Abstract
Syphilis is caused by Treponema pallidum an anaerobic filamentous spirochete. In recent years, striking outbreaks have occurred in USA, Canada, Russia, China and some areas of Central and Eastern Europe. Main epidemiology changes reflect sex industry, sexual promiscuity, decreasing use of barrier protection (i.e. condoms) due to false sense of security that nowadays sexually transmitted diseases are curable and lack of pertinent knowledge. Considering that the initial presentation of syphilis may be the oral cavity, it is of great relevance to include this disease in the differential diagnosis of unusual oral ulcerations and white patches. Primary syphilis is a highly infectious disease in which inappropriate treatment may be apparently curative while the patient remains highly infectious. It is then of pivotal importance that clinicians maintain a high clinical index of suspicion. At the present time, clinical-pathologic correlation together with serologic studies remain essential in establishing the diagnosis of syphilis.
Keywords: Syphilis, Oral cavity, Treponema pallidum
Introduction
Syphilis is an infectious disease caused by a spirochete called Treponema pallidum which has a tropism for several organs and tissues in the body causing complex clinical manifestations [1–3]. The precise origin of syphilis is unknown although two main theories prevail. One is that the disease was endemic to America and brought to Europe after Columbus discovery in 1492. The other one suggests that syphilis was endemic to central Africa and reached Europe before the voyage of Columbus [4]. Recently, a DNA-related strain of T. pallidum, which causes a disease with mix features of syphilis and yaws, has been fund in an isolated tribe living in Guyana. It has been suggested that this organism may represent the progenitor from which T. pallidum has evolved thousands of years ago [5].
Transmission of syphilis occurs mostly through sexual intercourse and sites of inoculations are usually genital but can also be extragenital [1–3]. Despite the introduction, in the mid-twentieth century, of penicillin it has been impossible to eradicate this disease and it remains a relevant problem among the ailments of mankind and its incidence is rapidly increasing in several parts of the world [6–9].
The aim of this paper is to review the epidemiologic and clinical aspects of syphilis, the oral manifestations and its interplay with HIV infection.
Epidemiology
In the USA, in the first half of the twentieth century, syphilis was a frequent infection (66.4 cases per 100.000 persons in 1947) and a leading cause of heart and neurological diseases [3, 6, 9]. In 1940s the introduction of penicillin therapy and prevention efforts made the disease rare. Since the 1950s in the USA periodic epidemics have been observed with peaks around the 1990 followed by a progressive decline of prevalence by the year 1993 until the year 2000 [10–14]. However, during that time syphilis still remained a problem in the Southern States where the disease used to spread especially among poor minority groups that did not have access to medical facilities [15–17]. In the USA, the incidence of syphilis continued to cycle reaching an all time low in 2000, with 2.1 cases per 100.000 persons [17]. During the last 8 years a significant resurgence of this disease has been reported in several countries such as the USA, Canada, England, France, Spain, Ireland, Eastern Europe, Russia and China [6–24]. Main epidemiology changes, at the basis of increasing prevalence, reflect sex industry, sexual promiscuity, decreasing use of barrier protection (i.e. condoms) due to a false sense of security that today sexually transmitted disease are curable and lack of pertinent knowledge.
Today, more than 50–60% of new cases of syphilis occur in men who have sex with men (MSM) and are strongly associated with HIV coinfection and high risk sexual behavior [6, 7, 25]. In the Russian Federation since the fall of the Soviet Union the incidence of syphilis has shown a rapid and substantial increase. The reasons are linked to changes in sexual behavior, drug abuse, increased travel and migration which all have created the conditions for a parallel epidemic of HIV infection [19]. Also in other European countries like Spain, syphilis has been linked in injecting drug users with high-risk sexual behavior [20]. In China cases of syphilis have been recently observed in an increasing number and in association with less education, alcohol use, unprotected anal sex with male partners and diagnosis of sexual transmitted diseases [8].
Etiology and Mechanism of Infection
The causative agent of syphilis is T. pallidum. There are other organisms which belong to the genus Treponema that are closely related antigenically to T. pallidum. Among these spirochetes that are pathogenic to humans there are: T. pallidum subsp. pertenue (causative of yaws); T. pallidum subsp. endemicum (causative of bejel, nonvenereal endemic syphilis); and T. carateum (causative of pinta) [2, 3].
Treponema pallidum has a slender, coiled morphology and when examined by dark field microscopy it moves with a drifting rotary motion (corkscrew). T. pallidum cannot survive outside its only known natural host (humans) because it has limited metabolic capacities in order to synthesize its own bionutrients. Syphilis spirochetes like other virulent treponemes cannot be cultivated in vitro [2, 3].
The primary mode of syphilis transmission is sexual contact. After T. pallidum penetrates through the genital mucosa or abraded skin, it enters the lymphatic and blood stream and disseminates to various organs including the CNS [1–3]. The incubation time is directly proportional to the size of the inoculum and may vary from 3 to 90 days. In about 10–20% of cases the primary lesion is intrarectal, perianal or oral. At difference with other sexually transmitted diseases (e.g. HIV) T. pallidum is easily transmissible by oral sex, kissing and close contact with an infectious lesion [1–3, 6].
An important common mode of transmission is in utero transmission. In addition, transmission can occur at delivery if the newborn comes in contact with a contagious lesion. Blood transfusion can be another mode of transmission although today quite rare. Healthcare workers and laboratory personnel can acquire the infection if their unprotected hands come in contact with the spirochetes [2, 3, 6].
The likelihood of a susceptible person who is exposed to an infected individual of developing syphilis is about 50% [1–3]. Spirochetes multiply locally at the site of entry and some spread to lymph nodes and systemically through the bloodstream. T. pallidum has an innate capacity to evade the host’s immune system. Evaluations of the outer membrane show only a small number of integral proteins thus the immune system cannot mount, in the absence of proper immunogenic targets, an efficient immune response capable of eradicating the infection. Serological tests detect antibodies to T. pallidum early in the primary stage which remain detectable during the infection and which are used to monitor the response to treatment. Cellular immune responses cause the resolution of both primary and secondary syphilis. However, despite these immune responses and in absence of antibiotic therapy T. pallidum is able to survive in the human host for decades [26–30].
The clinical manifestations of syphilis are quite protean and can be recapitulated into four principal stages: primary, secondary, latent and tertiary syphilis (see below). The pathologic changes associated with syphilis are characterized by obliterative endarteritis that is found in all stages of the disease. In the primary chancre an inflammatory infiltrate formed by polymorphonuclear leucocytes and macrophages and rich of treponemes is the typical feature. Gummas are agranulomatous lesions formed by a necrotic coagulated center and associated with small-vessels obliterative endarteritis. T. pallidum is difficult to demonstrate in tissue albeit it can be revealed by special silver stains [1–3, 28]. An individual with active or latent syphilis is resistant to superinfection with T. pallidum. If the disease is eradicated by adequate antibiotic treatment, the individual again becomes fully susceptible to infection [2].
Clinical Manifestations
Syphilis evolves through a series of four overlapping stages commonly known as primary syphilis, secondary syphilis, latent syphilis, and tertiary syphilis. Each stage has distinct clinical characteristics and degree of infectivity (Table 1) [1–3, 6]. Prenatal transmission may induce distinct clinical manifestations.
