Abstract
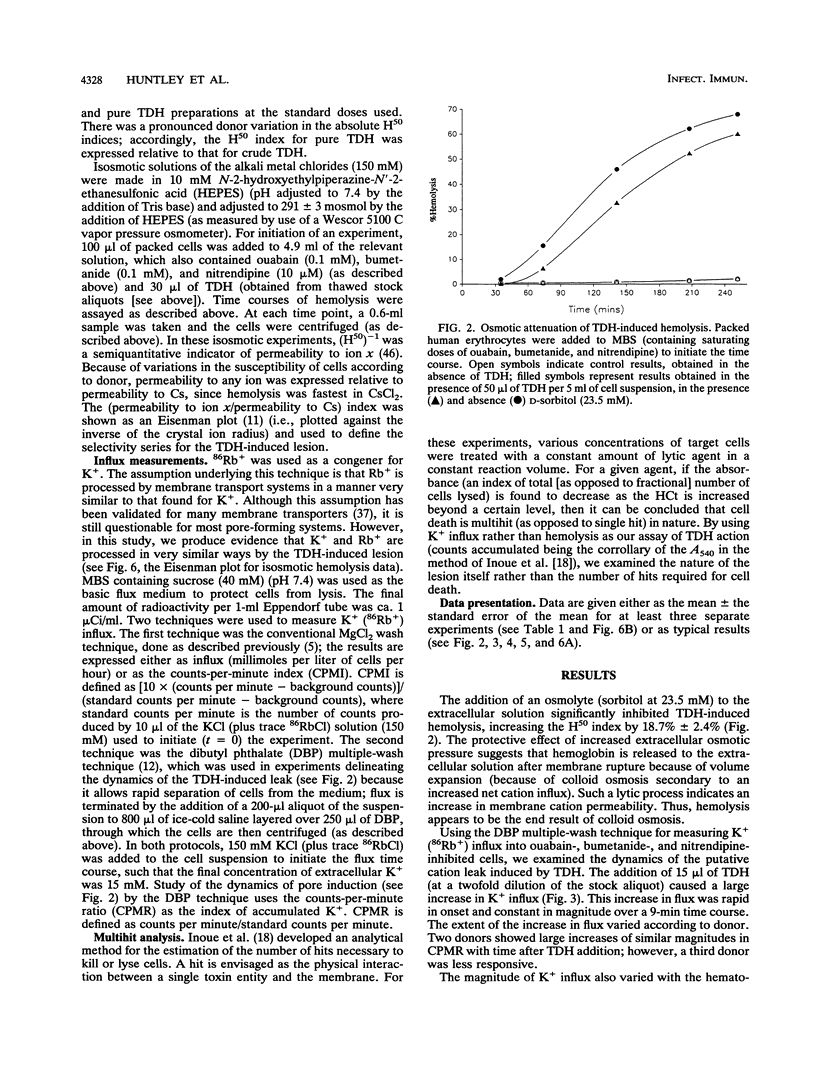
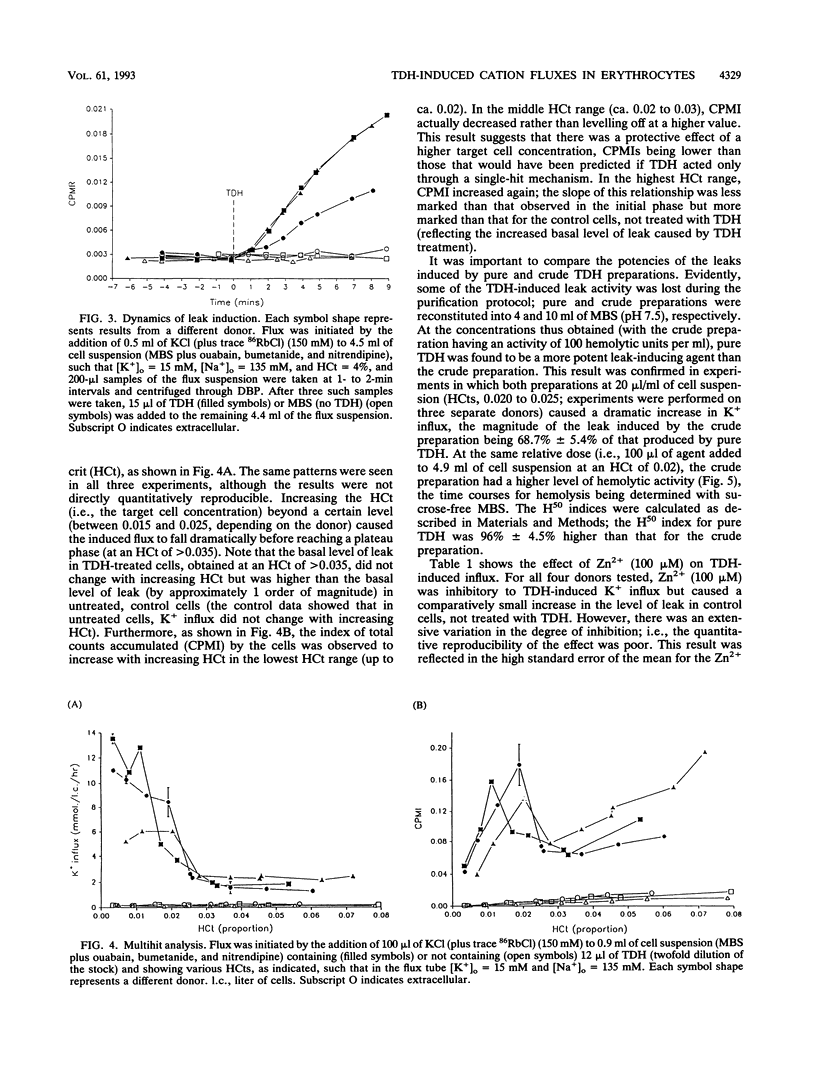
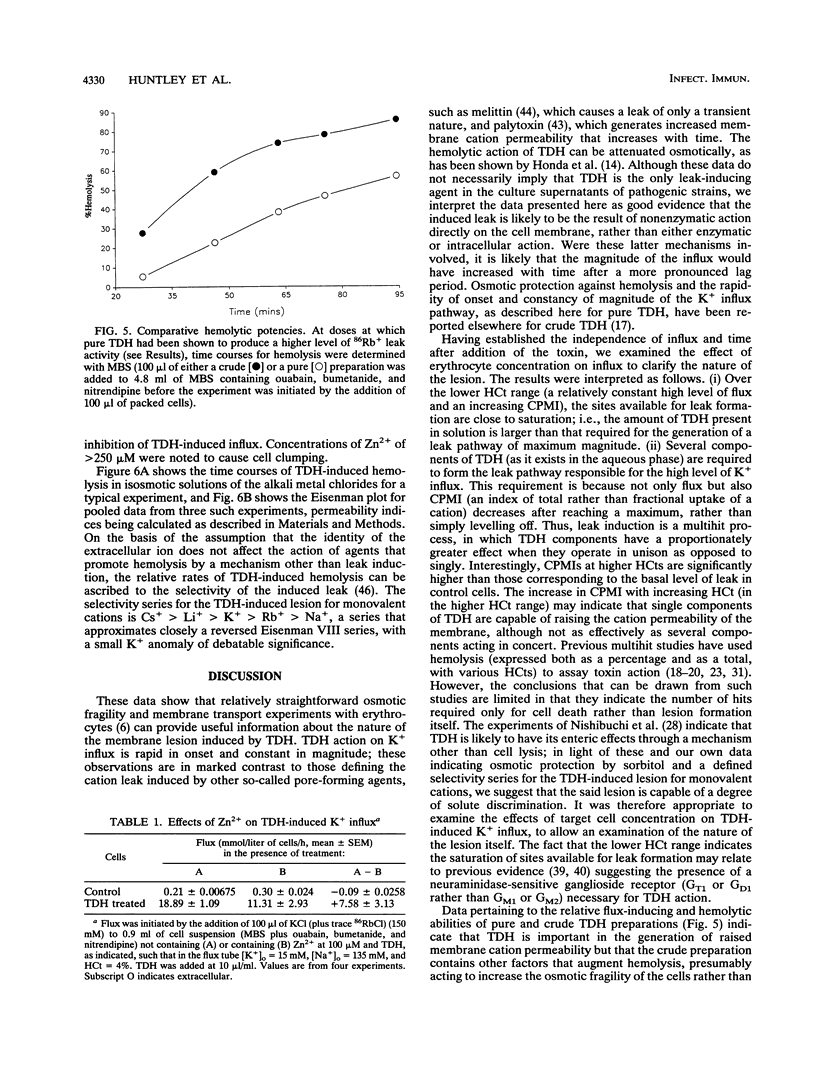
Vibrio parahaemolyticus, an important agent of seafood-borne gastroenteritis, expresses several putative virulence factors that could account for the disease symptoms of infected humans, namely, diarrhea, nausea, and abdominal cramps. The pathogenicity of V. parahaemolyticus correlates well with the Kanagawa phenomenon (the hemolytic ability of strains grown on Wagatsuma blood agar), implicating the thermostable direct hemolysin (TDH) as the predominant toxin responsible for pathogenicity. TDH-induced hemolysis could be inhibited by the addition of the osmolyte sorbitol to the extracellular solution, supporting the hypothesis that hemolysis occurs through colloid osmosis secondary to an increase in the cation permeability of the membrane. The effect of TDH on cation permeability was investigated by measuring K+ (congener, 86Rb+) influx into human erythrocytes in which the endogenous cation transporters had been blocked (by use of ouabain, bumetanide, and nitrendipine). TDH increased K+ influx into these cells; this increase was rapid in onset and constant in magnitude, suggesting a direct action by TDH on the membrane. The kinetics of leak generation were examined; the relationship between counts accumulated and hematocrit indicated that the TDH-induced lesion is multihit in nature. TDH-induced K+ influx was sensitive to Zn2+. Time courses of hemolysis in isosmotic solutions of monovalent cation chlorides were used to obtain the selectivity series for the TDH-induced leak: Cs+ > Li+ > K+ > Rb+ > Na+. Both the Zn2+ sensitivity and this selectivity series were obtained for crude culture supernatants, suggesting that TDH is the predominant leak-inducing agent. Thus, we have identified several features of the TDH-induced leak likely to be important in the diarrhetic action of V. parahaemolyticus in the human intestine.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baba K., Shirai H., Terai A., Kumagai K., Takeda Y., Nishibuchi M. Similarity of the tdh gene-bearing plasmids of Vibrio cholerae non-O1 and Vibrio parahaemolyticus. Microb Pathog. 1991 Jan;10(1):61–70. doi: 10.1016/0882-4010(91)90066-j. [DOI] [PubMed] [Google Scholar]
