Abstract
Human papilloma virus (HPV) is an etiologic agent in a subset of oropharyngeal squamous cell carcinomas (SCCs). The aim of this study was to sub-classify SCC of the oropharynx based upon histologic features into nonkeratinizing (NK) SCC, keratinizing (K) SCC, and hybrid SCC, and determine the frequency of HPV and patient survival in each group. Patients with oropharyngeal SCC with a minimum of 2 years of clinical follow-up were identified from radiation oncology databases from 1997 to 2004. All patients received either up front surgery with postoperative radiation or definitive radiation based therapy. In situ hybridization (ISH) for high-risk HPV subtypes and immunohistochemistry for p16, a protein frequently up-regulated in HPV-associated carcinomas, were performed. Overall and disease-specific survival were assessed. Of 118 cases, 46.6% were NK SCC, 24.6% K SCC and 28.8% hybrid SCC. NK SCC occurred in slightly younger patients that were more often male. It more frequently presented with lymph node metastases and was surgically resected compared to K SCC. NK SCC was significantly more likely to be HPV and p16 positive than KSCC (P < 0.001) and to have better overall and disease-specific survival (P = 0.0002; P = 0.0142, respectively). Hybrid SCC was also more likely than K SCC to be HPV and p16 positive (P = 0.003; P = 0.002, respectively) and to have better overall survival (P = 0.0105). Sub-classification of oropharyngeal SCC by histologic type provides useful clinical information. NK SCC histology strongly predicts HPV-association and better patient survival compared to K SCC. Hybrid SCC appears to have an intermediate frequency of HPV-association and patient survival.
Keywords: Human papillomavirus, p16, Oropharynx, Head and neck, Nonkeratinizing squamous cell carcinoma, Hybrid squamous cell carcinoma, Keratinizing squamous cell carcinoma, Intensity-modulated radiation therapy, Immunohistochemistry, In situ hybridization
Introduction
Human papillomavirus (HPV) infection is now recognized as an etiologic agent in a subset of head and neck squamous cell carcinomas (SCCs) that are clinically and biologically distinct [1–9]. HPV-related SCC occurs predominantly in the oropharynx and affects younger patients [3, 7, 10]. The risk factors are similar to those of HPV-related cervical SCC and include a high lifetime number of vaginal or oral-sex partners [9, 11–14]. This is unlike non-HPV related SCC, which is more commonly linked with heavy tobacco and alcohol use [14–16]. Importantly, in spite of a tendency to present at high stage, HPV-related SCC is associated with better patient survival [1, 4, 7]. Recent evidence suggests that unique molecular events in HPV-related SCC may, in fact, underlie their distinct biological behavior [5, 17–19].
Furthermore, El-Mofty et al. [20, 21] have noted that HPV-related SCC of the oropharynx has unique histologic features. The tumors tend to be nonkeratinizing squamous cell carcinomas (NK SCCs). They form sheets, nests and trabeculae that have pushing borders with little stromal response. Frequent mitoses and comedo necrosis are characteristic features. The cells are ovoid to spindle-shaped with hyperchromatic nuclei that lack prominent nucleoli and have indistinct cell borders (Fig. 1). These morphologic features are in contrast to those of non-HPV related SCC, which is keratinizing and composed of polygonal cells with abundant, mature cytoplasm, distinct cell borders and intercellular bridges. The growth pattern of these tumors is typically infiltrative with angulated nests that have tapered extensions and pronounced stromal desmoplasia (Fig. 2). Additionally, El-Mofty et al. [22] described an intermediate or “hybrid” histologic group with partial squamous maturation that also appears to be HPV-related (Fig. 3).
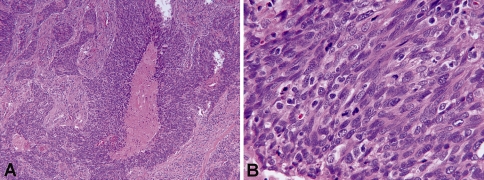
Fig. 1.
Histologic features of NK SCC (H&E stained sections). a Low-power image (×100) showing nest of tumor cells with pushing borders and comedo-type necrosis. In addition, there is a lack of stromal desmoplasia around the tumor nests. b High-power image (×600) showing oval to spindled, hyperchromatic tumor cells that lack prominent nucleoli and have indistinct cell borders
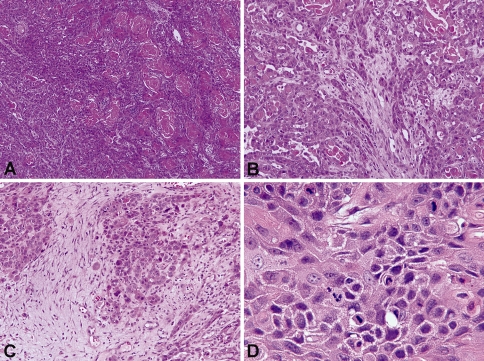
Fig. 2.