Table 1.
Syphilis: outline of stages and incubation times
Primary Syphilis
The primary stage of infection is known as syphilis chancre, which occurs at the site of inoculation. Chancre starts as a papule that may evolve into an indurated, painless, nonpurulent ulcer with clean base. The size varies from 0.3 to 3 cm and the borders appear marginated. Chancres can be single or multiple and usually regress (without treatment!) after 2–8 weeks. The majority of extragenital chancres occur in the mouth (40–75%) although they can be observed in any body parts, including hands of healthcare workers. Regional lymphadenopathy occurs in up to 80% of cases about 7–10 days after the development of the genital chancre [1–3, 31].
Secondary Syphilis
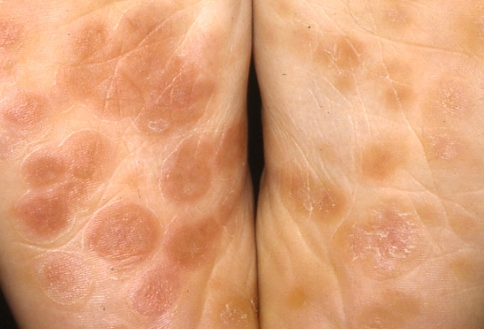
The secondary stage develops after 2–12 weeks after the first contact with the organism. This stage is the result of a hematogenous dissemination and the organism, in very high number, colonizes several organs giving constitutional and mucocutaneous manifestations (Table 2). A rash of varying extension is the most common presenting symptom [1–3, 31, 32]. The rash typically does not cause pruritus and develops as symmetrical 3–10 mm pink or red macules, which can progress to papular or pustular form (Fig. 1). If untreated, the rash resolves over several weeks without complications. Characteristic anatomical locations are the arms, palms, flanks and soles (Fig. 2). Occasionally, about 5–6% of patients may develop patchy hair loss of the beard, eyebrows and scalp localized alopecia (Fig. 3).
Table 2.
Constitutional and mucocutaneous manifestations of secondary syphilis
| Symptoms: fever, malaise, weight loss |
| Skin rash (symmetrical and generalized), alopecia |
| Condyloma latum in intertriginous areas |
| Lymphadenopathy |
| Oral involvement: multiple mucous patches covered by grayish, white pseudomembranes and surrounded by erythema |
| Ocular involvement: uveitis, iritis, optic neuritis |
| Arthritis, periostitis |
| Hepatitis |
| Glomerulonephritis |
| Neurologic involvement: headache, meningitis, cranial nerve paralysis, cerebrovascular accident |
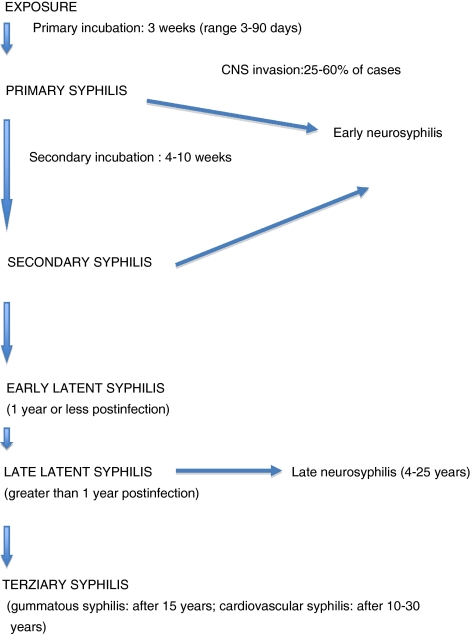
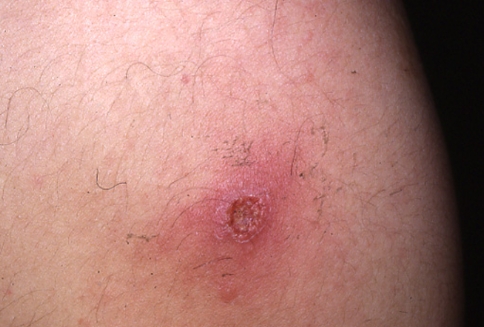
Fig. 1.
Secondary syphilis: maculopapular skin lesions of the neck
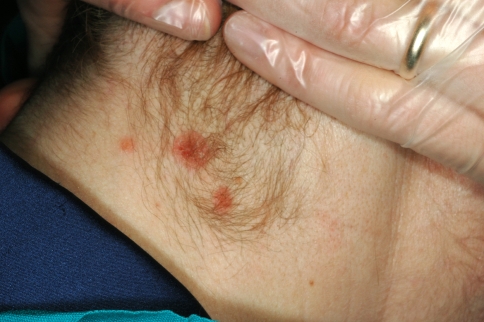
Fig. 2.
Secondary syphilis: maculopapular and scaly lesions of the plantar area
Fig. 3.
Moth-eaten alopecia in secondary syphilis
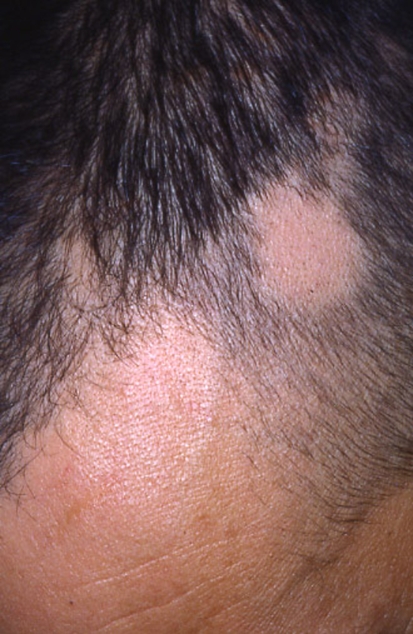
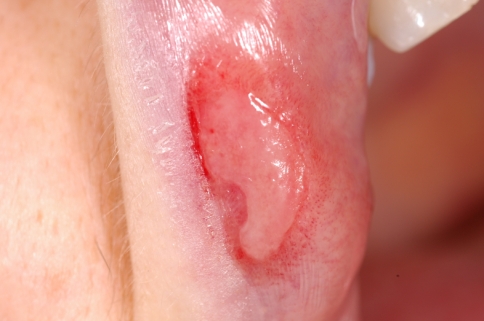
Condyloma lata (white gray mucous patches) are mainly found in the genital or anal area in 5–22% of patients; less frequently they may develop in the axilla, inframammary folds, face, oral commissures and toe webs (Fig. 4). Patients may also experience sore throats and hoarseness due to inflammatory involvement of the pharynx, larinx or the tonsils. Due to systemic hematogenous dissemination renal, ophthalmologic, hepatic, bone and joint diseases can be seen [2, 3, 32, 33]. During secondary syphilis CNS involvement can be seen under the form of meningitis (which usually occurs within the first 6 months of infection or during the secondary rash) or cranial nerve involvement. Deafness and optic neuritis may develop as sequelae [3, 6].
Fig. 4.