- Baba K., Yamasaki S., Nishibuchi M., Takeda Y. Examination by site-directed mutagenesis of the amino acid residues of the thermostable direct hemolysin of Vibrio parahaemolyticus required for its hemolytic activity. Microb Pathog. 1992 Apr;12(4):279–287. doi: 10.1016/0882-4010(92)90046-q. [DOI] [PubMed] [Google Scholar]
- Bashford C. L., Alder G. M., Graham J. M., Menestrina G., Pasternak C. A. Ion modulation of membrane permeability: effect of cations on intact cells and on cells and phospholipid bilayers treated with pore-forming agents. J Membr Biol. 1988 Jul;103(1):79–94. doi: 10.1007/BF01871934. [DOI] [PubMed] [Google Scholar]
- Bashford C. L., Rodrigues L., Pasternak C. A. Protection of cells against membrane damage by haemolytic agents: divalent cations and protons act at the extracellular side of the plasma membrane. Biochim Biophys Acta. 1989 Jul 24;983(1):56–64. doi: 10.1016/0005-2736(89)90380-5. [DOI] [PubMed] [Google Scholar]
- Bernhardt I., Hall A. C., Ellory J. C. Effects of low ionic strength media on passive human red cell monovalent cation transport. J Physiol. 1991 Mar;434:489–506. doi: 10.1113/jphysiol.1991.sp018482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer A. W. Assay of hemolytic toxins. Methods Enzymol. 1988;165:213–217. doi: 10.1016/s0076-6879(88)65033-6. [DOI] [PubMed] [Google Scholar]
- Bernheimer A. W., Rudy B. Interactions between membranes and cytolytic peptides. Biochim Biophys Acta. 1986 Jun 12;864(1):123–141. doi: 10.1016/0304-4157(86)90018-3. [DOI] [PubMed] [Google Scholar]
- Blake P. A., Weaver R. E., Hollis D. G. Diseases of humans (other than cholera) caused by vibrios. Annu Rev Microbiol. 1980;34:341–367. doi: 10.1146/annurev.mi.34.100180.002013. [DOI] [PubMed] [Google Scholar]
- Douet J. P., Castroviejo M., Dodin A., Bébéar C. Purification and characterization of Kanagawa haemolysin from Vibrio parahaemolyticus. Res Microbiol. 1992 Jul-Aug;143(6):569–577. doi: 10.1016/0923-2508(92)90114-4. [DOI] [PubMed] [Google Scholar]
- Eisenman G., Horn R. Ionic selectivity revisited: the role of kinetic and equilibrium processes in ion permeation through channels. J Membr Biol. 1983;76(3):197–225. doi: 10.1007/BF01870364. [DOI] [PubMed] [Google Scholar]
- Ellory J. C., Kirk K., Culliford S. J., Nash G. B., Stuart J. Nitrendipine is a potent inhibitor of the Ca(2+)-activated K+ channel of human erythrocytes. FEBS Lett. 1992 Jan 20;296(2):219–221. doi: 10.1016/0014-5793(92)80383-r. [DOI] [PubMed] [Google Scholar]