Histologic features of K SCC (H&E stained sections). a Low-power image (×100) showing sheets of tumor cells with keratin pearl formation. b Low-power image (×200) showing infiltrative nests of polygonal-shaped tumor cells with abundant, eosinophilic (“mature”) cytoplasm and distinct cell borders. c Low-power image (×200) showing desmoplastic stroma surrounding tumor nests. d High-power image (×600) highlighting intercellular bridges
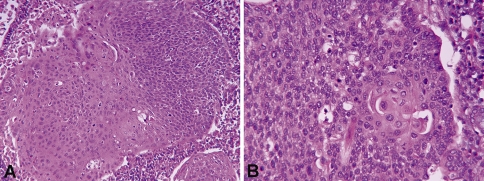
Fig. 3.
Histologic features of hybrid SCC (H&E stained sections). a Low-power image (×200) showing keratinizing SCC on the left and adjacent nonkeratinizing SCC on the right. b High-power image (×400) showing predominately ovoid to spindled, hyperchromatic cells. Focally, the cells have eosinophilic (“mature”) cytoplasm and distinct cell borders
Determination of HPV status in oropharyngeal carcinomas is becoming recognized as an important consideration in the management of these tumors and prediction of patient outcome. A variety of HPV detection methods are currently used, including PCR, ISH and immunohistochemistry. p16 is a tumor suppressor protein that is also considered to be a surrogate marker of HPV infection in squamous cell carcinomas of the cervix and head and neck [23]. However, false negatives and false positives are reported with each of these techniques. The use of RT-PCR for measurement of viral mRNA E6 and E7 in fresh tissue is the gold standard for HPV detection. Using techniques that are applicable to formalin-fixed paraffin-embedded tissue, there is often a lack of agreement as to what is considered evidence of pathophysiologically and clinically significant HPV involvement.
Histologic classification is simple and relatively straightforward. It also correlates well with HPV detection by ISH and p16 immunohistochemistry [20–22]. However, the use of histologic classification in determining HPV-association has not gained widespread acceptance. This may be, in part, due to the lack of studies that correlate histologic features directly with patient outcome.
The aim of this study was threefold: first, to sub-classify oropharyngeal carcinomas into three groups according to the above mentioned morphologic features: nonkeratinizing squamous cell carcinoma (NK SCC), keratinizing squamous cell carcinoma (K SCC) and hybrid squamous cell carcinoma (hybrid SCC); second, to determine HPV status of the tumors using HR HPV-ISH and p16 immunostaining; and third, to determine overall and disease-specific survival of patients in each histologic sub-group. The use of simple morphologic features to sub-classify SCC of the oropharynx is potentially useful, particularly if predictive of patient outcome.
Materials and Methods
Case Selection and Classification
Cases of oropharyngeal SCC were identified from a Radiation Oncology head and neck database at Barnes-Jewish Hospital from 1997 to 2004. This is an IRB approved combined retrospective/prospective database with a waiver for retrospective data collection and patient consent for prospective data collection. Radiation was either postoperative for patients managed with an up front surgical approach, or definitive for patients managed without surgery. Patients were treated exclusively with intensity modulated radiation therapy (IMRT) by a single radiation oncologist (WLT). Patients had a minimum of 2 years of clinical follow-up assessed from the end of radiation therapy with the exception of 5 patients who were lost to follow up within 2 years. The mean length of follow-up was 3.3 years (range of 5 months to 8 years). Only cases with primary and pre-treatment surgical pathology material available for review were included. The diagnosis of SCC was confirmed by slide review and all recognized variants such as verrucous, spindle cell, papillary, adenosquamous, undifferentiated, and basaloid squamous cell carcinomas were excluded. Particular care was taken to exclude cases of basaloid squamous cell carcinoma, which is histologically distinct from nonkeratinizing squamous cell carcinoma. Basaloid squamous cell carcinoma, as defined by Wain’s criteria, is intimately associated with keratinizing squamous cell carcinoma, and is composed of a lobular proliferation of small, crowded cells with scant cytoplasm and round, hyperchromatic nuclei [24]. In addition, it has cystic spaces with mucin-like material, coagulative necrosis and stromal hyalinosis with basement membrane-like material [22].
The cases were classified independently by three reviewers (RDC, SKEM, JSL), prior to HPV testing and without knowledge of clinical outcome, into the following three categories based upon histologic features: NK SCC, K SCC, and those with overlapping features (referred to as hybrid SCC). NK SCC was defined as forming sheets, nests or trabeculae with pushing borders, little stromal response, and having ovoid to spindled, hyperchromatic cells that lack prominent nucleoli and have indistinct cell borders (Fig. 1). Comedo-type necrosis and brisk mitotic activity were often present but were not considered requisite features. While varying from well to poorly differentiated, K SCC was defined as entirely composed of mature squamous cells without areas with NK SCC morphology (Fig. 2). Hybrid SCC showed nonkeratinizing morphology but with areas of squamous maturation (Fig. 3). Discrepant cases were collectively arbitrated around a multi-headed microscope by all three study pathologists and placed in a single category.