Macerated plaques (condylomata lata) of the toe webs
Secondary syphilis is clinically the least difficult to diagnose, although in some cases the clinical manifestations (especially the macular rash!) can be confused with a variety of skin diseases. Eczema, psoriasis, drug eruption, pityriasis, lichen planus and a syndrome with skin rash plus reactive arthritis, urethritis and conjunctivitis can be mimicked by syphilis [32, 34, 35]. In HIV infection things can be even more complicated considering that secondary syphilis may show totally unexpected features [3, 6].
Latent Syphilis
After the secondary stage, there is a latent period during which the patient does not show any clinical sign of infection. In this phase the diagnosis of syphilis can be made only through a serologic test [1–3].
Tertiary Syphilis
Late syphilis or tertiary disease develops in one third of untreated patients and is characterized by long-term complications. Late syphilis is also called gummatous syphilis with gummas that involve the skin, CNS, liver, spleen, bones and other organs [1–3]. Gummas are granulomatous-like lesions that are indolent and can range in size from tiny deposits to large masses. Skin manifestations may range from superficial tumefactive lesions to large granulomatous masses, which can be subjected to ulceration. Cardiovascular syphilis is today very rare and mainly represented by ascending aortitis. Neurosyphilis is caused by focal syphilitic endarteritis in the blood vessels of brain and spinal cord tissues. Clinical forms such as meningovascular syphilis and parenchymal neurosyphilis (tabes dorsalis) today are very rare due to the use of antibiotic treatment [1–3].
Congenital Syphilis
Congenital syphilis is observed when infection of the fetus in utero occurs in any untreated mother but is most likely to occur during the early stages of the infection [1–3, 6]. Transmission to the fetus is usually via the placenta, but may occur during delivery in the presence of maternal genital lesions. The risk of vertical transmission ranges from 70 to 100% for primary syphilis to 40% for early latent disease to 10% for late latent disease. The incidence of congenital syphilis has experienced a four to fivefold increase in the last 10 years. Worldwide the WHO estimates that maternal syphilis is responsible for 713,600–1,575,000 cases of congenital syphilis mainly in the developing world [36]. Recently, the WHO stated that the magnitude of the congenital syphilis burden, globally, equals that of HIV infection in neonates yet receives little attention. In the past 2 years, a WHO initiative has been implemented in order to eliminate congenital syphilis [37].
Depending on the severity of the disease, infection of the fetus may result in abortion, stillbirth, neonatal death or disease. The clinical manifestations of neonatal syphilis are quite variable and are a reflection of vasculitis. Congenital syphilis can be early or late with the early features that are similar to the manifestations of secondary syphilis in adults. These include generalized lymphadenopathy, maculopapular rash, hepatosplenomegaly, glomerulonephritis and bone alterations of tibia, hands, feet, clavicles, and skull. Late features of congenital syphilis include bone, teeth and nervous system alterations. A classic feature of congenital syphilis is the Hutchison’ triad formed by deafness, notched incisors and ocular interstitial keratitis [1–3, 6, 38].
HIV Infection and Syphilis
The interaction between HIV infection and syphilis is of great interest for the epidemiological and clinical implications that apply to both heterosexuals and MSM [39–42].
In the USA the increase in the rate of syphilis, between the years 1999–2005, has been mainly observed in men. This increase in the rate ratio of male to female patients was the result of a disproportionate number of cases among MSM. Large metropolitan communities of MSM have been particularly affected by concurrent epidemics of syphilis and HIV infection not only in the USA but also in Europe. In California more of 60% of MSM with syphilis are HIV infected, and it is estimated that, in major cities, 20–50% of MSM with syphilis have also HIV infection. Prevalence rate of HIV infection among syphilis cases has shown to be two or threefold higher than the prevalence of HIV infection in the general population of MSM in the USA [41]. The reasons underlying this upsurge are complex but include migration of people from high-prevalence areas, population mixing, reduction in safe sex in MSM who no longer feared AIDS, risk behavior such as use of the internet to meet partners and, use of recreational drugs. Recent findings have stressed, considering the high incidence of asymptomatic syphilis in HIV+ MSM, the importance of frequent (at least annually) routine syphilis testing in this group of patients [43].
Among MSM unprotected oral sex is considered a safe practice thus many of them acquire syphilis through oral sex. The fraction of syphilis transmission attributed to oral sex has been estimated between 20 and 46% in various literature reports coming from the USA and Europe (reviewed in Peterman and Furness [39]).
Several differences have been observed in the manifestations of syphilis in HIV+ patients. Primary disease seems less common than secondary syphilis and the infection shows a more aggressive behavior [40, 41, 44]. Furthermore, HIV itself or opportunistic infections may render problematic the interpretation of clinical and diagnostic findings. Primary syphilis in HIV+ patients is often symptomless and these patients frequently present with secondary or latent infection. It is relevant to mention that with the relative increase in importance of oral sex in transmission of syphilis the primary lesion may not be in the genitals [39].
In HIV+ patients syphilis presents an atypical clinical course with severe constitutional symptoms and unusual necrotic skin rashes (Fig. 5), organ involvement and a greater tendency to develop neurosyphilis and ocular involvement [40, 41, 44–46]. The skin lesions of syphilis show atypical features that may render the diagnosis difficult. Things can also be complicated by the fact that serology for syphilis in HIV infection can be falsely negative. Biopsy is commonly needed to establish the diagnosis but histology of secondary syphilis is highly variable even in the absence of HIV [47]. Course of syphilis may be more aggressive with increase frequency of lues maligna or the ulceronodular type [40, 41, 47–49]. In regard to neurosyphilis, this complication may present particular diagnostic and therapeutic challenges since both syphilis and HIV infection can have neurological involvement, which can be very variable (reviewed in Lynn and Lightman [40]). The commonest manifestation of ocular involvement is uveitis (intraocular inflammation) that, in these patients, may be more severe. Papillitis, optic neuritis, and retrobulbar optic neuritis may be additional presenting symptoms/ signs of coinfection with HIV [46].
Fig. 5.
Ulceronodular skin lesion of lue maligna
Oral Manifestations
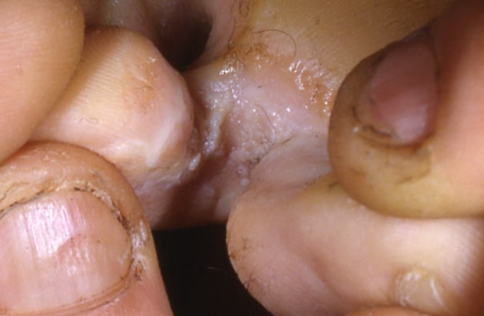
Oral chancres are observed in about 4–12% of patients with primary syphilis and occur at the site of penetration of the organism into the mucosa. Anatomical sites commonly affected are especially the tongue, gingiva, soft palate and lips (Fig. 6). Lesions appear as painless indurated ulcers associated with enlargement of the submandibular and cervical lymph nodes, lasting 3–7 weeks. The lesions are asymptomatic but are teaming with the spirochetes and are highly infectious. The lesion is usually single and heals spontaneously without scarring [50–53].
Fig. 6.