- Ginsburg H., Stein W. D. Biophysical analysis of novel transport pathways induced in red blood cell membranes. J Membr Biol. 1987;96(1):1–10. doi: 10.1007/BF01869329. [DOI] [PubMed] [Google Scholar]
- Honda T., Ni Y., Miwatani T., Adachi T., Kim J. The thermostable direct hemolysin of Vibrio parahaemolyticus is a pore-forming toxin. Can J Microbiol. 1992 Nov;38(11):1175–1180. doi: 10.1139/m92-192. [DOI] [PubMed] [Google Scholar]
- Honda T., Nishibuchi M., Miwatani T., Kaper J. B. Demonstration of a plasmid-borne gene encoding a thermostable direct hemolysin in Vibrio cholerae non-O1 strains. Appl Environ Microbiol. 1986 Nov;52(5):1218–1220. doi: 10.1128/aem.52.5.1218-1220.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K., Akiyama Y., Kinoshita T., Higashi Y., Amano T. Evidence for a one-hit theory in the immune bactericidal reaction and demonstration of a multi-hit response for hemolysis by streptolysin O and Clostridium perfringens theta-toxin. Infect Immun. 1976 Feb;13(2):337–344. doi: 10.1128/iai.13.2.337-344.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. K., Boese-Marrazzo D. Production and properties of heat-stable extracellular hemolysin from Pseudomonas aeruginosa. Infect Immun. 1980 Sep;29(3):1028–1033. doi: 10.1128/iai.29.3.1028-1033.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. K. Properties of purified pneumococcal hemolysin. Infect Immun. 1972 Nov;6(5):755–760. doi: 10.1128/iai.6.5.755-760.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S. W., Colwell R. R., Kaper J. B. Vibrio parahaemolyticus and related halophilic Vibrios. Crit Rev Microbiol. 1982;10(1):77–124. doi: 10.3109/10408418209113506. [DOI] [PubMed] [Google Scholar]
- Kothary M. H., Kreger A. S. Purification and characterization of an extracellular cytolysin produced by Vibrio damsela. Infect Immun. 1985 Jul;49(1):25–31. doi: 10.1128/iai.49.1.25-31.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y., Kato T., Obara Y., Akiyama S., Takizawa K., Yamai S. In vitro hemolytic characteristic of Vibrio parahaemolyticus: its close correlation with human pathogenicity. J Bacteriol. 1969 Nov;100(2):1147–1149. doi: 10.1128/jb.100.2.1147-1149.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y., Obara Y., Nikkawa T., Yamai S., Kato T., Yamada Y., Ohashi M. Simplified purification and biophysicochemical characteristics of Kanagawa phenomenon-associated hemolysin of Vibrio parahaemolyticus. Infect Immun. 1980 May;28(2):567–576. doi: 10.1128/iai.28.2.567-576.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos M., Jr, Nguyen N. Y., Liu T. Y. Reproducible high yield sequencing of proteins electrophoretically separated and transferred to an inert support. J Biol Chem. 1988 May 5;263(13):6005–6008. [PubMed] [Google Scholar]