Comorbidity Score
The Adult Comorbidity Evaluation (ACE-27) was used to a calculated comorbidity index [25]. The ACE-27 grades specific diseases and conditions into one of three groups, Grade 1, Grade 2, or Grade 3, according to the severity of organ decompensation and prognostic impact. An Overall Comorbidity Score, None, Mild, Moderate, or Severe, is assigned based on the highest ranked single ailment. In the cases where two or more Grade 2 ailments occur in different organ systems or disease groupings, the Overall Comorbidity Score is designated as Severe.
In Situ Hybridization for HPV
In Situ Hybridization (ISH) was performed on formalin-fixed, paraffin embedded, 4 μm tissue sections using ISH I View Blue Plus Detection Kit (Ventana Medical System, Inc., Tucson, AZ) according to the manufacturer’s instructions. The probes used hybridized with the high risk HPV (HR HPV) genotypes including types 16, 18, 33, 35, 45, 51, 52 56, and 66. Ventana Red Counterstain II (Ventana Medical System, Inc., Tuscon, AZ) was used. Positive staining was identified as blue nuclear dots. Any definitive nuclear staining in the tumor cells was considered positive. Cases were classified in a binary manner as either positive or negative.
Immunohistochemistry for p16
Immunoperoxidase staining was done on formalin-fixed paraffin embedded, 4 μm tissue sections using the DAKO LSAB2 horseradish peroxidase system (DAKO Corp., Carpentaria, CA) following the manufacturer’s instructions. Antigen retrieval was done by microwave heating for 10 min in 10 mM citrate buffer (pH 6.0); a p16 monoclonal antibody (1:40 dilution) was used (Novocastra Labs Ltd., UK). Cases were classified in a binary manner as either positive (any cells with nuclear and cytoplasmic staining) or negative.
Statistics
Fisher’s exact test and Student’s t-test for unpaired data were used to test differences in clinical characteristics, including comorbidity, as well as HR HPV and p16 results between histologic groups. Log-rank tests for the equality of survivor functions and Cox-proportional hazards regression analysis were used to test differences in survivorship between groups. A P value of <0.05 was considered statistically significant.
Results
A total of 118 cases of SCC of the oropharynx were classified: 55 (46.6%) as NK SCC, 29 (24.6%) as K SCC, and 34 (28.8%) as hybrid SCC. Clinical characteristics by histologic type are shown in Table 1. All tumors were more common in males than females, although significantly fewer females were present in the NK SCC group compared to either the K or hybrid SCC group (Fisher’s exact test, P = 0.018; P = 0.024). Patients with NK SCC were slightly younger than those with K SCC and had a mean age of 54.0 compared to 58.8 years (Student’s t-test, P = 0.0265). Although there were no differences in stage across histologic types, NK SCC was more likely than K or hybrid SCC to have lymph node metastases at presentation (Fisher’s exact test, P = 0.038; P = 0.036, respectively). Patients with NK SCC were more likely to have received up front surgery followed by postoperative radiation rather than definitive radiation compared to either K or hybrid SCC (Fisher’s exact test, P = 0.003; P = 0.017), a finding that may be due to the tendency of NK SCC to present as lymph node metastases with an occult primary tumor that is usually only detected operatively in the search of a primary site. One-third (33.9%) of patients also received chemotherapy, but there were no differences by histological type (Fisher’s exact test). Chemotherapy consisted of cisplatin and/or carboplatin/paclitaxel. Seven patients also received 5-fluorouracil and one patient, docetaxil.
Table 1.
