Abstract
The status of the cervical lymph nodes is the most important prognosticator in head and neck squamous cell carcinoma. The neck dissection is both a therapeutic and staging procedure and has evolved to include various types with standardized level designations (I–VI) for lymph node groups: the radical neck dissection, modified radical neck dissection, the selective neck dissection, and the extended neck dissection. The gross and histologic examination of a neck dissection should provide the critical information (size of metastasis, number of lymph nodes involved) for staging purposes. Additionally, extracapsular spread of lymph node metastasis must be reported because of its significance as an adverse prognosticator. Current dilemmas in nodal disease are the detection of micrometastases, isolated tumor cells, and molecular positivity. The significance of these categories of disease is still unclear, though they may explain a subset of the estimated 10% of the regional recurrences in the neck despite pathologic node negativity by traditional methods of evaluation. Sentinel lymph node biopsy has been recently applied to head and neck squamous cell carcinoma to enhance the management of the clinicoradiographically node negative patients. While still investigational, sentinel lymph node biopsy shows promise in selecting patients who require a neck dissection. Rapid highly automated real-time RT-PCR based platforms will allow for incorporation of molecular findings into the intraoperative evaluation of a sentinel lymph node.
Keywords: Neck dissection, Sentinel node, Micrometastasis, Squamous cell carcinoma, Head and neck, Isolated tumor cells
Introduction
The adverse impact of lymph node involvement in head and neck squamous cell carcinoma (HNSCC), with nodal metastases was known even by 19th century surgeons, and today, the cervical lymph node status remains the most important prognosticator in HNSCC reducing 5 year survival by 50%. Since the first documented neck dissection performed in 1888 by Franciszek Jawdyński and the first methodical description of the radical neck dissection by George Crile in 1906, neck dissection has evolved into a more refined set of procedures that now allow for a greater degree of conservation and reduced morbidity [1]. One of the basic yet perhaps most important contributions of the pathologist in the management of HNSCC patients is a thorough and accurate assessment of the lymph nodes. With the inclusion of immunohistochemical and molecular techniques as part of the pathologist’s arsenal, the ability to detect minimal disease in lymph nodes is better now than ever before. The evolution of surgical procedures and pathologic assessment of the neck dissection are thus by necessity linked and driven by the oncologic objective of regional therapeutic control and prognostication.
Discussion
Anatomic and Macroscopic Examination
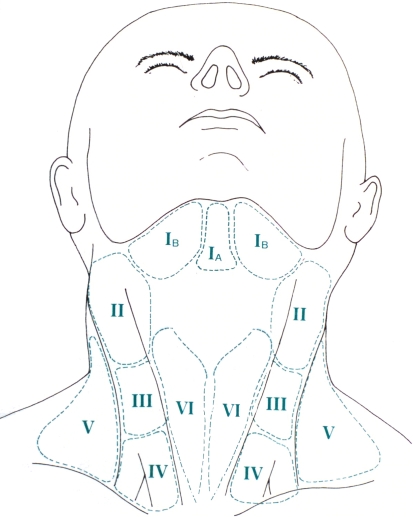
Over the past decade, the nomenclature and description of neck dissections has not changed, and if anything has become more simplified and standardized. According to the revised neck dissection classification proposed by the American Head and Neck Society and the American Academy of Otolaryngology–Head and Neck Surgery [2], the lymph nodes of the neck are now currently divided into six levels (I–VI) (Fig. 1; Table 1). Not included in this classification are lymph nodes of the superior mediastinum (often referred to as level VII). Lymph nodes outside the neck groupings are designated by their specific group (i.e. retropharyngeal, periparotid, buccinator).
Fig. 1.
Level designations of lymph node groups in the neck
Table 1.
