Abstract
This paper provides a review of the more common tumors to metastasize to 12 anatomic subsites of the head and neck, exclusive of cervical lymph nodes. Emphasis is placed on clinical rather than subclinical metastases discovered at autopsy.
Keywords: Metastases, Head and neck
Introduction
Metastases to the head and neck are infrequent but in 20–35% of cases may be the first indication of an otherwise occult malignancy. A review of the more common tumors to metastasize to 12 anatomic subsites of the head and neck is provided, exclusive of cervical lymph nodes.
Emphasis is placed on clinical rather than subclinical metastases discovered at autopsy. A metastasis in this review is defined as a secondary tumor derived from a non-contiguous and/or remote malignant neoplasm. Direct extension from an adjacent neoplasm and leukemia–lymphoma are excluded.
Orbit
Orbital metastases occur in 2–3% of patients with cancer and represent 5–10% of all orbital neoplasms [1–3]. In about 20% of cases, the metastasis is the first sign of malignancy.
The metastases are typically unilateral and equally distributed between the right and left orbits, but in 5% of cases they are bilateral. In a review of 469 cases, Shields et al. [1] observed the most frequent primary tumor sites to metastasize to the orbit, in descending order, to be the breast (92 cases), lung (44 cases), prostate (41 cases), GI tract (22 cases), kidney (15 cases) and skin (melanoma; 13 cases).
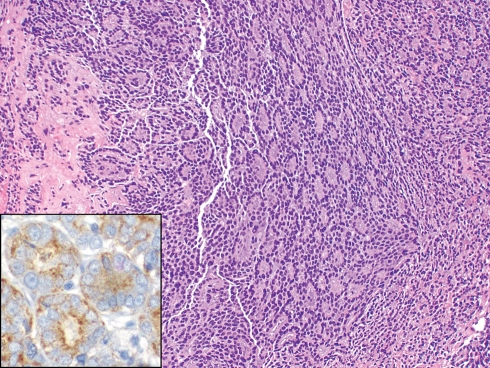
Most patients are over 40 years (mean 62 years), but children are also affected, especially from adrenal neuroblastomas. The metastases may be either localized (lung, kidney and melanoma) or diffuse (breast and prostate) and occasionally involve contiguous bone as well as soft tissue (Fig. 1).
Fig. 1.
Prostatic adenocarcinoma metastatic to orbit masquerading as a lacrimal sac neoplasm (H+E, ×100). The tumor is positive for prostate-specific antigen (inset, ×400)
Signs and symptoms, which often occur abruptly, include limited ocular mobility, proptosis, blepharoptosis, palpable mass, visual disturbances and pain [1]. Tumors that are associated with a prominent desmoplastic stroma frequently result in enophthalmos. Prognosis is poor with an overall mean survival of 15 months after orbital diagnosis [1].
Nasal Cavity and Paranasal Sinuses
Metastases to the nasal cavity and paranasal sinuses are rare. Kent and Majumdar [4] identified only two metastatic tumors of the maxillary sinus in their review of 250 malignant sinonasal tumors on file at their regional medical center. In 2001, only 169 cases were recorded in the world literature [5].
They may occur in any age group. In one review of 82 cases, the median age at the time of diagnosis of the metastatic tumor was 57 years (range 3 months to 76 years) and about 60% of the patients were males [6].
Metastases may be solitary or multifocal and ordinarily produce symptoms indistinguishable from those of a primary tumor. Among these include nasal obstruction, headache, facial pain, visual disturbances, exophthalmos, facial swelling, cranial nerve deficits and epistaxis (especially metastatic renal and thyroid carcinomas). In some instances, the metastasis may be the first manifestation of an otherwise clinically occult carcinoma.
The maxillary sinus is most frequently involved (33% of cases) followed by the sphenoid (22%), ethmoid (14%) and frontal (9%) sinuses. In 22% of cases, multiple sinuses are involved [5]. The most common tumor sites to disseminate to this region are the kidney (40%), lung (9%), breast (8%), thyroid (8%) and prostate (7%). The remaining 28% of cases include multiple miscellaneous sites [5]. In 10–15% of cases, the metastases are limited to the nasal cavity.
Although the eventual outcome is usually poor, prognosis depends, in part, on whether the sinonasal metastasis is isolated or part of widespread disseminated disease. If the metastasis to the nasal cavity and sinuses is localized and treated aggressively, Kent and Majumdar [4] indicated that the average survival following discovery of the metastasis may be as long as 20–30 months.
Ear and Temporal Bone
Berlinger et al. [7] indicate that the temporal bone may be involved with secondary malignant neoplasms by five distinct pathways: (1) isolated metastasis from a distant primary tumor, (2) direct extension from a regional primary tumor, (3) meningeal carcinomatosis, (4) leptomeningeal extension from an intracranial primary tumor, and (5) leukemia or lymphomatous infiltration. Most metastases (75%), however, are hematogenous and involve the first pathway as noted above [8].