Clinical Characteristics of Patients with Oropharyngeal Carcinoma by Histologic Type, Fisher’s exact test and t-test
| Histologic type (#) | K SCC (29) | NK SCC (55) | Hybrid SCC (34) | Total (118) |
|---|---|---|---|---|
| Age (Mean ± SD) | 58.8 (±9.2) | 54.0 (±8.4) | 57.1 (±8.4) | 56.1 (±9.5) |
| P value | – | 0.0265 | NS | |
| Sex (%) | ||||
| Male | 23 (79.3) | 53 (96.4) | 27 (79.4) | 103 (87.3) |
| Female | 6 (20.7) | 2 (3.6) | 7 (20.6) | 15 (12.7) |
| P value | 0.018 | – | 0.024 | |
| Smoker (%) | ||||
| Yes | 17 (58.6) | 30 (54.5) | 17 (50.0) | 64 (54.2) |
| No | 3 (10.3) | 10 (18.2) | 6 (17.6) | 19 (16.1) |
| Unknown | 9 (31.0) | 15 (27.3) | 11 (32.4) | 35 (29.7) |
| P value | – | NS | NS | |
| Stage (%) | ||||
| II | 5 (17.2) | 2 (3.6) | 1 (2.9) | 8 (6.8) |
| III | 6 (20.7) | 11 (20.0) | 6 (17.6) | 23 (19.5) |
| IVA | 17 (58.6) | 40 (72.7) | 23 (67.6) | 80 (67.8) |
| IVB | 1 (3.4) | 2 (3.6) | 4 (11.8) | 7 (5.9) |
| P value | – | NS | NS | |
| Lymph node metastases (%) | ||||
| Yes | 11 (37.9) | 39 (70.9) | 16 (47.1) | 66 (55.9) |
| No | 12 (41.4) | 14 (25.5) | 16 (47.1) | 42 (35.6) |
| Unknown | 6 (20.7) | 2 (3.6) | 2 (5.8) | 10 (8.5) |
| P value | 0.038 | – | 0.036 | |
| IMRT (%) | ||||
| Definitive | 14 (48.3) | 8 (14.5) | 12 (35.3) | 34 (28.8) |
| Post-operative | 14 (48.3) | 46 (83.6) | 20 (58.8) | 80 (67.8) |
| Both | 1 (3.4) | 1 (1.8) | 2 (5.9) | 4 (3.4) |
| P value | P = 0.003 | – | P = 0.017 | |
| Chemotherapy (%) | ||||
| Yes | 11 (37.9) | 18 (32.7) | 11 (32.4) | 40 (33.9) |
| No | 18 (62.1) | 37 (67.3) | 23 (67.6) | 78 (66.1) |
| P value | – | NS | NS | |
K SCC keratinizing squamous cell carcinoma, NK SCC nonkeratinizing squamous cell carcinoma, Hybrid SCC hybrid squamous cell carcinoma, IMRT intensity-modulated radiation therapy, NS not significant
Of the 118 cases, 89 had formalin-fixed paraffin embedded tissue available for HPV testing and p16 immunohistochemistry. Both NK SCC and hybrid SCC were much more likely to be HR HPV positive than K SCC (Fisher’s exact test, NK SCC, P < 0.001; hybrid SCC, P = 0.003; Table 2). Both NK SCC and hybrid SCC were also much more likely to be p16 positive than K SCC (Fisher’s exact test, NK SCC, P < 0.001; hybrid SCC, P = 0.002, respectively; Table 2). All NK SCCs were p16 positive and 69% were HR HPV positive. Hybrid SCCs were also frequently p16 and HR HPV positive (81.8 and 45.5%, respectively). In contrast, only 36% of K SCCs were p16 positive and 8% HR HPV positive. While HR HPV-ISH staining varied from focal to diffuse, p16 staining was strong, both nuclear and cytoplasmic, in all cases and diffuse in the majority (87%) of the cases. Examples of HR HPV and p16 positive and negative tumors are shown in Fig. 4.
Table 2.
HR HPV-ISH and p16 Status by Histologic Type, Fisher’s exact test
| Histologic Type | K SCC (25) | NK SCC (42) | Hybrid SCC (22) | Total (89) |
|---|---|---|---|---|
| HR HPV-ISH (%) | 2 (8%) | 29 (69%) | 10 (45.5%) | 41 (46.1%) |
| P value | – | <0.001 | 0.003 | |
| p16 IHC (%) | 9 (36%) | 42 (100%) | 18 (81.8%) | 69 (77.5%) |
| P value | – | <0.001 | 0.002 |
K SCC Keratinizing squamous cell carcinoma, NK SCC nonkeratinizing squamous cell carcinoma, Hybrid SCC hybrid squamous cell carcinoma, HR HPV-ISH high-risk human papilloma virus—in situ hybridization, IHC immunohistochemistry
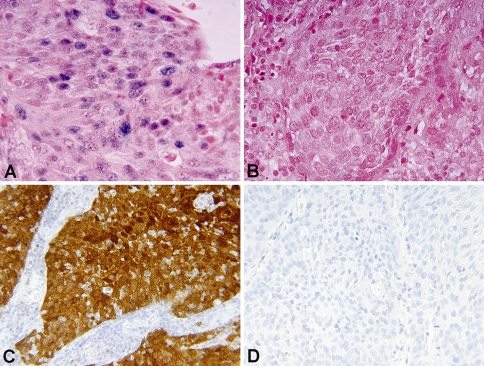
Fig. 4.