Lymph node groups in a neck dissection [2]
| Level | Superior border | Inferior border | Anteromedial border | Posterolateral border |
|---|---|---|---|---|
| IA (submental) | Symphysis of mandible | Body of hyoid | Anterior belly of contralateral digastric muscle | Anterior belly of ipsilateral digastric muscle |
| IB (submandibular) | Body of mandible | Posterior belly of digastric muscle | Anterior belly of digastric muscle | Stylohyoid muscle |
| IIA (upper jugular) | Skull base | Horizontal plane defined by the inferior body of the hyoid bone | Stylohyoid muscle | Vertical plane defined by the spinal accessory nerve |
| IIB (upper jugular) | Skull base | Horizontal plane defined by the inferior body of the hyoid bone | Vertical plane defined by the spinal accessory nerve | Lateral border of the sternocleidomastoid muscle |
| III (middle jugular) | Horizontal plane defined by the inferior body of the hyoid bone | Horizontal plane defined by the inferior border of the cricoid cartilage | Lateral border of the sternohyoid muscle | Lateral border of the sternocleidomastoid muscle |
| IV (lower jugular) | Horizontal plane defined by the inferior border of the cricoid cartilage | Clavicle | Lateral border of the sternohyoid muscle | Lateral border of the sternocleidomastoid muscle |
| VA (posterior triangle) | Apex of convergence of the sternocleidomastoid and trapezius muscle | Horizontal plane defined by the inferior border of the cricoid cartilage | Posterior border of the sternocleidomastoid muscle | Anterior border of the trapezius muscle |
| VB (posterior triangle) | Horizontal plane defined by the inferior border of the cricoid cartilage | Clavicle | Posterior border of the sternocleidomastoid muscle | Anterior border of the trapezius muscle |
| VI (anterior compartment) | Hyoid bone | Suprasternal | Common carotid artery | Common carotid artery |
In addition, levels I, II, and V are often further subdivided into A and B since this subdivision may be biologically important. For instance, oropharyngeal squamous cell carcinoma (SCC) has a higher propensity for metastasis to level IIB than oral or laryngeal SCC. Thus, level IIB may not be dissected in oral and laryngeal SCC unless there is clinically evident disease in this region. Additionally, dissection of level IIB does have some morbidity, namely an increased likelihood of spinal accessory nerve injury. Similarly, level 1A may not be removed unless the SCC involves the lip, floor of mouth or anterior skin of face [2].
The types of neck dissection are classified as follows: Radical neck dissection: levels I–V with associated sternocleidomastoid muscle, jugular vein and spinal accessory nerve; Modified radical neck dissection: levels I–V without one or more of the aforementioned non-lymphatic structures; Selective neck dissection: any dissection without one or more lymph node levels seen in a radical neck dissection (i.e. levels II–IV); and an extended neck dissection which includes one or more additional lymph node groups or non lymphatic structures in addition to those of a radical neck (i.e. periparotid lymph nodes and parotidectomy, superior mediastinal and level VI). The major therapeutic advance in the past two decades is the refinement of the various selective neck dissections to achieve oncologic control and minimize morbidity. Selective dissections, as alluded to above, can be tailored to some extent since there is now an awareness of the pattern of spread for each head and neck site. Table 2 summarizes the lymph node levels likely to be involved (and thus included in a selective dissection) based on site.
Table 2.
Common drainage patterns for tumors of various head and neck sites [2]
| Site | Drainage pattern |
|---|---|
| Oral cavity | Levels I–III (sometimes IV) |
| Oropharynx, hypopharynx, larynx | Levels II–IV (IIA only for some SCC of larynx and hypopharynx) |
| Larynx with subglottic involvement | Levels IV–VI |
| Thyroid | Level VI (level II–V if level V is clinically +) |
Gross orientation of neck dissections received in the pathology laboratory may be somewhat challenging since the anatomic landmarks for the division of levels are lost in most situations. Thus, the surgeon should divide each group and submit them to the pathology laboratory separately for optimal delineation of the lymph node levels. However, often, neck dissections are sent en bloc to the gross laboratory. Here, in the case of a radical neck dissection supero-inferior orientation is possible since the submandibular gland will denote level I, and superficial-deep orientation is possible since the sternocleidomastoid muscle is superficial, while the internal jugular vein is deep. Orientation becomes even more challenging with selective dissections that do not contain level I. Methods to provide orientation include submitting the neck dissection on a laminated template delineating each level, or using a suture to denote one end [3]. However, when these are not provided, orientation becomes essentially impossible, and even the surgeon may not be able to recreate the orientation after the specimen has been removed. If there is a clinically positive node on review of the history that is radiographically designated to be in a particular lymph node level (i.e. there is a 2.0 cm PET positive lymph node in level IV), this can serve as a landmark on the specimen. Additional elements, though not completely reliable, may also facilitate orientation of the neck. While the surface of the neck dissection on which the sternocleidomastoid rests is often smooth, the posteroinferior portion of a neck dissection that normally corresponds to the supraclavicular fossa typically consists of more loose, delicate fibroadipose tissue which can be useful in indicating level IV and VB. Additionally, anthracotic lymph nodes, when present in the neck, tend to be in the lower levels. Finally, accessory parotid tissue or salivary inclusions are more common in level II and can serve as yet another useful landmark.
