 10.1097/00000658-198512000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maringhini A, Uomo G, Patti R, et al. Pseudocysts in acute nonalcoholic pancreatitis: incidence and natural history. Dig Dis Sci. 1999;44:1669–1673. doi: 10.1023/a:1026691700511. [DOI] [PubMed] [Google Scholar]