Examples of HR HPV-ISH and p16 immunohistochemistry results. a HR HPV positive (blue nuclear dots) tumor by ISH (×600). b HR HPV negative tumor by ISH (×600). c p16 positive tumor by immunohistochemistry showing strong nuclear and cytoplasmic staining (×400). d p16 negative tumor by immunohistochemistry (×400)
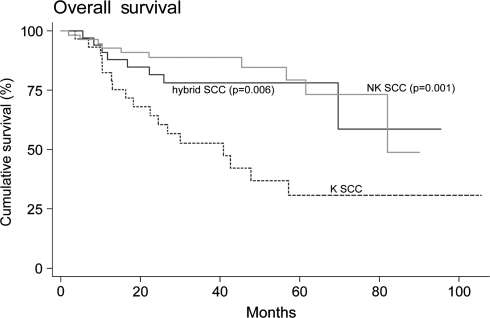
In all patients, overall survival was 69.3% (61/88) at 3 years. Overall survival was significantly better for patients with tumors classified as NK SCC or hybrid SCC than K SCC (log-rank test for equality of survivor functions, P = 0.0002; P = 0.0105, respectively). Specifically, 83.3% (30/36) of patients with NK SCC and 73.1% (19/26) of patients with hybrid SCC tumors were alive at 3 years compared to 46.2% (12/26) of patients with K SCC tumors. When survival analysis was then adjusted for age of the patients and for the type of primary therapy, surgery or definitive radiation, the overall survival of NK and hybrid SCC compared to K SCC remained significantly better (Cox-proportional hazards regression analysis, P = 0.001; P = 0.006, respectively; Fig. 5).
Fig. 5.
Kaplan–Meier curve of overall survival in patients by histologic type (Cox-proportional hazards regression analysis). P values are adjusted for age and surgical versus nonsurgical management
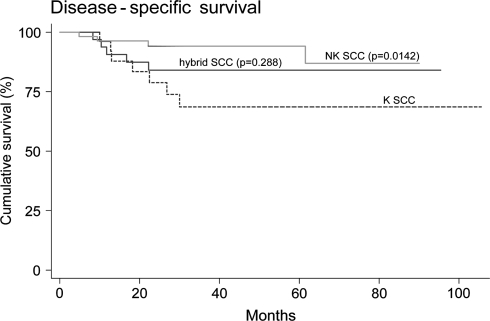
Disease-specific survival was also better for patients with NK SCC compared to K SCC (log-rank test for equality of survivor functions, P = 0.0142; Fig. 6). After adjusting for age of the patients and for the type of primary therapy, surgery or definitive radiation, the disease-specific survival of NK SCC was no longer significantly better than K SCC (Cox-proportional hazards regression analysis, P = 0.106), although there was still a trend. While there were no significant differences in disease-specific survival between hybrid SCC and either K SCC or NK SCC, the disease-specific survival of hybrid SCC appeared to be intermediate (Fig. 6).
Fig. 6.
Kaplan–Meier curve of disease-specific survival in patients by histologic type (log-rank tests for the equality of survivor functions). P values are unadjusted
The possibility that patient comorbidity may have influenced survival was also considered. The Adult Comorbidity Evaluation (ACE-27), a 27-item comorbidity index for use with cancer patients, was used to calculate a comorbidity index for the patients in the study [25]. Comorbidity information was available for all but 7 patients (6 with NK SCC and 1 with K SCC). However, there were no differences in comorbidity scores between the histologic types (Fisher’s exact test).
Finally, we examined survival within the NK SCC group. Since all NK SCC cases were p16 positive but only 69% were HR HPV positive, we evaluated whether or not there was a significant survival difference between those that were HR HPV positive and those that were negative. We found no difference in either overall or disease-specific survival (log-rank test for equality of survivor functions, P = 0.57 and P = 0.88, respectively), although the number of patients in each group was small: 29 HPV-negative NK SCC and 13 HPV-negative NK SCC.
Discussion
HPV infection occurs much more frequently in oropharyngeal SCC than in SCC of other head and neck sites. A meta-analysis of HPV in tonsillar carcinomas by Syrjanen et al. [26] showed a positivity rate of 51% at this site. This is in contrast to other head and neck sites where the HPV prevalence has been reported to average 20–25% [27]. Evidence suggests that HPV is not a bystander in the oropharynx but in fact drives tumor development there [5]. Furthermore, recent studies strongly suggest that HPV-driven oropharyngeal SCC is a distinct molecular, pathologic, and clinical disease entity [1–9, 14, 20, 21, 28]. This is not nearly as clear for HPV-positive SCC from other head and neck sites such as the larynx, hypopharynx, and oral cavity, where HPV may be present but is not necessarily promoting tumor development or “driving” its progression. In nonkeratinizing SCC of the nasal cavity and paranasal sinuses, however, HPV is an important factor [29].
HPV-positive and negative SCCs of the head and neck appear to have different molecular origins. HPV-negative SCCs are characterized by gross chromosomal deletions, while HPV-positive tumors show only occasional chromosomal loss [17]. This observation provides circumstantial evidence for the etiologic role of HPV in virus-harboring tumors, since tumors driven by viral infection and virus-induced signaling pathways theoretically do not require as many additional mutations in order for malignant transformation to occur.