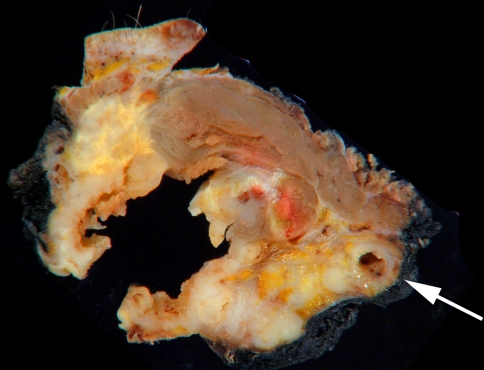
Lymph node or ‘N’ staging according to the AJCC 6th edition TNM staging manual is summarized in Table 3; [4]. Thus, in order to ensure accuracy, on gross examination, it is important to document the size, number and level location of the largest grossly suspicious lymph nodes and also the size range of all lymph nodes. All grossly negative lymph nodes should be entirely submitted—lymph nodes that are less than 0.4 cm thick can be simply placed into cassettes, while larger lymph nodes should be bisected or serially sectioned and submitted. Only one bisected lymph node should be placed in a cassette, since placing more can lead to confusion as to which halves belong to each other. For grossly positive nodes, one cross section with a thin rim of surrounding soft tissue is sufficient. When large mass potentially representing multiple matted lymph nodes is encountered, the specific ‘count’ is of minimal significance. By convention, it is often typically considered one ‘conglomerate’ nodal metastasis and only 2–3 sections are necessary. It is also important to indicate extranodal structures involved by a metastasis if present. At several institutions even margination of the neck dissection (i.e. internal jugular vein and deep soft tissue margins) are necessary to plan three dimensional conformal radiotherapy to the neck (Fig. 2) [5]. A neck dissection consisting of five levels yields about 31–55 lymph nodes on average depending on prosection skill and technique as well as the surgeons proficiency in removing the nodes [6, 7]. One method that includes total submission of a neck dissection is actually quite labor intensive, yielding up to 63 slides per neck dissection, though it may potentially increase the detection rate of micrometastases [8]. A variation on this theme includes using a fat dissolving fixative to minimize the number of cassettes required for total lymph node submission.
Table 3.
AJCC UICC N staging of HNSCC [4]
| NX | Cannot be assessed |
| N0 | Negative |
| All sites except nasopharynx | |
| N1 | Single ipsilateral metastasis 3 cm or less |
| N2a | Single ipsilateral metastasis greater than 3 cm, less than or equal to 6 cm |
| N2b | Multiple ipsilateral lymph nodes, less than or equal to 6 cm |
| N2c | Metastasis to bilateral or contralateral lymph nodes, less than or equal to 6 cm |
| N3 | Metastasis to a lymph node greater than 6 cm |
| Nasopharynx | |
| N1 | Unilateral metastases less than or equal to 6 cm |
| N2 | Bilateral metastases less than or equal to 6 cm |
| N3a | Metastasis to a lymph node greater than 6 cm |
| N3b | Extension into supraclavicular fossa (i.e. level IV and VB) |
Fig. 2.
Gross image of a cross section of matted nodes involving extranodal structures including the internal jugular vein (arrow). At many institutions the inked margin of neck dissection is relevant to help plan/map three dimensional conformal radiotherapy
Routine Microscopic Examination
Aside from its minor contribution to N staging, the number of positive lymph nodes is an independent adverse prognosticator [9]. A lymph node is specifically defined as encapsulated lymphoid tissue with a peripheral sinus; positive lymph nodes should be distinguished from tumor deposits with associated unencapsulated lymphoid aggregates. For assigning N stage, the size of the metastasis rather than the lymph node overall is what is important. For larger lymph nodes, generally these are equivalent. However, occasionally, a lymph node with concomitant lymphoma may be markedly enlarged over 3 cm but may only harbor a metastasis that is a few millimeters in size. Here size distinction becomes important as it is the difference between pN1 and pN2a.