Oral chancre in a promiscuous woman who had unprotected oral sex
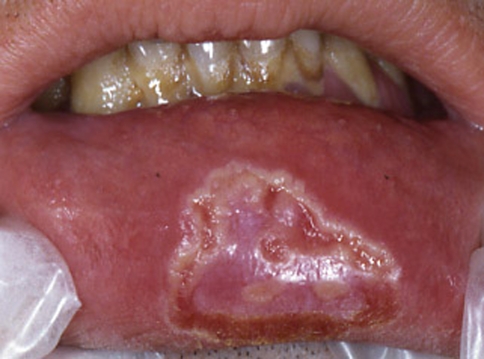
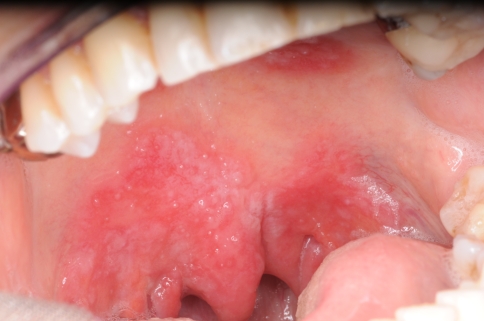
In secondary syphilis the oral manifestations are quite variable and may show highly aspecific features. The most well-recognized and characteristic lesions are multiple mucous patches that are slightly raised and covered by grayish, white pseudomembranes and surrounded by erythema. The typical sites are the soft palate and pillars, tongue and vestibular mucosa (Fig. 7) [31, 50, 51]. The cervical lymph nodes are usually enlarged and rubbery in consistency. Oral lesions are often painful and snail track ulcers result when multiple mucous patches become confluent. Often nonspecific pharyngitis, tonsillitis and laryngitis are associated (Fig. 8) [49–51, 53–55]. Syphilis presenting as isolated cervical lymphadenopathy has been also described [56].
Fig. 7.
Secondary oral syphilis: mucous patches covered by grayish, white pseudomembranes of the lower vestibular mucosa
Fig. 8.
Secondary oral syphilis with lesions on the soft palate. The patient also had pharyngitis and laryngitis
In HIV+ patients unusual oral, perioral and skin ulcers have been described under the term of lues maligna. In this situation the cutaneous manifestations are more florid with multiple large ulceronodular lesions localized on the face or other body parts [31, 47–49].
Tertiary syphilis manifests itself in the oral cavity as gumma localized mainly in the hard palate. Others sites may be the tongue, lips and soft palate. Gummas start as small ulcers that enlarge and may involve if left untreated the adjacent structures. An additional feature (although today of extremely rare observation!) is glossitis with mucosal atrophy, which may have a malignant potential [3, 50–53]; however, this point has been challenged by the consideration that instead tongue carcinomas in the past have been caused by arsenical compounds (formerly used to treat syphilis!) that notoriously are carcinogenetic.
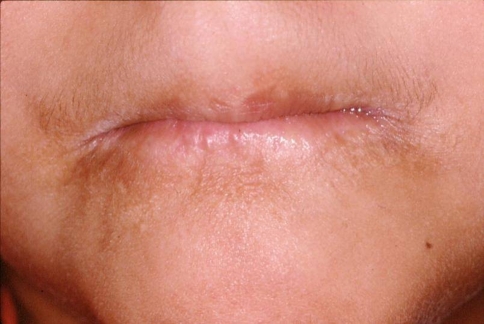
Congenital syphilis is mainly characterized by permanent cranial bone deformities such as saddle nose, palatal erosions, high- arched palate, protruding mandible and fontal bossing. Other features are alterations of the formation of both anterior and posterior teeth. The incisors (called Hutchinson’s teeth) show an increase of their width in the middle third of the crown and a central notch on the incisal edge. The molars (called mulberry molars) exhibit the occlusal surface with numerous small globular projections that resemble a mulberry. In some patients perioral fissuring (rhagades) can be seen (Fig. 9) [36, 53].
Fig. 9.
Perioral fissuring (rhagades) in a 3 year-old patient with congenital syphilis
Diagnosis
Laboratory Confirmation
Dark-Field Microscopy and Direct Fluorescence
The best ways to identify T. pallidum from primary or secondary lesions are the dark field illumination that shows the typical motile spirochetes and direct immunofluorescence, a method that uses a fluorescin-labeled antitreponeme serum [2, 3, 6, 57, 58]. Using these techniques it is possible to make the correct diagnosis in almost 100% of untreated primary syphilitic chancre. With the exception of macular lesions, all cutaneous and mucous membrane lesions of secondary syphilis contain treponemes. Thus, material obtained from an eroded moist lesion will almost always yield positive results [3, 57]. Nevertheless, limitations can be availability of a dark-field microscope and of a trained, experienced microscopist. Success is dependent on factors such as too little or too much fluid on the slide, thickness of smear, slide and cover slip, presence of refractile elements in the specimen, etc. The test lacks reliability in case of oral lesions because saprophytic treponemes that cannot be differentiated from T. pallidum are common in the oral cavity [3, 57].
For dark-field examination the surface of the lesion should be gently cleaned with sterile gauze soaked in 0.9% sterile saline avoiding bleeding as blood cells may masquerade the treponemes. The best result is obtained using the serous exudate that accumulates on the surface after squeezing the base of the lesion. The exudate is mixed with one or two drops of saline on a glass slide and covered with a plastic cover slip and then is examined under a dark-field microscope [2, 3, 57].
The direct fluorescent antibody test is the most specific test for the diagnosis of syphilis when lesions are present [57, 58]. The lesional exudate, after it has been smeared on a glass slide, is stained with fluorescein-labeled anti-T. pallidum immunoglobulin. In contrast to dark-field microscopic examination, this test is reliable for the evaluation of oral and anal lesions because only T. pallidum is stained. However, this test does not differentiate between T. pallidum and other pathogenic treponemes causing yaws, endemic syphilis and pinta [2, 3, 57, 58].
New specific and sensitive methods that employ reverse transcriptase PCR have been recently developed in order to detect very low numbers of spirochetes in clinical samples [58]. Although these methods are not standardized, they have been found to be highly sensitive, because able to detect as low as 1–10 organisms per specimen with high specificity. However, these methods have not yet reached the stage of widespread clinical application [57–59].
Serology
The serological laboratory tests that are used diagnostically are referred to as serologic tests for syphilis. These tests fall into two categories: nontreponemal tests for screening, and treponemal tests for confirmation [3, 6, 58]. The nontreponemal tests measure both IgG and IgM antiphospholipid antibodies formed by the host in response to lipoidal material released by damaged host cells early in infection and lipid from the cell surfaces of the treponeme itself. Instead, treponemal tests use T. pallidum or its components as the antigen. Despite their limitations and the complexity of interpretation, serological tests play a pivotal role in the diagnosis and follow-up of syphilis [3, 6, 58].
The nonspecific nontreponemal reaginic antibody tests (VDRL, RPR, ART) are inexpensive, rapid, and convenient for screening large number of sera and are also quite helpful as an indication of disease activity [1–3, 57, 58]. These tests, developed as economical tests for mass screening, are not specific for T. pallidum therefore a variety of acute or chronic ailments may result in biological false-positive reactions. Also, these tests are reactive in almost all patients with secondary and latent disease but are less sensitive in patients with primary syphilis.