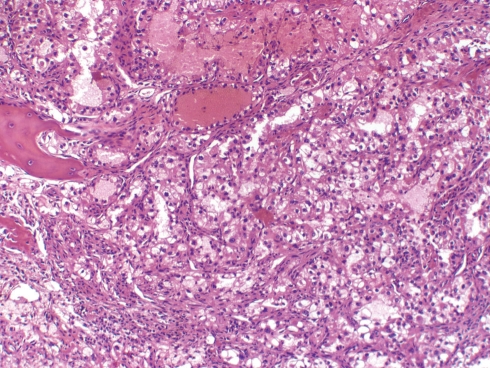
Although temporal bone metastasis may be the first indication of an underlying malignancy, the majority of patients have a known primary with widespread disease [9, 10]. In a review of 148 tumors metastatic to the temporal bone, Nelson and Hinojosa [11] observed the five most frequent primary sites to be breast (21%), lung (10%), kidney (7%), prostate (7%), and stomach (5%) (Fig. 2). Other studies have also confirmed the frequency of these tumor sites [12, 13]. Within the temporal bone, the preferred site of metastasis is, by far, the petrous apex followed equally by the mastoid and internal auditory canal [8]. The inner ear is an uncommon site. Patients have ranged from very young to very old with an equal gender distribution. While some are asymptomatic, others have experienced hearing loss, vertigo, facial asymmetry, tinnitus, otalgia, otorrhea, and/or a mass in the external canal. According to Maddox [14], the symptom triad of otalgia, preauricular swelling and facial nerve paresis in the presence of an intact tympanic membrane should be regarded as suspicious for a malignant neoplasm.
Fig. 2.
Renal cell carcinoma metastatic to temporal bone (H+E, ×200). Note the clear cells, focal hemorrhage and bone spicule
Subclinical metastases to the temporal bone, as expected, are not uncommon. In a review of autopsy records of 212 patients with malignant neoplasms who had their temporal bones removed, 47 (22%) had metastases to their temporal bones and, of these, 62% were bilateral [8].
Oral Soft Tissue and Jaws
Metastases to the oral soft tissues (OST) and jaws (mandible and maxilla) are uncommon, accounting for only 1–2% of all malignant neoplasms of these sites [15–18]. Most of these involve the jaws (65–75%) rather than the OST (25–35%), and in 25–35% of cases the metastasis may be the first sign of an otherwise occult malignancy [17, 19, 20].
When all sites are considered, the age and gender distribution range, respectively, from 9 months to 90 years (mean or median of 50–60 years) and from 1:1 to 1.3:1 in favor of males [17–20]. The average interval from diagnosis of the primary tumor to the detection of the oral metastasis is 40 months [19]. Once the diagnosis is confirmed, the prognosis is generally poor with an average survival time of 7 months [19].
Jaws
Most metastases to the jaws involve the mandible (80–85%) rather than the maxilla, but in 5% of cases both jaws may be involved [21, 22]. An explanation for this uneven distribution may be related to the larger amount of hematopoietic tissue in the mandible than the maxilla. The vascular spaces in hematopoietic tissue are sinusoidal, allegedly allowing more easily penetration by tumor cells.
According to Hirshberg et al. [21] the most frequent site of involvement in the mandible is the molar area (55%) followed by the premolar area (38%), angle-ramus (29%), condyle (3.5%) and coronoid process (1.6%). In the maxilla, the premolar-molar region predominates (55% of all cases). Whether the presence or absence of teeth has any impact on the distribution of metastases to the jaws is not clear. Hirshberg et al. [21] noted that 78% of their subjects were dentulous and 22% edentulous. Although the frequency of maxillary metastases was low, it appeared to depend largely on the presence of teeth, which were reported in 56 out of 60 cases (93%).
Most metastases to the jaws appear on imaging as poorly defined osteolytic lesions. Mixed osteolytic-osteoblastic and pure osteoblastic (especially prostate) metastases are uncommon. A negative image, however, does not rule out the possibility of small foci of tumor.
Patients typically complain of pain, paresthesia, and/or swelling. Pathologic fractures (4%), loosening of the teeth (4%) and trismus (4%) are uncommon [19].
In men, the four most common tumor sites that metastasize to the jaws, in descending order of frequency, are lung, prostate, kidney and liver and in women, breast, adrenal, female genital organs (uterus, cervix, ovaries), and colorectum [19]. In the first decade of life, tumors of the adrenal gland (neuroblastomas) most frequently spread to the jaws and in the second decade, bone primaries prevail (osteosarcoma, Ewing’s/PNET).
Oral Soft Tissues
Metastases to the OST most often involve the gingiva (55%), followed by the tongue (27%), tonsil (8%), palate (4%), lip (3%), buccal mucosa (1%) and floor of mouth (<1%) [23]. Gingival lesions are equally distributed between the maxilla and mandible and clinically resemble hyperplastic reactive conditions.
In men, the four most common primary tumor sites to involve the OST, in descending order of frequency, are lung, kidney, skin (melanoma) and liver, and in women, breast, female genital organs (uterus, cervix, and ovaries), kidney and lung. [19].
Postextraction Sites
Metastatic tumors involving sites of previous tooth extractions are uncommon [24]. It is not always apparent whether the metastatic tumor existed prior to tooth extraction or whether the extraction site resulted in fertile grounds for subsequent metastases.
The most common extracted teeth to be involved by metastases are the mandibular premolars followed by the mandibular molars. In one series, the mean interval between extraction and discovery of the metastases was 2 months (range 1 day to 30 months) [24]. The extraction site typically fails to heal and is associated with swelling, pain and/or paresthesia. Lung and breast are the two most common primary sites to involve extraction sites.