Among the most important adverse prognosticators is extracapsular spread of a lymph node metastasis. Unlike other organ sites, there is insufficient evidence to support measuring the actual extent beyond the capsule. In fact, the few studies on this subject suggest that microscopic and macroscopic extent have the same adverse prognostic impact [10]. As suggested above, occasionally, tumor deposits may be present (~10% of all neck dissections) with or without associated lymphoid tissue. There are currently no guidelines for the reporting and classification of these deposits with respect to the TNM classification. Evidence suggests, however, that they are prognostically equivalent to extracapsular spread, and in fact, many of the nodular deposits may indeed be completely effaced lymph nodes [11]. Our practice is to count nodular deposits as completely effaced lymph nodes with extracapsular spread, and irregular deposits as soft tissue deposits that are counted separately—with a comment indicating that they should be considered the prognostic equivalent of extracapsular spread.
With the increasing use of adjuvant radiotherapy and monitoring using PET scan, neck dissection may be performed on patients with previously irradiated necks. This poses a unique problem in the detection of metastatic disease—the treated metastasis. Often the tumor in an irradiated lymph node may be effaced by extensive fibrosis with some or no entrapped tumor cells. There are no strict guidelines as to how extensive a search for residual tumor cells is necessary as the standard of care. Additionally, in this context, the significance of isolated tumor cells (see below) is unclear. Our recommendation is to generously sample a grossly evident treated node and, if possible, entirely submit it for histologic evaluation (feasible under 3.0 cm). Histologically, if there are no suspicious areas, we do not feel additional immunohistochemical staining will be useful and in fact, may be misleading due to occasional spurious reactivity of myofibroblasts with cytokeratin. Suspicious areas can of course be immunostained for confirmation of tumor. As far as reporting the nodal status, the AJCC staging manual allows for the prefix “y” to precede the staging in cases treated with chemoradiation. Thus a treated fibrotic lymph node metastasis can be considered “y”pN0. Our preference is to in some way acknowledge in the report that this fibrotic lymph node likely represents a treated metastasis.
Incidental findings in lymph nodes that may cause diagnostic challenges or invoke further patient workup include salivary inclusions, thyroid inclusions/micrometastasis from papillary thyroid carcinoma, occult lymphoma, intranodal melanocytic nevi, or granulomatous disease. These may be present in up to 5% of neck dissections and should not be overlooked.
Micrometastases
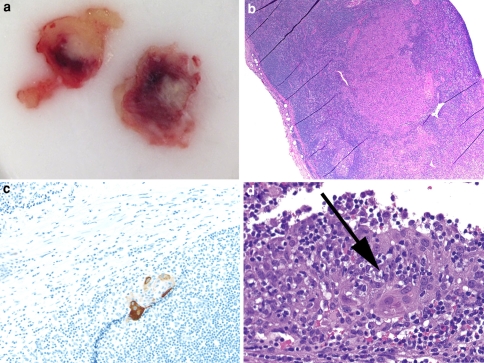
As in other organ sites, the concepts of micrometastases and isolated tumor cells are emerging for HNSCC. Up to 10% of pathologically node negative (pN0) cases will have a regional recurrence in the neck [12]. Potential explanations include a surgical failure to excise all lymph nodes, non lymphatic spread (i.e. venous) to the neck soft tissue, and failure to detect occult disease by routine gross examination and light microscopy. With respect to the latter, the identification of micrometastases is of interest. In order to standardize the definitions of these terms, it is suggested that the same definition already in use for breast carcinomas should be applied to HNSCC, namely, micrometastases are tumor metastases that are between 0.2 mm and 2.0 mm in greatest dimension while isolated tumor cells are groups of tumor cells that are less than 0.2 mm in greatest dimension (Fig. 3); [13].