The treponemal tests are considered today the most sensitive and specific [3, 58]. These tests are the fluorescent treponemal antibody (FTA-ABS) and the T. pallidum hemagglutination (TPHA) and the MHA-TP which is a modified version of TPHA. The former is able to reveal syphilis antibodies in the patient’s serum in the early stage, while the last two become positive somewhat later during the course of infection. The MHA-TP test is highly specific (>98%) and sensitive in patients with secondary syphilis. The T. pallidum immobilization (TPI) test reveals specific antibodies after the second week of infection but is rarely employed because it requires live treponemes and is expensive.
The diagnosis of secondary syphilis remains largely clinical with support by serologic methods. The RPR and VDRL are uniformly positive (with high dilution: at least 1:32) in secondary syphilis; thus a negative or non reactive test in a patient with a suspect of syphilitic rash in reality indicates the absence of the infection [3, 57, 58]. In Table 3 is reported an interpretation scheme of the serological tests for syphilis.
Table 3.
Interpretative outline of serological tests
| Nontreponemal tests | Treponemal tests | Significance |
|---|---|---|
| + | + | Syphilis, yaws or pinta |
| + | − | Absence of syphilis; false positivity |
| − | + | Primary or latent syphilis; prior, treated or untreated syphilis; yaws or pinta |
| − | − | Incubating syphilis; absence of syphilis |
Neurosyphilis is diagnosed in cerebrospinal fluid using the same methods [3, 58].
Histopathology
The diagnosis of syphilis is often based on clinical and serological findings without the employment of biopsy. However, because of the multiform clinical manifestations, rarity of oral lesions and unusual presentation in HIV+ patients, histological examination may be pivotal to confirm the diagnosis [2, 3, 60–62].
In primary lesions, the epidermis shows changes such as acanthosis, spongiosis and exocytosis of lymphocytes and neutrophils. In the center of the chancre, the epidermis is thinner or completely absent and, when present, shows permeation of inflammatory cells. The connective tissue appears edematous with a dense perivascular and interstitial lymphohistiocytic and plasmacellular infiltrate which occupies the whole dermis. Also an obliterative endarteritis characterized by endothelial swelling and mural edema is observed [60, 61]. Most of the features described above are comparable to those observed in lesions of secondary syphilis. By silver staining or immunofluorescent techniques, spirochetes are usually identified at the dermal-epidermal junction and around the capillaries [60, 61].
In secondary syphilis of the skin ulceration may be present and the surface epithelium often demonstrates hyperplasia with significant spongiosis and exocytosis. The most common histologic changes of secondary syphilis are: (a) superficial and deep perivascular infiltrate containing plasma cells; (b) lichenoid infiltrate obscuring the epithelial-lamina propria junction; (c) lichenoid as well as superficial and deep perivascular pattern; (d) epithelial hyperplasia, and (e) thickening and/or dilatation of lamina propria blood vessels. Of interest, a perineural plasmacellular infiltrate can be seen in about 2/3 of cases of skin or mucosal lesions [53–56, 60, 61].
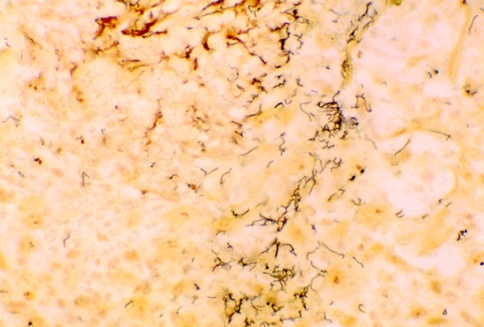
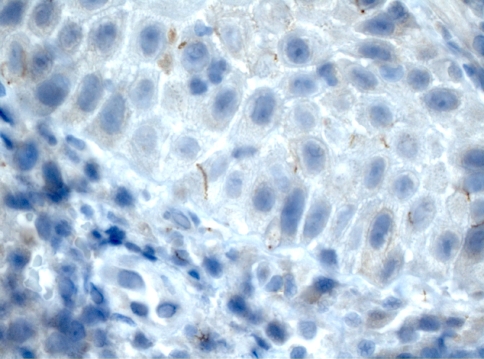
The usual method for detecting spirochetes in tissue sections is the silver stain (Fig. 10); however, they are often difficult to detect due to marked background staining frequently seen with this technique. Alternative methods for tissue detection of spirochetes are immunofluorescence using both fresh and fixed paraffin-embedded tissue [62–64]. Another method is the use of the immunoperoxidase method [65]. Hoang et al. [62] have found that immunostaining with polyclonal or monoclonal antibodies against T. pallidum is a more sensitive and specific method in comparison to the silver stain method for detecting spirochetes in formalin-fixed, paraffin-embedded tissue of secondary syphilis. Organism demonstrated by this method can be seen, due to the near absence of background staining, in the epithelial lamina, at the junction or in the connective tissue around capillaries (Fig. 11).
Fig. 10.
Silver stain of syphilis showing the corkscrew-shaped treponemes (×40)
Fig. 11.
Immunohistochemical method: the spirochetes are visible in the lower epithelial lamina (×40)
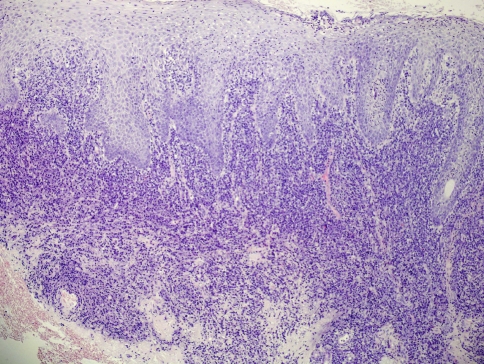
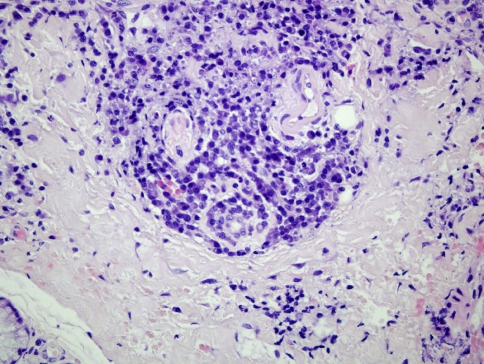
Regarding oral syphilis, few papers have reported detailed studies of its histological features and this may be due to the rarity of the oral manifestations or that biopsy is rarely taken [60]. Several Authors [60, 66] have stressed on the fact that a key microscopic feature is plasma cell infiltration and proliferative endarteritis, at least in primary and secondary disease. Endothelial cells proliferate within small arteries and arterioles, producing a concentric layering of cells that result in a narrowed lumen. Plasma cell, along with lymphocytes and macrophages can be found in a perivascular distribution or as a band-like infiltrate in the lamina propria (Fig. 12). The presence of plasma cells in skin is unusual and immediately raises the possibility of syphilis, particularly if there is a perivascular distribution [61]. However, it should be stressed that plasma cells are common in oral biopsies then their significance must be critically evaluated [60, 66]. The presence of a plasma cell infiltrate which extents deeply into the lamina propria and around the capillaries is a finding that should suggests the diagnosis of syphilis. As for skin syphilis a perineural plasmacellular infiltrate has also been described in oral lesions (Fig. 13) [60].
