Abstract
Host cell-derived protein impurities may be present at low levels in biopharmaceutical products. Antibodies to host cell proteins are present in individuals with no known exposure to these products. In this study, antibodies to drug process-specific Chinese hamster ovary host cell-derived proteins (CHO-HCP) were measured in unexposed individuals using a validated enzyme-linked immunosorbent assay. Samples that tested positive for anti-CHO-HCP reactivity were further characterized to determine the isotypes and IgG subclasses expressed in each positive individual. The specificity of the detected anti-CHO-HCP antibody isotypes was confirmed by the competitive inhibition assay and the uncoated plate specificity testing. These antibody characterization experiments revealed that the prevalent anti-CHO-HCP antibody subclasses were predominantly IgG1 (present in 66.6% of individuals) and IgG2 (60%), with 33.3% positive for IgG3 while IgG4 was detected in low abundance. Forty percent (40%) of the individuals with IgG type reactivity were also identified as IgM-positive. Anti-CHO-HCP-specific IgE was not detected in the assays used in this study. Some individuals exhibited single isotype anti-CHO-HCP reactivity; others contained a combination of multiple antibody isotypes. Data presented in this study demonstrate the isotypic complexity and the high prevalence of anti-CHO-HCP antibodies in human serum with no known exposure to CHO cell-derived therapeutic biologics.
Key words: antibody isotypes, Chinese hamster ovary cells, host cell protein, immunogenicity response characterization, specificity
INTRODUCTION
Biopharmaceutical products can be produced from various host cell systems, including bacteria, yeast, insect cells, and mammalian cells. Today, 60–70% of recombinant protein pharmaceuticals and almost all currently approved therapeutic antibodies are produced in mammalian cells due to the advantage these cells have with regard to providing more sophisticated protein folding, secretion, and posttranslational modifications (1). Chinese hamster ovary (CHO) cells have gained regulatory approval for commercial use and are the most commonly used mammalian cell lines for protein therapeutics production (2–4). In addition to the desirable protein folding and posttranslational modifications, CHO cells are amenable to genetic manipulations and volumetric scalability, allowing for expression of recombinant biologics in large scale. More than two decades of commercial production proved CHO cells as safe host cells for the production of biologics compatible and bioactive in humans (5).
The recombinant therapeutics produced from CHO host cells, especially in scale-up production, can contain residual components of the host cells. Some levels of the host cell proteins (HCP) may potentially be associated with the therapeutic products even after multiple steps of purification. Since the HCP are foreign to humans, there is a potential to induce an immune response to HCP in patients dosed with the drug (6–8). Although many companies are taking bioanalytical measures to test for the HCP levels in the final products and release the batches with low impurity content, there is no explicit requirement for the types of testing and the acceptable levels of HCP in products (9). Recognizing the complexity of each drug manufacturing process and the variable levels of HCP associated with each process, the need to characterize the anti-host cell protein antibody response in humans may be considered under specific circumstances. Antibodies to CHO-HCP were found present in normal individuals with no known exposure to therapeutic drugs (10, unpublished data). In this study, additional normal human serum samples collected from unexposed individuals were analyzed for the reactivity to CHO-HCP. The antibody isotypes that constitute the reactivity were further characterized and confirmed in the specificity testing.
MATERIALS AND METHODS
Anti-CHO-HCP Antibody Detection
A validated enzyme-linked immunosorbent assay (ELISA) was used to screen normal human serum samples collected from healthy donors (Bioreclamation INC.) for the anti-CHO-HCP antibody reactivity. Briefly, 96-well microtiter plates (Costar) were coated with CHO-HCP (Wyeth Research) at a concentration of 0.5 μg/ml in 0.1 M carbonate/bicarbonate buffer (pH 9.6). CHO-HCP was partially purified from CHO cells grown under drug manufacturing processes. After incubation at 4°C overnight, plates were blocked with phosphate-buffered saline with 0.05% Tween-20 (PBST)/5% bovine serum albumin (BSA). After washing with the Tris high-salt buffer (50 mM Tris, 1 mM glycine, and 0.5 M NaCl) containing 0.05% Tween-20 (THST), plates were incubated with human serum samples at a dilution of 1:50 for 2 h at room temperature. A pool of sheep anti-CHO-HCP serum (Wyeth Research) was used as the assay positive control. Pooled normal human serum with low reactivity for CHO-HCP was used as the assay negative control. After washing with THST buffer, the bound anti-CHO-HCP immunoglobulins (Igs) were detected with a mixture of protein A and protein G horseradish peroxidase (HRP) conjugates (Invitrogen). 2,2′-Azino-di-(3-ethyl-benzthiazoline-6-sulfonate) (ABTS, KPL) was used as an enzymatic substrate for signal generation and colorimetric readout. Plates were read at wavelength 405 nm in a plate reader. Plate cut point ODs were calculated by multiplying the negative control mean OD value on each plate by the multiplication factor of 2. Samples with OD values above the cut points were reported as positive. Samples with OD values below the cut points were reported as negative.
Antibody Isotyping Detector Reagents Specificity Analysis
The specificity of the detector reagents for isotyping the anti-CHO-HCP antibodies was tested by coating 96-well microtiter plates (Costar) with purified Ig isotypes including human myeloma IgG1, IgG2, IgG3, IgG4 (MP Biomedical), IgM (Thermo Scientific), and IgE (Biodesign) for overnight incubation at 4°C. The plates were washed, blocked, and incubated with the detector reagents mouse anti-human IgG-HRP (Southern Biotech, clone JDC-10), mouse anti-human IgG1-HRP (Southern Biotech, clone 4E3), mouse anti-human IgG2-HRP (Southern Biotech, clone 31-7-4), mouse anti-human IgG3-HRP (Southern Biotech, clone HP6050), mouse anti-human IgG4-HRP (Southern Biotech, clone HP6025), mouse anti-human IgM-HRP (Southern Biotech, clone SA-DA4), mouse anti-human IgE-HRP (Southern Biotech, clone B3102E8), and biotin mouse anti-human IgG4 (BD Pharmingen, clone JDC-14) for 1 h at room temperature, respectively. For the biotin mouse anti-human IgG4 detector, a subsequent incubation with avidin-D-HRP was applied. The plates were developed in accordance with the standard procedures described above.