Fig. 3.
a OCT embedded cut surface of a micrometastasis in 3 mm lymph node. SCC notoriously illicits a desmoplastic stromal reaction that allows for gross detection in many cases, especially if a lymph node is sliced thinly at ~1 mm as shown here. b H&E demonstrating a micrometastasis (40×). c ITC in a lymph node detected by a cytokeratin immunostain (100×). d On close examination in that area, these cells are evident on the corresponding H&E as well (400×)
Generally, SCC metastases to lymph nodes tend to ‘telegraph’ their presence because of desmoplasia or keratin debris, thus even micrometastases can be detected by standard light microscopy more readily perhaps than other tumor types. Nonetheless, the literature suggests that the use of serial sectioning and immunohistochemical stains for cytokeratins potentially increase the detection rate of micrometastasis by 15% [14] in a pN0 case by standard light microscopy. Despite the improved ability to detect micrometastatic disease, it is still unclear whether the subgroups of micrometastases and isolated tumor cells will be clinically relevant.
Molecular Approach
Molecular PCR based techniques have recently garnered interest as an even more sensitive technique to detect minimal nodal involvement. The majority of techniques are reverse transcriptase PCR (RT-PCR) based and evaluate lymph nodes for mRNA transcript. As such, in order to ensure adequate RNA quality, parts of lymph nodes evaluated in this fashion are required to be frozen. The two basic classes of markers that have been used in this fashion include the transcripts for proteins that are markers of squamous differentiation (i.e. CK5, CK14, CK19 mRNA) and markers that are known to be differentially expressed between HNSCC and normal irrespective of their function (i.e. E48, SCCA, PVA, PTHrP) [15–17]. While initial studies evaluated simply the presence or absence of transcript [16, 17] as denoting positivity, with the introduction of quantitative RT-PCR [12, 15] validated cutoff levels discriminating between positive and negative results can be generated. Using these approaches, ‘molecular positive’ lymph nodes can be seen in as many as 20–30% of cases that are negative by light microscopy and immunohistochemistry [12, 14–17]. A small fraction of these cases have been validated by further serial sectioning to detect micrometastases or ITC previously not identified by initial H&E.
However, one unresolved issue is the specificity of a ‘positive’ molecular result. A disadvantage to PCR based methodology is the loss of morphologic correlation. The concern is that a false positive result may arise from various benign epithelial inclusions. Additionally, whether the presence of transcript is indicative of tumorigenic metastatic disease rather than dead or dying tumor cells in circulation is unclear. Two approaches that ameliorate these issue are quantitative PCR, as mentioned above, which can establish a threshold for defining positivity, and a multiplex approach utilizing more than one marker to define a positive lymph node [15]. Both approaches require a statistically sound validation using an adequately powered set of positive and negative lymph nodes. However, in the end, a positive result with a set of markers rather than an individual marker is less likely to be a spurious result whether from a benign inclusion or non-viable tumor cells.
The applicability of a molecular approach is obviously not feasible on all lymph nodes in a neck dissection just as serial sectioning and immunohistochemical staining of all lymph nodes in a neck dissection is impractical. Thus the utility of these extended approaches would be in select cases in which a patient undergoes a sentinel node biopsy (see below).
Sentinel Nodes
Sentinel lymph node biopsy involves the use of 99Tcm-labeled colloid radiotracer and/or dye to initial draining lymph node—the assumption is that metastases, if present, will be seen in this sentinel node, and that negativity in this node is a strong predictor of negativity in the neck overall. Sentinel node biopsy has become standard of care for breast carcinomas and cutaneous melanomas. The potential application of sentinel node biopsy (SLNB) to the neck in HNSCC was first documented in 1996. The current standard of care for a clinically (c) N0 patient is to offer elective neck dissection since current clinical and radiographic techniques may not accurately predict lymph node status. However, neck dissection has considerable mortatility and since only 25–30% of clinically node negative patients are actually pathologically node positive, up to three quarters of these patients are overtreated [15]. However, the repercussions of undertreatment are severe; cases that appear clinically negative but present later as bulky neck disease have increased morbidity and mortality. Thus SLNB as a more accurate predictor of nodal status is an appealing concept.