Fig. 12.
Secondary oral syphilis. Plasma cell, along with lymphocytes and macrophages are visible as a band-like infiltrate in the lamina propria and with a perivascular distribution (EE, ×20)
Fig. 13.
Secondary oral syphilis. A perineural and perivascular plasmacellular infiltrate is visible in the deep lamina propria (EE, ×40)
Oral tertiary syphilis typically shows absence of the epithelial lamina with peripheral hyperplasia of pseudoepitheliomatous type. The lamina propria contains foci of granulomatous inflammation (large central zone of acellular necrosis) with well-circumscribed collections of histiocytes and multinucleated giant cells. Despite the use of special stains, the spirochetes are not found in gummas [3, 60, 66].
In conclusion, although there is no single microscopic feature specific to syphilis if the clinical information are matched with the microscopic features the combination of the two can provide a logical basis for further laboratory investigations able to rule out the infection.
Principles of Treatment
Drugs of Choice
Benzathine penicillin G or aqueous procaine penicillin G remains the drug of choice for all forms of syphilis. A concentration of penicillin above 0.018 mg/L is considered treponemicidal. However, serum concentration equivalent to the maximally effective in vitro level (0.36 mg/L) is recommended for at least 7–10 days in early syphilis and longer in late stage [67]. This drug is very effective in early syphilis and less effective in late stages. The principal contraindication is hypersensitivity to the penicillins [3, 6, 67].
Oral tetracyclines are also effective in the treatment of syphilis for patients who are allergic to penicillin. Tetracycline, 500 mg orally four time daily for 14 days, or doxycycline 100 mg orally twice for 14 days are effective against T. pallidum. These regimens are valid for primary, secondary, and early latent syphilis. In syphilis of more than 1 year’s duration (or unknown duration), treatment is given for 28 days in the same dosage. Recent data suggest that both ceftriaxone and azithromycin are effective for the treatment of early syphilis [2, 3]. A problem with azithromycin is increasing resistance of T. pallidum [6, 67].
Primary Syphilis
Benzathine penicillin G, 2, 4 million units intramuscularly in the gluteal area, is given once. In penicillin-allergic patients: tetracycline, 500 mg orally four time daily for 14 days, or doxycycline 100 mg orally twice for 14 days.
Secondary Syphilis
Therapy is as for primary syphilis. If neurosyphilis is present then treatment must be more aggressive (see below).
Latent and Tertiary Syphilis
These forms are treated with benzathine penicillin G intramuscularly (2.4 million units weekly for 3 consecutive weeks). In case of CNS involvement the same regimen as neurosyphilis applies (see below).
Neurosyphilis
Present recommendations for the treatment of neurosyphilis are the use of high doses of short-acting penicillin to obtain better penetration and higher levels of drug in the cerebrospinal fluid [1–3, 6, 66]. Recommended regimen is 3–4 million units of aqueous crystalline penicillin G intravenously every 4 h or as a continuous infusion for 10–14 days. An alternative regimen is 2–4 million units of procaine penicillin intramuscularly once daily along with 500 mg of probenecid orally four time daily, both for 10–14 days. Several Authors recommended additional administration of 2.4 million units of benzathine penicillin intramuscularly once weekly for 3 weeks at the end of the classic regimen [3, 6, 67].
Syphilis in HIV+ Patients
Treatment of HIV+ patients with primary and secondary syphilis is the same as for HIV-negative patients. However, because syphilis in HIV+ patients has a more aggressive course and treatment failure with benzathine penicillin may occur more commonly, some Authors recommend a more aggressive treatment as 3 doses of 2.4 million units of benzathine penicillin intramuscularly at weekly intervals instead of a single administration [68, 69]. In HIV+ patients, accurate clinical and serologic follow-up should be done at 3, 6, 9, 12, and 24 months [3, 6].
Comment
In recent years, syphilis outbreaks have been reported in several parts of the world. The current epidemic in the US and Europe has largely involved HIV+ MSM. Since a misdiagnosis can have serious consequences for the patients clinicians should be aware of the protean manifestations of syphilis and difficulties in the diagnosis of the disease. Young clinicians in particular are no longer familiar with the variable clinical symptoms and signs of syphilis.
Acknowledgement
The authors thank Prof. Oslei Paes de Almeida, FOP-UNICAMP, Brazil, for performing the immunohistochemistry reactions in Fig. 11.
References
- 1.Hook EW, III, Marra CM. Acquired syphilis in adults. New Engl J Med. 1992;326:1060–1068. doi: 10.1056/NEJM199204163261606. [DOI] [PubMed] [Google Scholar]
- 2.Tramont EC. Treponema pallidum (syphilis) In: Mandell GL, Benett JF, Dolin R, editors. Principles and practice of infectious diseases. 6. Orlando, FL: Churchill Livingstone; 2005. pp. 2768–2784. [Google Scholar]
- 3.Sanchez MR. Syphilis. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick’s dermatology in general medicine. 7. New York: Mc Graw Hill; 2008. pp. 1955–1977. [Google Scholar]
- 4.Tramont EC. Syphilis in adults: from Christopher Columbus to Sir Alexander Fleming to AIDS. Clin Infect Dis. 1995;21:1361–1369. doi: 10.1093/clinids/21.6.1361. [DOI] [PubMed] [Google Scholar]
- 5.Zimmer C. Isolated tribe gives clues to the origins of syphilis. Science. 2008;319:272. doi: 10.1126/science.319.5861.272. [DOI] [PubMed] [Google Scholar]
- 6.Kent ME, Romanelli F. Reexamining syphilis: an update on epidemiology, clinical manifestations, and management. Ann Pharmacother. 2008;42:22–36. doi: 10.1345/aph.1K086. [DOI] [PubMed] [Google Scholar]
- 7.Buchaz K, Greenberg A, Onorato I, et al. Syphilis epidemics and human immunodeficiency virus (HIV) incidence among men who have sex with men in the United States: implications for HIV prevention. Sex Transm Dis. 2005;32:S73–S79. doi: 10.1097/01.olq.0000180466.62579.4b. [DOI] [PubMed] [Google Scholar]