HPV-positive SCC is also characterized by a distinct molecular profile. Specifically, HPV-positive SCC typically overexpresses the tumor suppressor p16, lacks p53 mutations, and is less likely to show EGFR amplification [5, 18, 19, 23, 30]. In particular, the overexpression of p16 has emerged as an important biomarker of HPV positivity in SCC. Detection of p16 expression by immunohistochemistry approaches 100% in HPV-positive oropharyngeal carcinomas in some studies [5, 18]. While other mechanisms may coexist, it is thought that HPV oncoprotein E7-mediated inactivation of phosphorylated retinoblastoma protein leads to a loss of inhibition of p16 transcription with its subsequent overexpression. p16 overexpression is also observed in HPV-related cervical SCC [31]. In contrast, p16 is usually inactivated in HPV-negative SCC of the head and neck, leading to minimal, if any, detectable protein by immunohistochemistry [5, 18].
Many authors have also noted that HPV-positive oropharyngeal carcinomas have distinct histologic features. Although the molecular mechanism for the divergent growth pattern is unknown [2, 20, 21, 28], they are likely related to the viral oncogenic process. The histologic features have been variably described as “poorly differentiated” or “basaloid” [2, 32]. However, the use of these terms may be misleading, as HPV-negative SCCs may be poorly differentiated and basaloid SCC is a defined subtype that is morphologically distinct and, in contrast to HPV-positive SCC, is associated with an aggressive clinical course [24, 33]. We therefore prefer the term nonkeratinizing squamous cell carcinoma to describe HPV-related squamous cell carcinoma of the oropharynx.
El-Mofty et al. [20, 21] previously described the specific histologic features that characterize HPV-positive oropharyngeal SCCs, noting most to be nonkeratinizing tumors composed of monomorphic, ovoid and spindle-shaped hyperchromatic cells with indistinct cell borders forming sheets, nests and cords and demonstrating frequent mitoses, apoptosis and comedo-type necrosis. Oropharyngeal SCC with the described features was strongly correlated with p16 expression and the presence of HR HPV DNA. In a subsequent study, El-Mofty et al. [22] showed that the nonkeratinizing histology in neck lymph node metastases is associated both with HPV positivity by in situ hybridization and very strongly with the primary site of origin being in the oropharynx. Furthermore, a hybrid histologic group was described which showed some squamous maturation that also appeared to be HPV-related [22].
We have currently used the same histologic features to classify SCC of the oropharynx and confirmed the strong HPV-association of the nonkeratinizing type both by ISH for HR HPV subtypes and by p16 immunohistochemistry. Specifically, we classified tumors into three groups: nonkeratinizing squamous cell carcinoma, keratinizing squamous cell carcinoma, and those with overlapping features (hybrid squamous cell carcinoma). In our study, NK SCC was strongly associated with HR HPV and p16 positivity (69 and 100%, respectively) compared to K SCC (8 and 36%, respectively). Hybrid SCC was an intermediate group with 45.5% HR HPV and 81.8% p16 positivity. The discrepancy between HR HPV positivity by ISH and p16 positivity by immunohistochemistry may be due to imperfect sensitivity of HR HPV-ISH or, alternatively, viral shedding at later stages of tumor progression.
It is widely accepted that HPV-positive SCC of the head and neck and, in particular, of the oropharynx is associated with better overall and disease-specific patient survival than HPV-negative SCC [1, 2, 4, 6, 7, 9]. Better outcome has been demonstrated both in patients treated with surgery or with radiation therapy [6, 7, 34]. However, clear characterization of the histologic features is not widely known, and an association between SCC histology alone and patient outcome has not been previously demonstrated.
In the present study, we have shown that histologic features can be used to classify oropharyngeal carcinomas into outcome related groups. NK SCC was associated with better overall and disease-specific survival compared to K SCC. While hybrid SCC was also associated with better overall survival compared to K SCC, the disease-specific survival appeared to be intermediate. When survival analysis was adjusted for age and type of primary management, the differences in overall survival remained. However, the difference in disease-specific survival between NK and K SCC was no longer significant, although there was still a trend (P = 0.106). This may be in part due to the low number of disease-specific deaths in any of the histologic groups. Seven of 22 patients with K SCC versus 4 of 51 patients with NK SCC died of disease. A prospective study including a larger sample size may resolve this finding. It is important to note that the survival advantage of NK SCC and hybrid SCC can not be attributed to better overall health of these patients since significant co-morbidity differences were not detected among the groups.
In addition, within the NK SCC group, we found no difference in survival between those that are HR HPV-positive and those that are negative. While this finding will need confirmation in a larger cohort of patients, it may reflect the imperfect sensitivity of HR HPV-ISH or shedding of virus at later stages of tumor progression. It also supports the concept that NK SCC histology, which is associated with p16 positivity in 100% of cases, may be more important in prediction of outcome than actual HPV status.
In summary, we have shown that histologic classification of squamous cell carcinoma of the oropharynx is clinically useful. It identifies a group of tumors (NK SCC) for which HPV-association is essentially implied and which have better patient survival. Hybrid and K SCC histology indicate sequentially lesser frequencies of HPV positivity. It is likely that hybrid SCC also represents an intermediate prognostic group between NK and K SCC.