Oropharynx
Metastases to this region are mainly restricted to the base of the tongue and tonsils. Data indicate that one-third of all metastases to the tongue involve the base, and, most of these are from lung, kidney and skin (melanoma) [16, 25]. The mean survival after discovery of the lingual metastasis is only 10 months.
Metastases to the tonsils represent 0.8% of all malignant neoplasms at this site, with <100 cases recorded in the literature as of 2008 [26, 27]. Skin (melanoma), lung, breast and kidney are the most frequent tumor sites to involve the tonsil (Fig. 3). Rare examples of bilateral metastases have been reported and, interestingly, most of these have been from gastric adenocarcinomas [28]. The mean survival after the appearance of metastases is 9 months.
Fig. 3.
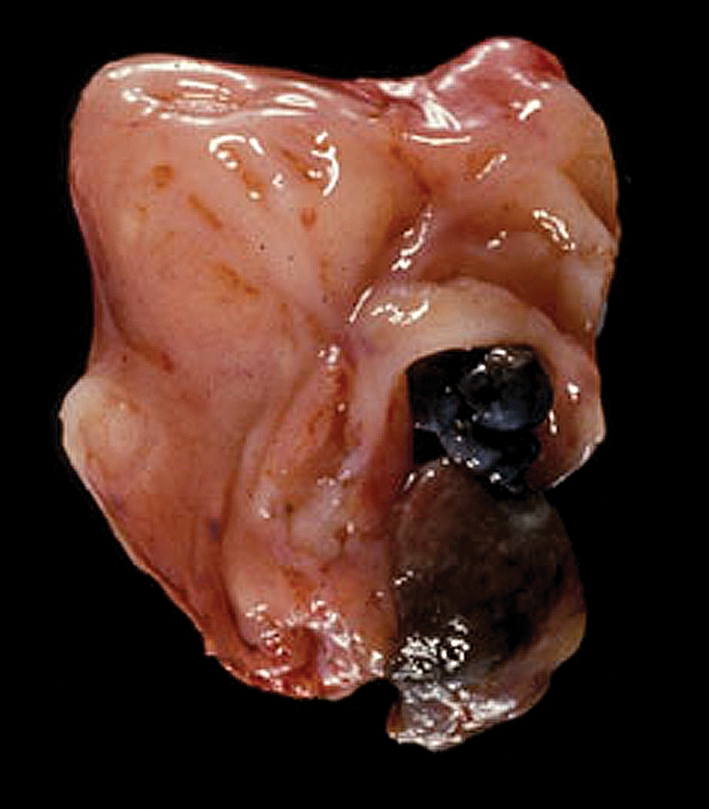
Melanoma of shoulder metastatic to tonsil
Nasopharynx
Metastases to the nasopharynx may be asymptomatic or masquerade as a primary tumor with nasal obstruction, epistaxis, unilateral serous otitis media and/or otalgia. Most patients are over 50 years. Reported tumors and/or primary sites have included melanoma (9 cases), kidney (3 renal cell, 1 Wilms), lung (4 cases) and one case each of breast, colon, and cervix [29].
Larynx
Metastases to the larynx are uncommon. Batsakis et al. [30] observed only 11 cases over a 20 year interval at the M. D. Anderson Hospital in Houston, Texas. Ferlito identified 8 additional cases in a series of more than 4,000 malignant neoplasms of the larynx at the University of Padua, Italy [31]. In 1993, 134 cases were recorded in the world literature and by 2008, the number had increased to at least 174 (7 cases of lymphoma excluded from a reported 181 cases) [31, 32].
Most metastases to the larynx are through the systemic circulation, often following the route of the inferior vena cava, right heart, lungs, left heart, aorta, external carotid artery, superior thyroid artery and laryngeal artery. Spread may also take place via the retrograde circulation of the paravertebral venous plexus or thoracic lymphatic duct. The infrequent occurrence of metastases to the larynx may therefore be related to the terminal location of the larynx in the lymphatic-vascular circulation. Known differences in regional lymphatic and vascular supply of the larynx determine the distribution of metastases within the larynx.
The majority of tumors that metastasize to the larynx are either melanomas (29% of all cases) or carcinomas, especially renal (25%), G-I (14%), lung (9%), breast (8%), and prostate (5%) [32]. Less than 5% are from mesenchymal tumors (bone and soft tissue sarcomas).
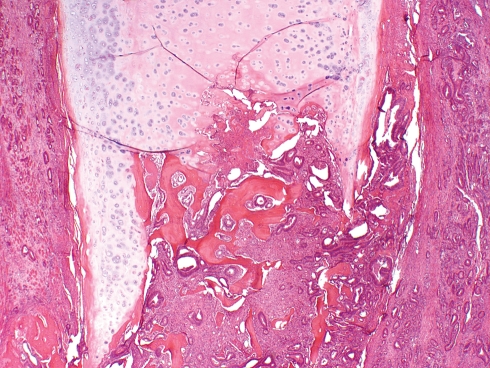
Metastases may be submucosal, cartilaginous or both (Fig. 4). If cartilage is involved, it is usually only in the portion which has undergone ossification. The most frequently affected site is the supraglottis (35–40% of all cases) followed by the subglottis (10–20%) and glottis (5–10%). Synchronous involvement of multiple laryngeal sites, however, is common and observed in about 35% of all cases [30, 33].