- Nishibuchi M., Fasano A., Russell R. G., Kaper J. B. Enterotoxigenicity of Vibrio parahaemolyticus with and without genes encoding thermostable direct hemolysin. Infect Immun. 1992 Sep;60(9):3539–3545. doi: 10.1128/iai.60.9.3539-3545.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishibuchi M., Kaper J. B. Nucleotide sequence of the thermostable direct hemolysin gene of Vibrio parahaemolyticus. J Bacteriol. 1985 May;162(2):558–564. doi: 10.1128/jb.162.2.558-564.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishibuchi M., Taniguchi T., Misawa T., Khaeomanee-Iam V., Honda T., Miwatani T. Cloning and nucleotide sequence of the gene (trh) encoding the hemolysin related to the thermostable direct hemolysin of Vibrio parahaemolyticus. Infect Immun. 1989 Sep;57(9):2691–2697. doi: 10.1128/iai.57.9.2691-2697.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberley T. D., Duncan J. L. Characteristics of streptolysin O action. Infect Immun. 1971 Dec;4(6):683–687. doi: 10.1128/iai.4.6.683-687.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai J., Bahavar M. A., Jinguji Y., Miwatani T. Interaction of thermostable direct hemolysin of Vibrio parahaemolyticus with human erythrocytes. Biken J. 1975 Dec;18(4):187–192. [PubMed] [Google Scholar]
- Sakurai J., Honda T., Jinguji Y., Arita M., Miwatani T. Cytotoxic effect of the thermostable direct hemolysin produced by Vibrio parahaemolyticus on FL cells. Infect Immun. 1976 Mar;13(3):876–883. doi: 10.1128/iai.13.3.876-883.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai J., Matsuzaki A., Miwatani T. Purification and characterization of thermostable direct hemolysin of Vibrio parahaemolyticus. Infect Immun. 1973 Nov;8(5):775–780. doi: 10.1128/iai.8.5.775-780.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons T. J. A method for estimating free Ca within human red blood cells, with an application to the study of their Ca-dependent K permeability. J Membr Biol. 1982;66(3):235–247. doi: 10.1007/BF01868498. [DOI] [PubMed] [Google Scholar]