The proposed classification of oropharyngeal carcinomas into nonkeratinizing, hybrid, and keratinizing squamous cell carcinoma parallels the current World Health Organization classification of nasopharyngeal carcinomas into nonkeratinizing and keratinizing types with the former being strongly associated with Epstein-Barr virus [35]. We believe that, as in the case of nasopharyngeal carcinomas, the diagnosis of virally related oropharyngeal carcinomas can be based, to a large extent, on their morphologic features with adjuvant HPV-ISH and p16 immunostaining in selected cases, specifically those with hybrid or keratinizing morphology. For tumors with NK SCC morphology, adjuvant testing is not necessary as 100% are p16 positive and HR HPV-ISH status does not appear to further stratify tumor behavior. It is hoped that increased familiarity with the histologic features of oropharyngeal carcinomas may lead to the use of simple light microscopic examination as an integral part of the algorithm for the detection of HPV-related carcinomas.
Acknowledgments
We thank Rodney Brown and Kevin Keith for their expert technical assistance with immunohistochemistry and in situ hybridization experiments. We also thank Jean Zhang for assistance with statistical analysis.
References
- 1.Dahlgren L, Dahlstrand H, Lindquist D, et al. Human papilloma virus is more common in base of tongue than in mobile tongue cancer and is a favorable prognostic factor in base of tongue cancer patients. Int J Cancer. 2004;112:1015–1019. doi: 10.1002/ijc.20490. [DOI] [PubMed] [Google Scholar]
- 2.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 200;92(9):709–720. [DOI] [PubMed]
- 3.Hammarstedt L, Lindquist D, Dahlstrand H, et al. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;119:2620–2623. doi: 10.1002/ijc.22177. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman M, Gorogh T, Gottschlich S, et al. Human papillomavirus in head and neck cancer: 8 year-survival-analysis of 73 patients. Cancer Lett. 2005;218:199–206. doi: 10.1016/j.canlet.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 5.Kim SH, Koo BS, Kang S, et al. HPV integration begins in the tonsillar crypt and leads to the alteration of p16, EGFR and c-myc during tumor formation. Int J Cancer. 2007;120:1418–1425. doi: 10.1002/ijc.22464. [DOI] [PubMed] [Google Scholar]
- 6.Licitra L, Perrone F, Bossi P, et al. High-risk human papillomavirus affects prognosis in patients with surgically treated squamous cell carcinoma. J Clin Oncol. 2006;24(36):5630–5636. doi: 10.1200/JCO.2005.04.6136. [DOI] [PubMed] [Google Scholar]
- 7.Mellin H, Friesland S, Lewensohn R, et al. Human papillomavirus (HPV) DNA in tonsillar cancer: clinical correlates, risk of relapse, and survival. Int J Cancer. 2000;89:300–304. doi: 10.1002/1097-0215(20000520)89:3<300::AID-IJC14>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 8.Mork J, Lie AK, Glattre E, et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344(15):1125–1131. doi: 10.1056/NEJM200104123441503. [DOI] [PubMed] [Google Scholar]
- 9.Ritchie JM, Smith EM, Summersgill KF, et al. Human papillomavirus infection as a prognostic factor in carcinomas of the oral cavity and oropharynx. Int J Cancer. 2003;104:336–344. doi: 10.1002/ijc.10960. [DOI] [PubMed] [Google Scholar]
- 10.Paz IB, Cook N, Odom-Maryon T, et al. Human papillomavirus in head and neck cancer: an association of HPV 16 with squamous cell carcinoma of Waldeyer’s tonsillar ring. Cancer. 1997;79:595–604. doi: 10.1002/(SICI)1097-0142(19970201)79:3<595::AID-CNCR24>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 11.Gillison ML, D’Souza G, Westra WH. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 12.Reeves WC, Brinton LA, Garcia M, et al. Human papillomavirus infection and cervical cancer in Latin America. N Engl J Med. 1989;320(22):1437–1441. doi: 10.1056/NEJM198906013202201. [DOI] [PubMed] [Google Scholar]
- 13.Lenselink CH, Melchers WJ, Quint WG, et al. Sexual behavior and HPV infections in 18 to 29 year old women in the pre-vaccine era in The Netherlands. PLoS ONE. 2008;3(11):e3743. doi: 10.1371/journal.pone.0003743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Souza G, Kreimer AR, Viscidi R, et al. Case–control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 15.Applebaum KM, Furniss CS, Zeka A, et al. Lack of association of alcohol and tobacco with HPV16-associated head and neck cancer. J Natl Cancer Inst. 2007;99:1801–1810. doi: 10.1093/jnci/djm233. [DOI] [PubMed] [Google Scholar]
- 16.Maier H, Dietz A, Gewelke U, et al. Tobacco and alcohol and the risk of head and neck cancer. Clin Investig. 1992;70(3–4):320–327. doi: 10.1007/BF00184668. [DOI] [PubMed] [Google Scholar]