Fig. 4.
Adenocarcinoma of colon metastatic to larynx. The tumor involves the ossified portion of the thyroid cartilage and the adjacent soft tissue (H+E, ×100)
Laryngeal metastases increase with age (median 58 years, range 24–83 years) and are more common in males by a ratio of 2:1 [30, 33].
The signs and symptoms are similar to those exhibited by primary tumors of the larynx and vary according to location. Supraglottic lesions present with a sensation of a foreign body in the throat, dysphagia or a change in quality of the voice, while glottic deposits manifest as hoarseness and subglottic lesions as compromise of the airway. Richly vascular tumors, such as renal cell carcinomas and thyroid carcinomas, often result in hemoptysis. On rare occasions, the metastasis is the only evidence of an otherwise occult primary tumor.
The prognosis is generally poor and associated with terminal widespread disseminated disease. In a few instances, the metastases may be isolated or localized, and, with appropriate therapy, a prolonged survival may be achieved.
Trachea
Metastases to the trachea are exceptional and usually manifest as cough, stridor, wheezing, dyspnea, and/or hemoptysis. In a review of 12 tumors metastatic to the trachea, the primary site of origin was breast (4 cases), colon (3 cases), skin (melanoma;2 cases), uterine cervix (1 case), endometrium (1 case) and ovary (1 case) [34–39].
Major Salivary Glands
Metastases to the major salivary glands, particularly the parotid gland, are relatively common and vary in frequency according to geographic location. In North America they represent 1–4% of all salivary neoplasms and about 8–16% of all malignant salivary tumors [40, 41]. In Australia where solar-related skin cancers (squamous cell carcinoma and melanoma) are a major public health problem, metastases represent 75% of all malignant neoplasms of the parotid gland [42, 43]. Metastases to the submandibular gland, in contrast, are rare and involvement of the sublingual gland is non-existent.
The parotid gland contains, on average, 20 lymph nodes which are concentrated in the superficial lobe. Accordingly, metastases to this gland may be either through the lymphatics or the blood stream. The submandibular gland lacks lymph nodes and metastases are almost exclusively hematogenous.
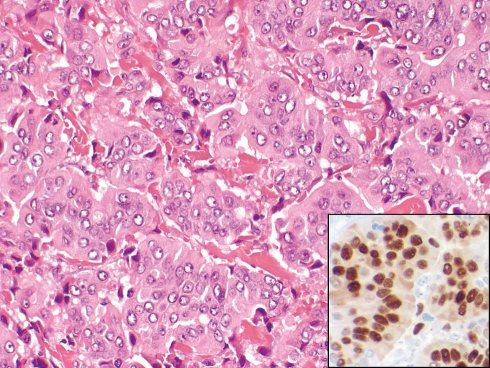
About 70% of all metastases to the parotid gland are from squamous cell carcinoma and melanoma involving cutaneous sites of the head and neck [40]. An additional 15% are from non-cutaneous head and neck sites and the remaining 15% are from sites below the clavicles, especially lung, kidney and breast (Fig. 5). Patients who have cutaneous squamous cell carcinomas of the head and neck with the following features are at increased risk for developing metastases: (1) large size (<2 cm), (2) more than 4 mm of invasion, (3) incomplete excision, (4) history of local recurrence, (5) high grade or desmoplastic tumors, (6) perineural invasion, (7) angiolymphatic invasion in primary tumor, (8) location on ear, temple, forehead or anterior scalp, and (9) immunosuppressed state [44].
Fig. 5.
Ductal adenocarcinoma of breast metastatic to superficial lobe of parotid gland (H+E, ×200). The tumor is positive for estrogen receptor (inset, ×400)
Metastases to the submandibular gland, in contrast, are rarely from cutaneous sites of the head and neck but arise predominantly (75% of all cases) from infraclavicular tumors, particularly breast, kidney and lung [40].
Metastasis to the salivary glands is usually an ominous sign and, especially so, if the primary tumor is located below the clavicles. Patients with parotid metastases may also have disease in the cervical lymph nodes. It has been demonstrated that in the case of cutaneous squamous cell carcinoma with parotid metastasis, that 15–35% of individuals will also have either clinical or occult synchronous spread to adjacent level I–V lymph nodes which must be taken in consideration when treating these patients [45–47].
Thyroid Gland
The thyroid gland is one of the most vascular organs in the human body, yet clinical metastases to this gland are uncommon, accounting for only 1–3% of malignant neoplasms of the thyroid [48–51]. Data derived from autopsies indicate that the thyroid is the site of metastasis in 1.25% of unselected autopsies (the thyroid gland is not routinely examined in many autopsies) and up to 24% in patients with disseminated malignancies [52].
In most series, renal cell carcinoma is the most common clinical tumor to involve the thyroid followed by lung and breast [49, 51–54]. While sarcomas, rarely spread to the thyroid, leiomyosarcoma is by far the most common. The frequency of various tumors, however, depends on geographic location. In Hong Kong, Lam and Lo observed in their series of 79 metastatic tumors to the thyroid that lung was by far the most common primary site followed by breast and stomach [48]. In autopsies, breast, lung and melanoma are the dominant tumors [50, 55].