- Takeda R., Honda T., Sakural J., Otomo N. Inhibition of hemolytic activity of the thermostable direct hemolysin of Vibrio parahaemolyticus by ganglioside. Infect Immun. 1975 Oct;12(4):931–933. doi: 10.1128/iai.12.4.931-933.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y., Takeda T., Honda T., Miwatani T. Inactivation of the biological activities of the thermostable direct hemolysin of Vibrio parahaemolyticus by ganglioside Gt1. Infect Immun. 1976 Jul;14(1):1–5. doi: 10.1128/iai.14.1.1-5.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y. Thermostable direct hemolysin of Vibrio parahaemolyticus. Pharmacol Ther. 1982;19(1):123–146. doi: 10.1016/0163-7258(82)90044-4. [DOI] [PubMed] [Google Scholar]
- Terai A., Baba K., Shirai H., Yoshida O., Takeda Y., Nishibuchi M. Evidence for insertion sequence-mediated spread of the thermostable direct hemolysin gene among Vibrio species. J Bacteriol. 1991 Aug;173(16):5036–5046. doi: 10.1128/jb.173.16.5036-5046.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda H., Sakiyama F., Yoh M., Honda T., Miwatani T. Tryptophan 65 is essential for hemolytic activity of the thermostable direct hemolysin from Vibrio parahaemolyticus. Toxicon. 1991;29(7):837–844. doi: 10.1016/0041-0101(91)90220-l. [DOI] [PubMed] [Google Scholar]
- Tosteson M. T., Halperin J. A., Kishi Y., Tosteson D. C. Palytoxin induces an increase in the cation conductance of red cells. J Gen Physiol. 1991 Nov;98(5):969–985. doi: 10.1085/jgp.98.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosteson M. T., Holmes S. J., Razin M., Tosteson D. C. Melittin lysis of red cells. J Membr Biol. 1985;87(1):35–44. doi: 10.1007/BF01870697. [DOI] [PubMed] [Google Scholar]
- Tsunasawa S., Sugihara A., Masaki T., Sakiyama F., Takeda Y., Miwatani T., Narita K. Amino acid sequence of thermostable direct hemolysin produced by Vibrio parahaemolyticus. J Biochem. 1987 Jan;101(1):111–121. doi: 10.1093/oxfordjournals.jbchem.a121882. [DOI] [PubMed] [Google Scholar]
- Weiner R. N., Schneider E., Haest C. W., Deuticke B., Benz R., Frimmer M. Properties of the leak permeability induced by a cytotoxic protein from Pseudomonas aeruginosa (PACT) in rat erythrocytes and black lipid membranes. Biochim Biophys Acta. 1985 Nov 7;820(2):173–182. doi: 10.1016/0005-2736(85)90110-5. [DOI] [PubMed] [Google Scholar]
- Yanagase Y., Inoue K., Ozaki M., Ochi T., Amano T. Hemolysins and related enzymes of Vibrio parahaemolyticus. I. Identification and partial purification of enzymes. Biken J. 1970 Jun;13(2):77–92. [PubMed] [Google Scholar]