On a smaller scale, sentinel node biopsy has been applied to mucosal head and neck squamous cell carcinoma. Despite the increasing number of prospective observational studies over the past 5 years, SLNB remains an investigational procedure. Nonetheless, it is currently used in lieu of elective neck dissection in select cases at several institutions. The recommended indications for SLNB as proposed by ‘The Second International Conference on Sentinel Node Biopsy in Mucosal Head and Neck Cancer’ [18] are as follows: (1) staging of the ipsilateral neck in unilateral cT1/2 cN0 tumors, (2) staging of the ipsilateral and contralateral neck in midline tumors or tumors crossing the midline (cT1/2 cN0), and (3) staging of the contralateral neck in midline tumors or tumors crossing the midline (cT1/2 cN+; ipsilateral). Here it is shown to be quite sensitive at 97% on average (range: 96–100%); however, there is still a small false negative rate (average 4%, range: 0–12%) [18–20]. Potential explanations for this are skip metastases and ‘masking’ of a sentinel node by adjacent radiotracer uptake from carcinomas of the floor of mouth particularly in level I. The latter issue has been somewhat ameliorated by the utilization of three dimentional SPECT/CT [21]. Sentinel lymph node biopsy is also of limited value in cT3/4 patients since these tumors often drain to multiple lymph node basins, making it difficult to pinpoint the sentinel node(s).
The advantage of SLNB is a reduction in the number of lymph nodes to be examined histologically in patients for whom this is performed—on average, only about two sentinel nodes per patient are procured by the surgeon. This decreased workload allows for a more careful examination of the sentinel nodes which is required in order to maximize the performance characteristics of SLNB. The recommended standard for gross processing of lymph nodes is that they should be serially section along the long axis at 2.5 mm intervals regardless of size to maximize the surface area available for examination. In fact, at our institution we serially section these nodes at 1 mm intervals, if possible. For histologic examination, the recommendations from ‘The Second International Conference on Sentinel Node Biopsy in Mucosal Head and Neck Cancer’ are that the lymph nodes should be step sectioned in their entirety at 150 μm. Each level will consist of four sections with the first stained by H&E, the second immunostained by a cytokeratin, and the third and fourth retained as blanks for additional potential studies. In our practice (and likely many other practices in the US), there is admittedly resistance to this extensive protocol for lymph node evaluation since it is still quite labor intensive, is not perceived as ‘the standard of care,’ and the detection of ITC or micrometastases is still of unclear significance. In our practice, we utilize a modification of this procedure where we perform 2–3 iterations of this step sectioning, but we do not exhaust the block.
One major issue is the utility of intraoperative assessment of sentinel nodes. Node positivity in the intraoperative setting would allow the surgeon to complete the neck dissection in one operation rather than two. However, the sensitivity of frozen section analysis even in breast carcinoma and melanoma sentinel nodes may be as low as 30–40%. Experience with frozen section analysis of head and neck squamous cell carcinoma sentinel nodes yields similar results [15, 19]. Reasons for this include:
Technical—since lymph nodes are often fatty, flat sections equivalent to permanent section quality lymph nodes are difficult to obtain
Sampling—standard sentinel lymph node processing protocols often obtain serial levels through the paraffin block to more thoroughly examine sentinel nodes. However, in the intraoperative setting, sectioning through the tissue block is not feasible
Interpretive—frozen sections are more difficult to assess for micrometastasis than permanent sections
Alternatives to frozen section include touch imprint cytology. This technique is quicker and tissue sparing. However, it also may not be sufficiently sensitive, and these imprints are prone to artifacts typical of cytology specimens, such as air drying. Evidence in breast carcinomas suggests that both techniques are comparable from a performance standpoint [22].
Thus to a large extent, it is surgeon preference as to whether or not there will be an intraoperative assessment. The pathologist’s goal in this setting is to provide histologic assessment without compromising the final permanent sections. Thus tissue should NOT be exhausted in the intraoperative setting. As mentioned above, fundamental to all approaches is to maximize the surface area visible for evaluation. Lymph nodes should thus be serially sliced at 1 mm intervals perpendicular to the long axis. Imprints may be made from all these slices simultaneously. Alternatively, these serial sections may be embedded in 1–2 blocks for frozen section analysis. Again tissue conservation is important, and it is recommended that sectioning should extend only to the first complete section with all slices, rather than beyond. Regardless of approach, gross examination of these slices is important since even micrometastases may be visualized as a result of the desmoplastic response (Fig. 3a). In such a case, frozen section of that slice alone, or even scrape cytologic preparation may be adequate to confirm positivity.