Metastases to the thyroid are more common in women (55%) and occur over a broad age range with a mean of 62 years [54]. Signs and symptoms range from an asymptomatic mass to those associated with hoarseness, dysphagia and/or pain. Exceptionally some metastases may result in transient hyperthyroidism, presumably due to the destruction of thyroid parenchyma and release of hormones [56]. In about one-third of cases, the metastasis may be the first manifestation of a primary undiagnosed malignancy.
Most clinical metastases present as solitary nodules (especially renal cell carcinoma) and are equally distributed between the right and left lobes [48, 53, 57] (Fig. 6). Some are multifocal, either unilateral or bilateral, while others are only microscopic. It has been stated that thyroid glands previously altered by thyroiditis, follicular nodules, adenomas and/or neoplasms may be more receptive to metastases than normal glands [53, 56]. This statement, however, must be tempered by the fact that these conditions are common in otherwise healthy individuals and whether they predispose to metastases remains to be determined.
Fig. 6.
Endometrioid adenocarcinoma of ovary metastatic to right lobe of thyroid
Prognosis is determined by the type of tumor and the extent of dissemination. Some tumors, particularly renal cell carcinoma, with isolated metastasis may have a relative good prognosis.
Parathyroid Glands
Horwitz et al. [58] have reported two patients with disseminated carcinoma of the breast who developed terminal hypoparathyroidism. At autopsy at least 70% of their parathyroid glandular tissue was replaced by metastatic tumor. Other than these exceptional cases, patients with metastases to the parathyroid glands are almost invariably asymptomatic with the abnormal gland(s) discovered at the time of postmortem examination [59, 60].
Large autopsy studies of patients who have died of carcinomatosis and in which the parathyroid glands were specifically examined indicate that about 5–10% of parathyroid glands will harbor metastatic tumor, especially from primary breast and occasionally melanoma, lung, soft tissue sarcoma and renal neoplasms [58, 61, 62].
Skin of Head and Neck
Cutaneous metastases occur in 0.7–10% of all patients with cancer and, of these, about 20–25% involve the head and neck [63–65]. Most present as painless, rapidly enlarging nodules, either solitary or as small aggregates, covered by an intact epidermis. The scalp and neck are the most common sites and melanoma, breast, lung and kidney are the usual culprits. Some tumors exhibit unique features. Breast, for example, may involve the eyelid and masquerade as a histiocytic lesion while prostatic carcinoma rarely involves the skin. Follicular carcinomas of the thyroid rarely metastasize to the skin, but when they do, the scalp is the site of predilection [66].
Conclusions
Metastases to the head and neck are infrequent.
Most are associated with disseminated disease.
In about 20–35% of cases, the metastasis may be the first manifestation of an otherwise occult malignancy.
With the exception of the parotid gland, most metastases are of hematogenous origin.
Any malignant tumor may disseminate to the head and neck.
Type and frequency of metastatic tumors vary according to geographic location and generally correlate with the prevalence of malignant tumors in the host country.
In the US, lung, breast, kidney and skin (melanoma) predominate.
Metastases to the head and neck can occur at any age. In the first two decades of life adrenal neuroblastoma, osteosarcoma, and Ewing’s/PNET are more common.
Signs and symptoms of metastatic tumors are similar to those of primary tumors.
Prognosis is generally poor.
Solitary metastases, however, may have an unexpected good prognosis.
References
- 1.Shields JA, Shields CL, Brotman HK, Carvalho C, Perez N, Eagle RC., Jr Cancer metastatic to the orbit: the 2000 Robert M. Curts lecture. Ophthal Plast Reconstr Surg. 2001;17:346–354. doi: 10.1097/00002341-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Font RL, Croxatto JO, Rao NA. Tumors of the orbit. In: Font RL, Croxatto JO, Rao NA, editors. Tumors of the eye and ocular adnexa. Washington DC: American Registry of Pathology; 2006. pp. 306–307. [Google Scholar]
- 3.Henderson JW, Campbell RJ, Farrow GM, Garrity JA. Metastatic carcinomas. In: Henderson JW, Campbell RJ, Farrow GM, Garrity JA, editors. Orbital tumors. 3. New York: Raven Press; 1994. pp. 361–375. [Google Scholar]
- 4.Kent SE, Majumdar B. Metastatic tumours in the maxillary sinus. A report of two cases and a review of the literature. J Laryngol Otol. 1985;99:459–462. doi: 10.3109/00016488509108938. [DOI] [PubMed] [Google Scholar]