- 17.Boudewijn JM, Braakhuis BJM, Snijders PJF, et al. Genetic patterns in head and neck cancers that contain or lack transcriptionally active human papillomavirus. J Natl Cancer Inst. 2004;96:998–1006. doi: 10.1093/jnci/djh183. [DOI] [PubMed] [Google Scholar]
- 18.Klussmann JP, Gultekin E, Weissenborn SJ, et al. Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am J Pathol. 2003;162(3):747–753. doi: 10.1016/S0002-9440(10)63871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus—associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24:736–747. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 20.El-Mofty SK, Lu DW. Prevalence of human papillomavirus type 16 DNA in squamous cell carcinoma of the palatine tonsil, and not the oral cavity, in young patients: a distinct clinicopathologic and molecular disease entity. Am J Surg Pathol. 2003;27(11):1463–1470. doi: 10.1097/00000478-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 21.El-Mofty SK, Patil S. Human Papillomavirus (HPV)-related oropharyngeal nonkeratinizing squamous cell carcinoma: characterization of a distinct phenotype. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:339–345. doi: 10.1016/j.tripleo.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 22.El-Mofty SK, Zhang MQ, Davila RM. Histologic identification of Human Papillomavirus (HPV)-related squamous cell carcinoma in cervical lymph nodes: a reliable predictor of the site of an occult head and neck primary carcinoma. Head Neck Pathol. 2008;2:163–168. doi: 10.1007/s12105-008-0066-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strati K, Pitot HC, Lambert PF. Identification of biomarkers that distinguish human papillomavirus (HPV)-positive versus HPV-negative head and neck cancers in a mouse model. 38. PNAS. 2006;103:14152–14157. doi: 10.1073/pnas.0606698103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wain SL, Kier R, Vollmer RT, et al. Basaloid-squamous carcinoma of the tongue, hypopharynx and larynx: report of 10 cases. Hum Pathol. 1986;17(11):1158–1166. doi: 10.1016/S0046-8177(86)80422-1. [DOI] [PubMed] [Google Scholar]
- 25.Piccirillo J, Tierney RM, Costas I, et al. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291(20):2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 26.Syrjanen S. HPV infections and tonsillar carcinoma. J Clin Pathol. 2004;57:449–455. doi: 10.1136/jcp.2003.008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kreimer AR, Clifford GM, Boyle P, et al. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14(2):467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 28.Wilczynski SP, Lin BTY, Xie Y, et al. Detection of human papillomavirus DNA and oncoprotein overexpression are associated with distinct morphological patterns of tonsillar squamous cell carcinoma. Am J Surg Pathol. 1998;152(1):145–156. [PMC free article] [PubMed] [Google Scholar]
- 29.El-Mofty SK, Lu DW. Prevalence of high-risk human papillomavirus DNA in nonkeratinizing (cylindrical cell) carcinoma of the sinonasal tract: a distinct clinicopathologic and molecular disease entity. Am J Surg Pathol. 2005;29(10):1367–1372. doi: 10.1097/01.pas.0000173240.63073.fe. [DOI] [PubMed] [Google Scholar]
- 30.Begum S, Cao D, Gillison M, et al. Tissue distribution of human papillomavirus 16 DNA integration in patients with tonsillar carcinoma. Clin Cancer Res. 2005;11(16):5694–5699. doi: 10.1158/1078-0432.CCR-05-0587. [DOI] [PubMed] [Google Scholar]
- 31.Sano T, Oyama T, Kashiwabara K, et al. Expression status of p16 protein is associated with human papillomavirus oncogenic potential in cervical and genital lesions. Am J Pathol. 1998;153(6):1741–1748. doi: 10.1016/S0002-9440(10)65689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dahlstrom KR, Adler-Storthz K, Etzel CJ, et al. Human papillomavirus type 16 infection and squamous cell carcinoma of the head and neck in never-smokers: a matched pair analysis. Clin Cancer Res. 2003;9:2620–2626. [PubMed] [Google Scholar]
- 33.Soriano E, Faure C, Lantuejoul S, et al. Course and prognosis of basaloid squamous cell carcinoma of the head and neck: a case–control study of 62 patients. Eur J Cancer. 2008;44(2):244–250. doi: 10.1016/j.ejca.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Lindel K, Beer KT, Laissue J, et al. Human papillomavirus positive squamous cell carcinoma of the oropharynx: a radiosensitive subgroup of head and neck carcinoma. Cancer. 2001;92:805–813. doi: 10.1002/1097-0142(20010815)92:4<805::AID-CNCR1386>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 35.Chan JKC, Bray F, McCarron P, et al. Nasopharyngeal carcinoma. In: Barnes L, Eveson JW, Reichart PA, Sidransky D, et al., editors. Pathology and genetics of head and neck tumours (world health organization classification of tumours) Lyon, France: International Agency for Research on Cancer; 2005. [Google Scholar]