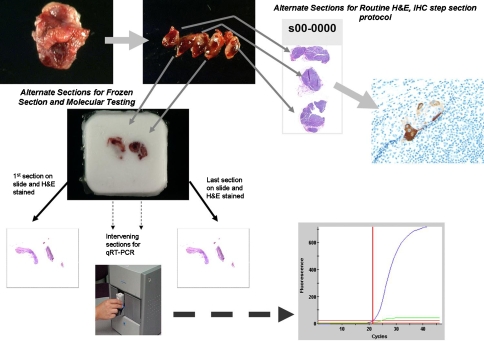
Future analyses may also incorporate molecular methods into the intraoperative setting. Recently, platforms have been developed that can successfully apply a highly automated multiplex panel to a lymph node to perform quantitative analysis within 40 min. One such platform is currently being validated at our and other institutions. Figure 4 depicts a possible protocol for evaluation of a SLN in the intraoperative setting that would incorporate molecular techniques [15].
Fig. 4.
Schematic representation of a future triaging algorithm for sentinel lymph node evaluation. Such a protocol would involve fixing alternate slices and evaluating for micrometastasis on permanent section using a traditional sentinel lymph node protocol consisting of levels and immunostains for cytokeratin. The other slices should be embedded in OCT for molecular analysis. H&E frozen section stains as morphologic quality assurance should be performed followed by submission of sections directly into a nucleic acid fixative to be placed on an rapid automated platform such as the GeneXpert© system depicted here for real-time RT-PCR analysis. In the ideal state, the frozen section and molecular component of this procedure should take ~40 min, which, if positive, allows the surgeon to perform the neck dissection in one procedure rather than reoperating pending the permanent results
Conclusions
For over a century, the cervical lymph node status has been a central focus in HNSCC. Over the past few decades, surgical approach has been refined to yield a variety of neck dissection types with less morbidity. Pathologic assessment of node positivity and extracapsular spread remains among the most important roles of the pathologist in management of HNSCC. The ability to detect micrometastases and ITC by serial section or immunohistochemical stains and the use of RT-PCR based molecular techniques for minimal disease are an ongoing challenge to incorporate into routine pathologic practice, though these methodologies may prove to improve the prognostic accuracy of nodal status. To this end, a standard nomenclature and reporting format should be utilized. Ultimately, the utility of these extended techniques have the most impact on clinically N0 patients for whom the SLNB may eventually become the standard of care.
References
- 1.Rinaldo A, Ferlito A, Silver CE. Early history of neck dissection. Eur Arch Otorhinolaryngol. 2008;265(12):1535–1538. doi: 10.1007/s00405-008-0706-9. [DOI] [PubMed] [Google Scholar]
- 2.Robbins KT, Clayman G, Levine PA, et al. Neck dissection classification update: revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology–Head and Neck Surgery. Arch Otolaryngol Head Neck Surg. 2002;128(7):751–758. doi: 10.1001/archotol.128.7.751. [DOI] [PubMed] [Google Scholar]
- 3.Avery CM, Laugharne D. A laminated template to aid orientation of neck dissection specimens. Br J Oral Maxillofac Surg. 2008;46(6):510–511. doi: 10.1016/j.bjoms.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 4.AJCC Cancer Staging Manual. 6th ed. Springer; 2002.