- 5.Prescher A, Brors D. Metastases to the paranasal sinuses: case report and review of the literature. Laryngorhinootologie. 2001;80:583–594. doi: 10.1055/s-2001-17835. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein JM, Montgomery WW, Balogh K., Jr Metastatic tumors to the maxilla, nose, and paranasal sinuses. Laryngoscope. 1966;76:621–650. doi: 10.1288/00005537-196604000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Berlinger NT, Koutroupas S, Adams G, Maisel R. Patterns of involvement of the temporal bone in metastatic and systemic malignancy. Laryngoscope. 1980;90:619–627. doi: 10.1288/00005537-198004000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Gloria-Cruz TI, Schachern PA, Paparella MM, Adams GL, Fulton SE. Metastases to temporal bones from primary nonsystemic malignant neoplasms. Arch Otolaryngol Head Neck Surg. 2000;126:209–214. doi: 10.1001/archotol.126.2.209. [DOI] [PubMed] [Google Scholar]
- 9.Sahin AA, Ro JY, Ordonez NG, Luna MA, Weber RS, Ayala AG. Temporal bone involvement by prostatic adenocarcinoma: report of two cases and review of the literature. Head Neck. 1991;13:349–354. doi: 10.1002/hed.2880130414. [DOI] [PubMed] [Google Scholar]
- 10.Davis GL. Secondary tumors. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization classification of tumours, pathology and genetics, head and neck tumors. Lyon: IARC Press; 2005. p. 360. [Google Scholar]
- 11.Nelson EG, Hinojosa R. Histopathology of metastatic temporal bone tumors. Arch Otolaryngol Head Neck Surg. 1991;117:189–193. doi: 10.1001/archotol.1991.01870140077010. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi K, Igarashi M, Ohashi K, McBride RA. Metastatic seminoma of the temporal bone. Arch Otolaryngol Head Neck Surg. 1986;112:102–105. doi: 10.1001/archotol.1986.03780010104021. [DOI] [PubMed] [Google Scholar]
- 13.Cumberworth VL, Friedmann I, Glover GW. Late metastasis of breast carcinoma to the external auditory canal. J Laryngol Otol. 1994;108:808–810. doi: 10.1017/s0022215100128208. [DOI] [PubMed] [Google Scholar]
- 14.Maddox HE., III Metastatic tumors of the temporal bone. Ann Otol Rhinol Laryngol. 1967;76:149–165. doi: 10.1177/000348946707600111. [DOI] [PubMed] [Google Scholar]
- 15.Meyer I, Shklar G. Malignant tumors metastatic to mouth and jaws. Oral Surg Oral Med Oral Pathol. 1965;20:350–362. doi: 10.1016/0030-4220(65)90167-2. [DOI] [PubMed] [Google Scholar]
- 16.Baden E, Duvillard P, Micheau C. Metastatic papillary endometrial carcinoma of the tongue. Case report and review of the literature. Arch Pathol Lab Med. 1992;116:965–968. [PubMed] [Google Scholar]
- 17.Waal RI, Buter J, Waal I. Oral metastases: report of 24 cases. Br J Oral Maxillofac Surg. 2003;41:3–6. doi: 10.1016/S0266-4356(02)00301-7. [DOI] [PubMed] [Google Scholar]
- 18.Disibio G, French SW. Metastatic patterns of cancers: results from a large autopsy study. Arch Pathol Lab Med. 2008;132:931–939. doi: 10.5858/2008-132-931-MPOCRF. [DOI] [PubMed] [Google Scholar]
- 19.Hirshberg A, Shnaiderman-Shapiro A, Kaplan I, Berger R. Metastatic tumours to the oral cavity—pathogenesis and analysis of 673 cases. Oral Oncol. 2008;44:743–752. doi: 10.1016/j.oraloncology.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Shen ML, Kang J, Wen YL, Ying WM, Yi J, Hua CG, Tang XF, Wen YM. Metastatic tumors to the oral and maxillofacial region: a retrospective study of 19 cases in West China and review of the Chinese and English literature. J Oral Maxillofac Surg. 2009;67:718–737. doi: 10.1016/j.joms.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 21.Hirshberg A, Leibovich P, Buchner A. Metastatic tumors to the jawbones: analysis of 390 cases. J Oral Pathol Med. 1994;23:337–341. doi: 10.1111/j.1600-0714.1994.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 22.D’Silva NJ, Summerlin DJ, Cordell KG, Abdelsayed RA, Tomich CE, Hanks CT, Fear D, Meyrowitz S. Metastatic tumors in the jaws: a retrospective study of 114 cases. J Am Dent Assoc. 2006;137:1667–1672. doi: 10.14219/jada.archive.2006.0112. [DOI] [PubMed] [Google Scholar]
- 23.Hirshberg A, Leibovich P, Buchner A. Metastases to the oral mucosa: analysis of 157 cases. J Oral Pathol Med. 1993;22:385–390. doi: 10.1111/j.1600-0714.1993.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 24.Hirshberg A, Leibovich P, Horowitz I, Buchner A. Metastatic tumors to postextraction sites. J Oral Maxillofac Surg. 1993;51:1334–1337. doi: 10.1016/s0278-2391(10)80138-7. [DOI] [PubMed] [Google Scholar]