- 8.Ruan Y, Luo F, Jia Y, et al. Risk factors for syphilis and prevalence of HIV, hepatitis B and C among men who have sex with men in Beijing, China: implications for HIV prevention. AIDS Behav. 2008. doi: 10.1007/s10461-008-9503-0. [DOI] [PMC free article] [PubMed]
- 9.Velicko I, Arneborn M, Blaxhult A. Syphilis epidemiology in Sweden: re-emergence since 2000 primarily due to spread among men who have sex with men. Euro Surveill. 2008;13:1–5. doi: 10.2807/ese.13.50.19063-en. [DOI] [PubMed] [Google Scholar]
- 10.Kilmarx PH, St Louis ME. The evolving epidemiology of syphilis. Am J Public Health. 1995;85:1053–1054. doi: 10.2105/AJPH.85.8_Pt_1.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sexually transmitted disease surveillance, 1993. Georgia: Centers for Disease Control and Prevention; 1994. [Google Scholar]
- 12.Druskin LM. Syphilis makes a comeback. Int J STD AIDS. 1996;7:7–9. doi: 10.1258/0956462961917122. [DOI] [PubMed] [Google Scholar]
- 13.Nakashima AK, Rolfs RT, Flock ML, et al. Epidemiology of syphilis in the United States, 1941–1993. Sex Transm Dis. 1996;23:16–23. doi: 10.1097/00007435-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Aral SO. The social context of syphilis persistence in the southeastern United States. Sex Transm Dis. 1996;23:9–15. doi: 10.1097/00007435-199601000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Mushinski M. Sexually transmitted diseases: United States, 1995. Stat Bull Metrop Insur Co. 1997;78:10–17. [PubMed] [Google Scholar]
- 16.Garnett GP, Aral SO, Hoyle DV. The natural history of syphilis. Implications for transmission dynamics and control of infection. Sex Transm Dis. 1997;24:185–200. doi: 10.1097/00007435-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Center for Diseases Control and Prevention Primary and secondary syphilis—US, 200-2001-MMWR. Morb Mortal Wkly Rep. 2002;51:971–973. [PubMed] [Google Scholar]
- 18.Sexually transmitted infections in the United Kingdom, new episodes seen at genitourinary medicine clinics, 1991–001. London: Public Health Laboratory Service; 2002. [Google Scholar]
- 19.Tichonova L, Borisenko K, Ward H, et al. Epidemics of syphilis in the Russian Federation: trends, origins, and priorities for control. Lancet. 1997;350:210–213. doi: 10.1016/S0140-6736(97)01382-2. [DOI] [PubMed] [Google Scholar]
- 20.Muga R, Roca J, Tor J, et al. Syphilis in injecting drug users: clues for high-risk sexual behavior in female IDUs. Int J STD AIDS. 1997;8:225–228. doi: 10.1258/0956462971919967. [DOI] [PubMed] [Google Scholar]
- 21.Dallabetta G, Diomi MC. Treating sexually transmitted diseases to control HIV transmission. Curr Opin Infect Dis. 1997;10:22–25. doi: 10.1097/00001432-199702000-00006. [DOI] [Google Scholar]
- 22.D’Souza G, Lee JH, Paffel IM. Outbreak of syphilis among men who have sex with men in Houston, Texas. Sex Transm Dis. 2003;30:872–873. doi: 10.1097/01.OLQ.0000091144.72555.13. [DOI] [PubMed] [Google Scholar]
- 23.Hopkis S, Lyons F, Coleman C, et al. Resurgence in infections syphilis, in Ireland: an epidemiological study. Sex Transm Dis. 2004;31:317–321. doi: 10.1097/01.OLQ.0000123653.84940.59. [DOI] [PubMed] [Google Scholar]
- 24.Leber A, MacPherson P, Lee BC. Epidemiology of infectious syphilis in Ottawa. Recurring themes revisited. Can J Public Health. 2008;99:401–405. doi: 10.1007/BF03405250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bissessor M, Fairley K, Guingand D, et al. Delay in the diagnosis of early syphilis among men who have sex with men: need for greater community and health provider education. Int J STD AIDS. 2009;20:52–53. doi: 10.1258/ijsa.2008.008254. [DOI] [PubMed] [Google Scholar]
- 26.Fraser CM, Norris SJ, Weinstock GM, et al. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science. 1998;281:375–388. doi: 10.1126/science.281.5375.375. [DOI] [PubMed] [Google Scholar]
- 27.Radolf JD, Norgard MV, Schulz WW. Outer membrane ultrastructure explains the limited antigenicity of virulent Treponema pallidum. Proc Natl Acad Sci USA. 1989;86:2051–2055. doi: 10.1073/pnas.86.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peeling RW, Hook EW. The pathogenesis of syphilis: the great mimicker, revisited. J Pathol. 2006;208:224–232. doi: 10.1002/path.1903. [DOI] [PubMed] [Google Scholar]
- 29.Cox DL, Chang P, McDowall AW, Radolf JD. The outer membrane, not a coat of host proteins, limits antigenicity of virulent Treponema pallidum. Infect Immun. 1992;60:1076–1083. doi: 10.1128/iai.60.3.1076-1083.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lukehart SA. Scientific monogamy: thirty years dancing with the same bug. 2007 Thomas Parran Award lecture. Sex Transm Dis. 2008;35:2–7. doi: 10.1097/OLQ.0b013e318162c4f2. [DOI] [PubMed] [Google Scholar]
- 31.Domantay-Apostol GP, Handog EB, Gabriel MTG. Syphilis: the international challenge of the great imitator. Dermatol Clin. 2008;26:191–202. doi: 10.1016/j.det.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Baughn RE, Musher DM. Secondary syphilitic lesions. Clin Microbiol Rev. 2005;18:205–216. doi: 10.1128/CMR.18.1.205-216.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenstone CL, Saint S, Moseley RH. A hand-carried diagnosis. N Engl J Med. 2007;356:2407–2411. doi: 10.1056/NEJMcps062271. [DOI] [PubMed] [Google Scholar]
- 34.Monastirli A, Pasmatzi E, Georgiou S, Vryzaki E, Tsambaos D. Lichen-planus-like secondary syphilis in an 83-year-old woman. Clin Exp Dermatol. 2008;33:780–1. doi: 10.1111/j.1365-2230.2008.02831.x. [DOI] [PubMed] [Google Scholar]
- 35.Kishimoto M, Lee MJ, Mor A, et al. Syphilis mimicking Reiter’s syndrome in an HIV-positive patient. Am J Med Sci. 2006;332:90–92. doi: 10.1097/00000441-200608000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Simms I, Broutet N. Congenital syphilis re-emerging. JDDG. 2008;6:269–272. doi: 10.1111/j.1610-0387.2008.06490.x. [DOI] [PubMed] [Google Scholar]
- 37.Schimid G, Stoner BP, Hawkes S, et al. The need and plan for global elimination of congenital syphilis. Sex Transm Dis. 2007;34:S5–S10. doi: 10.1097/01.olq.0000261456.09797.1b. [DOI] [PubMed] [Google Scholar]
- 38.Chakraborty R, Luck S. Syphilis is on the increase: the implications for child health. Arch Dis Child. 2008;93:105–109. doi: 10.1136/adc.2006.103515. [DOI] [PubMed] [Google Scholar]