- 5.Beldi D, Jereczek-Fossa BA, D’Onofrio A, et al. Role of radiotherapy in the treatment of cervical lymph node metastases from an unknown primary site: retrospective analysis of 113 patients. Int J Radiat Oncol Biol Phys. 2007;69(4):1051–1058. doi: 10.1016/j.ijrobp.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 6.Morton RP, Gray L, Tandon DA, et al. Efficacy of neck dissection: are surgical volumes important? Laryngoscope. 2009;119(6):1147–1152. doi: 10.1002/lary.20167. [DOI] [PubMed] [Google Scholar]
- 7.Friedman M, Lim JW, Dickey W, et al. Quantification of lymph nodes in selective neck dissection. Laryngoscope. 1999;109(3):368–370. doi: 10.1097/00005537-199903000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Jose J, Coatesworth AP, MacLennan K. Cervical metastases in upper aerodigestive tract squamous cell carcinoma: histopathologic analysis and reporting. Head Neck. 2003;25(3):194–197. doi: 10.1002/hed.10194. [DOI] [PubMed] [Google Scholar]
- 9.Le Tourneau C, Velten M, Jung GM, et al. Prognostic indicators for survival in head and neck squamous cell carcinomas: analysis of a series of 621 cases. Head Neck. 2005;27(9):801–808. doi: 10.1002/hed.20254. [DOI] [PubMed] [Google Scholar]
- 10.Woolgar JA, Rogers SN, Lowe D, et al. Cervical lymph node metastasis in oral cancer: the importance of even microscopic extracapsular spread. Oral Oncol. 2003;39(2):130–137. doi: 10.1016/S1368-8375(02)00030-1. [DOI] [PubMed] [Google Scholar]
- 11.Jose J, Moor JW, Coatesworth AP, et al. Soft tissue deposits in neck dissections of patients with head and neck squamous cell carcinoma: prospective analysis of prevalence, survival, and its implications. Arch Otolaryngol Head Neck Surg. 2004;130(2):157–160. doi: 10.1001/archotol.130.2.157. [DOI] [PubMed] [Google Scholar]
- 12.Becker MT, Shores CG, Yu KK, et al. Molecular assay to detect metastatic head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130(1):21–27. doi: 10.1001/archotol.130.1.21. [DOI] [PubMed] [Google Scholar]
- 13.Ferlito A, Rinaldo A, Devaney KO, et al. Detection of lymph node micrometastases in patients with squamous carcinoma of the head and neck. Eur Arch Otorhinolaryngol. 2008;265(10):1147–1153. doi: 10.1007/s00405-008-0715-8. [DOI] [PubMed] [Google Scholar]
- 14.Devaney KO, Rinaldo A, Ferlito A. Micrometastases in cervical lymph nodes from patients with squamous carcinoma of the head and neck: should they be actively sought? Maybe. Am J Otolaryngol. 2007;28(4):271–274. doi: 10.1016/j.amjoto.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 15.Ferris RL, Xi L, Raja S, et al. Molecular staging of cervical lymph nodes in squamous cell carcinoma of the head and neck. Cancer Res. 2005;65(6):2147–2156. doi: 10.1158/0008-5472.CAN-04-3717. [DOI] [PubMed] [Google Scholar]
- 16.Hamakawa H, Fukizumi M, Bao Y, et al. Genetic diagnosis of micrometastasis based on SCC antigen mRNA in cervical lymph nodes of head and neck cancer. Clin Exp Metastasis. 1999;17(7):593–599. doi: 10.1023/A:1006732911057. [DOI] [PubMed] [Google Scholar]
- 17.McDonald LA, Walker DM, Gibbins JR. Cervical lymph node involvement in head and neck cancer detectable as expression of a spliced transcript of type II keratin K5. Oral Oncol. 1998;34(4):276–283. [PubMed] [Google Scholar]
- 18.Stoeckli SJ, Pfaltz M, Ross GL, et al. The second international conference on sentinel node biopsy in mucosal head and neck cancer. Ann Surg Oncol. 2005;12(11):919–924. doi: 10.1245/ASO.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 19.Devaney KO, Rinaldo A, Rodrigo JP, et al. Sentinel node biopsy and head and neck tumors-where do we stand today? Head Neck. 2006;28(12):1122–1131. doi: 10.1002/hed.20443. [DOI] [PubMed] [Google Scholar]
- 20.Paleri V, Rees G, Arullendran P, et al. Sentinel node biopsy in squamous cell cancer of the oral cavity and oral pharynx: a diagnostic meta-analysis. Head Neck. 2005;27(9):739–747. doi: 10.1002/hed.20228. [DOI] [PubMed] [Google Scholar]
- 21.Stoeckli SJ, Alkureishi LW, Ross GL. Sentinel node biopsy for early oral and oropharyngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2009;266(6):787–793. doi: 10.1007/s00405-009-0955-2. [DOI] [PubMed] [Google Scholar]
- 22.Vanderveen KA, Ramsamooj R, Bold RJ. A prospective, blinded trial of touch prep analysis versus frozen section for intraoperative evaluation of sentinel lymph nodes in breast cancer. Ann Surg Oncol. 2008;15(7):2006–2011. doi: 10.1245/s10434-008-9944-8. [DOI] [PubMed] [Google Scholar]