- 25.Azam F, Abubakerr M, Gollins S. Tongue metastasis as an initial presentation of renal cell carcinoma: a case report and literature review. J Med Case Reports. 2008;2:249–253. doi: 10.1186/1752-1947-2-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hyams VJ. Differential diagnosis of neoplasia of the palatine tonsil. Clin Otolaryngol Allied Sci. 1978;3:117–126. doi: 10.1111/j.1365-2273.1978.tb00674.x. [DOI] [PubMed] [Google Scholar]
- 27.Struijs B, Bree R, Groeningen CJ, Mooi WJ, Leemans CR. Tonsillar metastasis of oesophageal adenocarcinoma. Eur Arch Otorhinolaryngol. 2008;265:127–129. doi: 10.1007/s00405-007-0402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallo A, Pescarmona E, Crupi J, Corsetti GL, Vincentiis M. Bilateral tonsillar metastasis of gastric adenocarcinoma. Head Neck. 1992;14:55–57. doi: 10.1002/hed.2880140112. [DOI] [PubMed] [Google Scholar]
- 29.McKay MJ, Carr PJ, Jaworski R, Kalnins I. Cancer of distant primary site relapsing in the nasopharynx: a report of two cases and review of the literature. Head Neck. 1989;11:534–537. doi: 10.1002/hed.2880110611. [DOI] [PubMed] [Google Scholar]
- 30.Batsakis JG, Luna MA, Byers RM. Metastases to the larynx. Head Neck Surg. 1985;7:458–460. doi: 10.1002/hed.2890070604. [DOI] [PubMed] [Google Scholar]
- 31.Ferlito A. Secondary neoplasms. In: Ferlito A, editor. Neoplasms of the larynx. Edinburgh: Churchill Livingston; 1993. pp. 349–360. [Google Scholar]
- 32.El-Naggar AK. Secondary neoplasms of the larynx. In: Fried MP, Ferlito A, editors. The larynx. 3. San Diego: Pleural Publishing; 2009. pp. 719–728. [Google Scholar]
- 33.Ferlito A, Caruso G, Recher G. Secondary laryngeal tumors. Report of seven cases with review of the literature. Arch Otolaryngol Head Neck Surg. 1988;114:635–639. doi: 10.1001/archotol.1988.01860180049028. [DOI] [PubMed] [Google Scholar]
- 34.Baumgartner WA, Mark JB. Metastatic malignancies from distant sites to the tracheobronchial tree. J Thorac Cardiovasc Surg. 1980;79:499–503. [PubMed] [Google Scholar]
- 35.Westerman DE, Urbanetti JS, Rudders RA, Fanburg BL. Metastatic endotracheal tumor from ovarian carcinoma. Chest. 1980;77:798–800. doi: 10.1378/chest.77.6.798. [DOI] [PubMed] [Google Scholar]
- 36.Mackenzie IJ, Morgan JM, Mitchell JF. Secondary tracheal carcinoma. J Laryngol Otol. 1981;95:973–978. doi: 10.1017/S0022215100091702. [DOI] [PubMed] [Google Scholar]
- 37.Nicolai P, Peretti G, Cappiello J, Renaldini G, Cavaliere S, Morassi ML. Melanoma metastatic to the trachea and nasal cavity: description of a case and review of the literature. Acta Otorhinolaryngol Ital. 1991;11:85–92. [PubMed] [Google Scholar]
- 38.Conti JA, Kemeny N, Klimstra D, Minsky B, Rusch V. Colon carcinoma metastatic to the trachea. Report of a case and a review of the literature. Am J Clin Oncol. 1994;17:227–229. doi: 10.1097/00000421-199406000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Koyi H, Branden E. Intratracheal metastasis from malignant melanoma. J Eur Acad Dermatol Venereol. 2000;14:407–408. doi: 10.1046/j.1468-3083.2000.00100.x. [DOI] [PubMed] [Google Scholar]
- 40.Gnepp DR. Metastatic disease to the major salivary glands. In: Ellis GL, Auclair PL, Gnepp DR, editors. Surgical pathology of the salivary glands, major problems in pathology series. Philadelphia: WB Saunders; 1991. pp. 560–569. [Google Scholar]
- 41.Ellis GL, Auclair PL. Secondary tumors. In: Ellis GL, Auclair PL, editors. Tumors of the salivary glands. Washington DC: American Registry of Pathology; 2008. pp. 471–479. [Google Scholar]
- 42.O’Brien CJ, McNeil EB, McMahon JD, Pathak I, Lauer CS, Jackson MA. Significance of clinical stage, extent of surgery, and pathologic findings in metastatic cutaneous squamous carcinoma of the parotid gland. Head Neck. 2002;24:417–422. doi: 10.1002/hed.10063. [DOI] [PubMed] [Google Scholar]
- 43.Bron LP, Traynor SJ, McNeil EB, O’Brien CJ. Primary and metastatic cancer of the parotid: comparison of clinical behavior in 232 cases. Laryngoscope. 2003;113:1070–1075. doi: 10.1097/00005537-200306000-00029. [DOI] [PubMed] [Google Scholar]
- 44.Veness MJ. High-risk cutaneous squamous cell carcinoma of the head and neck. J Biomed Biotechnol. 2007;2007:80572–80577. doi: 10.1155/2007/80572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Brien CJ, McNeil EB, McMahon JD, Pathak I, Lauer CS. Incidence of cervical node involvement in metastatic cutaneous malignancy involving the parotid gland. Head Neck. 2001;23:744–748. doi: 10.1002/hed.1106. [DOI] [PubMed] [Google Scholar]