- 39.Peterman TA, Furness BW. The resurgence of syphilis among men who have sex with men. Curr Opin Infect Dis. 2007;20:54–59. doi: 10.1097/QCO.0b013e32801158cc. [DOI] [PubMed] [Google Scholar]
- 40.Lynn WA, Lightman S. Syphilis and HIV: a dangerous combination. Lancet Infect Dis. 2004;4:456–466. doi: 10.1016/S1473-3099(04)01061-8. [DOI] [PubMed] [Google Scholar]
- 41.Zetola NM, Klausner JD. Syphilis and HIV infection: an update. Clin Infect Dis. 2007;44:1222–1228. doi: 10.1086/513427. [DOI] [PubMed] [Google Scholar]
- 42.Blocker ME, Levine W, St Lou ME. HIV prevalence in patients with syphilis. United States. Sex Transm Dis. 2000;27:53–59. doi: 10.1097/00007435-200001000-00011. [DOI] [PubMed] [Google Scholar]
- 43.Branger J, Meer JTM, Ketel RJ, et al. High incidence of asymptomatic syphilis in HIV-infected MSM justifies routine screening. Sex Transm Dis. 2009;36:84–85. doi: 10.1097/OLQ.0b013e318186debb. [DOI] [PubMed] [Google Scholar]
- 44.O’Mahony C, Rodgers CA, Mendelsohn SS, et al. Rapidly progressive syphilis in early HIV infection. Int J STD AIDS. 1997;8:275–277. doi: 10.1258/0956462971919912. [DOI] [PubMed] [Google Scholar]
- 45.Tomberlin MG, Holton PD, Owens JL, et al. Evaluation of neurosyphilis in HIV-infected individuals. Clin Infect Dis. 1994;18:288–294. doi: 10.1093/clinids/18.3.288. [DOI] [PubMed] [Google Scholar]
- 46.Aldave AJ, King JA, Cunnigham ET., Jr Ocular syphilis. Curr Opin Ophtalmol. 2001;12:433–441. doi: 10.1097/00055735-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 47.Angus J, Langan SM, Staway A, et al. The many faces of secondary syphilis: a re-emergence of an old disease. Clin Exp Dermatol. 2006;31:741–745. doi: 10.1111/j.1365-2230.2006.02163.x. [DOI] [PubMed] [Google Scholar]
- 48.Ficarra G, Zaragoza AM, Stendardi L, et al. Early oral presentation of lues maligna in a patient with HIV infection. A case report. Oral Surg Oral Med Oral Pathol. 1993;75:728–732. doi: 10.1016/0030-4220(93)90431-3. [DOI] [PubMed] [Google Scholar]
- 49.Watson KMT, White JML, Salisbury JR, et al. Lues maligna. Clin Exp Dermatol. 2004;29:625–627. doi: 10.1111/j.1365-2230.2004.01630.x. [DOI] [PubMed] [Google Scholar]
- 50.Bruce AJ, Rogers RS. Oral manifestations of sexually transmitted diseases. Clin Dermatol. 2004;22:520–527. doi: 10.1016/j.clindermatol.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 51.Scott CM, Flint SR. Oral syphilis-re-emergence of an old disease with oral manifestations. Int J Oral Maxillofac Surg. 2005;34:58–63. doi: 10.1016/j.ijom.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 52.Leao JC, Gueiros LA, Porter SR. Oral manifestations of syphilis. Clinics. 2006;61:161–166. doi: 10.1590/S1807-59322006000200012. [DOI] [PubMed] [Google Scholar]
- 53.Bacterial infections. In: Neville BW, Damm DD, Allen CM, Bouquot JE, editors. Oral and maxillofacial pathology. 3rd ed. Philadelphia: Saunders Elsevier Company; 2009. p. 181–212.
- 54.Junkins-Hopkins JM. Multiple painful oral ulcerations. Secondary syphilis. Arch Fam Med. 1996;5:379–380. doi: 10.1001/archfami.5.7.379. [DOI] [PubMed] [Google Scholar]
- 55.Oddò D, Carrasco G, Capdeville F, et al. Syphilitic tonsillitis presenting as an ulcerated tonsillar tumor with ipsilateral lymphadenopathy. Ann Diagn Pathol. 2007;11:353–357. doi: 10.1016/j.anndiagpath.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 56.Crevel R, Grefte JMM, Doorninck D, et al. Syphilis presenting as isolated cervical lymphadenopathy: two related cases. J Infect. 2009;58:76–78. doi: 10.1016/j.jinf.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 57.Cummings MC, Lukehart SA, Marra C, et al. Comparison of methods for the detection of treponema pallidum in lesions of early syphilis. Sex Transm Dis. 1996;23:366–369. doi: 10.1097/00007435-199609000-00004. [DOI] [PubMed] [Google Scholar]
- 58.Ratnam S. The laboratory diagnosis of syphilis. Can J Infect Dis Med Microbiolol. 2005;16:45–51. doi: 10.1155/2005/597580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Centurion-Lara A, Castro C, Shaffer JM, et al. Detection of Treponema pallidum by sensitive reverse transcriptase PCR. J Clin Microbiol. 1997;35:1348–1352. doi: 10.1128/jcm.35.6.1348-1352.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barrett AW, Villarroel Dorrego M, Hodgson TA, et al. The histopathology of syphilis of the oral mucosa. J Oral Pathol Med. 2004;33:286–291. doi: 10.1111/j.0904-2512.2004.00099.x. [DOI] [PubMed] [Google Scholar]
- 61.Crowson AN, Magro C, Mihim M. Treponemal diseases. In: Elder D, Elenitas R, Jaworsky C, Johnson B Jr, editors. Lever’s Histopathology of the skin. 8. Philadelphia: Lippincott-Raven; 1997. pp. 503–515. [Google Scholar]
- 62.Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004;31:595–599. doi: 10.1111/j.0303-6987.2004.00236.x. [DOI] [PubMed] [Google Scholar]
- 63.Yobs AR, Brown L, Hunter EF. Fluorescent antibody techniques in early syphilis. As applied to the demonstration of T. pallidum in lesions in the rabbit and in the human. Arch Pathol. 1964;77:220–225. [PubMed] [Google Scholar]
- 64.Al-Sammarai HT, Henderson WG. Immunofluorescent staining of Treponema pallidum and Treponema pertenue in tissues fixed by formalin and embedded in paraffin wax. Br J Vener Dis. 1977;53:1–11. doi: 10.1136/sti.53.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beckett JH, Bigbee JW. Immunoperoxidase localization of Treponema pallidum: its use in formaldehyde-fixed and paraffin embedded tissue sections. Arch Pathol Lab Med. 1979;103:135–138. [PubMed] [Google Scholar]
- 66.Meyer I, Shklar G. The oral manifestations of acquired syphilis. A study of eighty-one cases. Oral Surg Oral Med Oral Pathol. 1967;23:45–57. doi: 10.1016/0030-4220(67)90484-7. [DOI] [PubMed] [Google Scholar]
- 67.Center for Disease Control and Prevention Sexually transmitted diseases treatment guidelines: 2006 MMWR Morb Mortal Wkly Rep 20065522–50.17183239 [Google Scholar]
- 68.Yinnon AM, Coury-Doniger P, Polito R, et al. Serologic response of syphilis in patients with HIV infection. Arch Intern Med. 1996;156:321–325. doi: 10.1001/archinte.156.3.321. [DOI] [PubMed] [Google Scholar]
- 69.Musher DM, Hamill RJ, Baughin RE. Effect of human immunodeficiency virus (HIV) on the course of syphilis and on the response to treatment. Ann Intern Med. 1990;113:872–876. doi: 10.7326/0003-4819-113-11-872. [DOI] [PubMed] [Google Scholar]