- 46.Vauterin TJ, Veness MJ, Morgan GJ, Poulsen MG, O’Brien CJ. Patterns of lymph node spread of cutaneous squamous cell carcinoma of the head and neck. Head Neck. 2006;28:785–791. doi: 10.1002/hed.20417. [DOI] [PubMed] [Google Scholar]
- 47.Hinerman RW, Indelicato DJ, Amdur RJ, Morris CG, Werning JW, Vaysberg M, Kirwan J, Mendenhall WM. Cutaneous squamous cell carcinoma metastatic to parotid-area lymph nodes. Laryngoscope. 2008;118:1989–1996. doi: 10.1097/MLG.0b013e318180642b. [DOI] [PubMed] [Google Scholar]
- 48.Lam KY, Lo CY. Metastatic tumors of the thyroid gland: a study of 79 cases in Chinese patients. Arch Pathol Lab Med. 1998;122:37–41. [PubMed] [Google Scholar]
- 49.Mirallie E, Rigaud J, Mathonnet M, Gibelin H, Regenet N, Hamy A, Bretagnol F, Calan L, Le Neel JC, Kraimps JL. Management and prognosis of metastases to the thyroid gland. J Am Coll Surg. 2005;200:203–207. doi: 10.1016/j.jamcollsurg.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 50.Wood K, Vini L, Harmer C. Metastases to the thyroid gland: the Royal Marsden experience. Eur J Surg Oncol. 2004;30:583–588. doi: 10.1016/j.ejso.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 51.Cichon S, Anielski R, Konturek A, Barczynski M, Cichon W. Metastases to the thyroid gland: seventeen cases operated on in a single clinical center. Langenbecks Arch Surg. 2006;391:581–587. doi: 10.1007/s00423-006-0081-1. [DOI] [PubMed] [Google Scholar]
- 52.Nakhjavani MK, Gharib H, Goellner JR, Heerden JA. Metastasis to the thyroid gland. A report of 43 cases. Cancer. 1997;79:574–578. doi: 10.1002/(SICI)1097-0142(19970201)79:3<574::AID-CNCR21>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 53.Heffess CS, Wenig BM, Thompson LD. Metastatic renal cell carcinoma to the thyroid gland: a clinicopathologic study of 36 cases. Cancer. 2002;95:1869–1878. doi: 10.1002/cncr.10901. [DOI] [PubMed] [Google Scholar]
- 54.Thompson LDR. Tumors metastatic to the thyroid. In: Nikiforov YE, Biddinger PW, Thompson LDR, editors. Diagnostic pathology and molecular genetics of the thyroid. A comprehensive guide for practicing thyroid pathology. Philadelphia: Wolters Kluwer; 2009. pp. 348–356. [Google Scholar]
- 55.McCabe DP, Farrar WB, Petkov TM, Finkelmeier W, O’Dwyer P, James A. Clinical and pathologic correlations in disease metastatic to the thyroid gland. Am J Surg. 1985;150:519–523. doi: 10.1016/0002-9610(85)90167-9. [DOI] [PubMed] [Google Scholar]
- 56.DeLellis R. Secondary tumours of the thyroid. In: DeLellis RA, Lloyd RV, Heitz PU, Eng C, editors. World Health Organization classification of tumours, pathology and genetics, tumors of endocrine organs. Lyon: IARC Press; 2004. pp. 122–123. [Google Scholar]
- 57.Gerges AS, Shehata SR, Gouda IA. Metastasis to the thyroid gland; unusual site of metastasis. J Egypt Natl Canc Inst. 2006;18:67–72. [PubMed] [Google Scholar]
- 58.Horwitz CA, Myers WP, Foote FW., Jr Secondary malignant tumors of the parathyroid glands. Report of two cases with associated hypoparathyroidism. Am J Med. 1972;52:797–808. doi: 10.1016/0002-9343(72)90086-1. [DOI] [PubMed] [Google Scholar]
- 59.Gattuso P, Khan NA, Jablokow VR, Kathuria S. Neoplasms metastatic to parathyroid glands. South Med J. 1988;81:1467. doi: 10.1097/00007611-198811000-00039. [DOI] [PubMed] [Google Scholar]
- 60.Venkatraman L, Kalangutkar A, Russell CF. Primary hyperparathyroidism and metastatic carcinoma within parathyroid gland. J Clin Pathol. 2007;60:1058–1060. doi: 10.1136/jcp.2005.035352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.la Monte SM, Hutchins GM, Moore GW. Endocrine organ metastases from breast carcinoma. Am J Pathol. 1984;114:131–136. [PMC free article] [PubMed] [Google Scholar]
- 62.Bumpers HL, Hassett JM, Jr, Penetrante RB, Hoover EL, Holyoke ED. Endocrine organ metastases in subjects with lobular carcinoma of the breast. Arch Surg. 1993;128:1344–1347. doi: 10.1001/archsurg.1993.01420240052009. [DOI] [PubMed] [Google Scholar]
- 63.Brownstein MH, Helwig EB. Metastatic tumors of the skin. Cancer. 1972;29:1298–1307. doi: 10.1002/1097-0142(197205)29:5<1298::AID-CNCR2820290526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 64.Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228–236. doi: 10.1016/0190-9622(93)70173-Q. [DOI] [PubMed] [Google Scholar]
- 65.Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33:161–182. doi: 10.1016/0190-9622(95)90231-7. [DOI] [PubMed] [Google Scholar]
- 66.Quinn TR, Duncan LM, Zembowicz A, Faquin WC. Cutaneous metastases of follicular thyroid carcinoma: a report of four cases and a review of the literature. Am J Dermatopathol. 2005;27:306–312. doi: 10.1097/01.dad.0000164606.33779.6f. [DOI] [PubMed] [Google Scholar]