Isotyping of the Anti-CHO-HCP Antibodies
The general assay procedure for isotyping of the anti-CHO-HCP antibodies is similar to that for the anti-CHO-HCP antibody detection in human serum. Briefly, 96-well microtiter plates were coated with purified CHO-HCP at a concentration of 0.5 μg/ml in 0.1 M carbonate/bicarbonate buffer (pH 9.6). After incubation at 4°C overnight, plates were blocked with PBST/5% BSA and washed with the THST buffer. Human serum samples at a dilution of 1:50 were incubated on the plates for 2 h at room temperature. After a washing with THST buffer, the bound anti-CHO-HCP antibodies were incubated with the isotyping detector reagents at a dilution of 1:1,000 for 1 h at room temperature. The plates were developed in accordance with the standard procedures.
For the detection of IgG4 and IgE type reactivity, a method using the biotinylated anti-human IgG4 or IgE antibody detectors was evaluated. In this method, the plate-bound anti-CHO-HCP antibodies in human serum samples were incubated with 1 μg/ml of biotin mouse anti-human IgG4 or 1 μg/ml of biotin mouse anti-human IgE (BD Pharmingen) for 1 h at room temperature. After washing, the plates were incubated with avidin-D-HRP conjugate (Vector) for 1 h at room temperature, then proceeded with plate development.
Specificity Testing
Competitive Inhibition ELISA
The competition ELISA was performed essentially as described for the ELISAs for detection of the anti-CHO-HCP antibody and the antibody isotypes, except that samples were pre-incubated with the purified CHO-HCP. The human serum samples were spiked with CHO-HCP at concentrations of 500, 100, 10, and 0 μg/ml, respectively. After 1-h incubation at room temperature, the spiked and unspiked samples were diluted to 1:50 and then added to the CHO-HCP-coated plates for 2-h incubation at room temperature. The ability of the varying amounts of the CHO-HCP in serum samples to compete with the plate-bound CHO-HCP for binding to anti-CHO-HCP antibody or antibody isotypes was evaluated based on the amount of reduction of OD values.
Uncoated Plate Method
The antibody specificity was confirmed by demonstrating that a signal generated by a sample with positive anti-CHO-HCP signal on a CHO-HCP-coated plate decreases or scores negatively when CHO-HCP was not coated onto the plate. The CHO-HCP-uncoated plates were treated with the coating buffer (0.1 M carbonate/bicarbonate buffer (pH 9.6)) only and blocked under the same conditions as CHO-HCP-coated plates. Samples were analyzed on the uncoated and the coated plates in parallel.
RESULTS
Prevalence of Anti-CHO-HCP Reactivity in Human Serum Samples
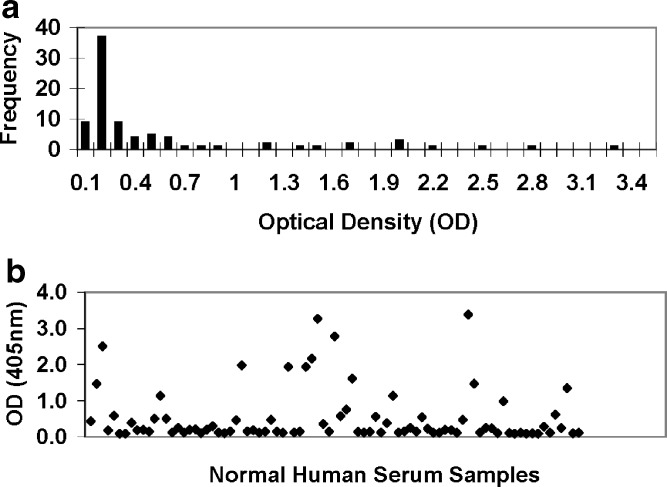
A total of 83 normal human serum samples from 83 individuals with no known prior exposure to therapeutic biologics were screened for the anti-CHO-HCP reactivity. The anti-CHO-HCP antibodies present in human serum was captured by the immobilized CHO-HCP and detected with a mixture of protein A and protein G HRP conjugates to ensure all human immunoglobulin isotypes be detected. The sheep anti-CHO-HCP anti-serum (positive control) and the pooled normal human serum with low reactivity to CHO-HCP (negative control) were included in the screening assay to monitor the assay performance. As shown in Fig. 1a, b, the tested normal human individual serum samples exhibited a wide distribution of the anti-CHO-HCP reactivity, with OD readings ranging from 0.086 to 3.433. The mean OD values of the negative control ranged from 0.071 to 0.083. Based on the defined plate cut point values (two times the mean of the negative control OD values), 45 out of the 83 tested samples (54%) scored positive for anti-CHO-HCP reactivity in the conditions of the assay. The mean OD values generated from the negative control were significantly different from the mean values produced by individual normal serum samples since the negative control was selectively pooled from the serum samples that have low reactivity to CHO-HCP. Based on the ANOVA (single factor), the p value was 0.04. Given the high prevalence of samples with specific anti-CHO-HCP reactivity, application of a statistically defined floating cut point (11) was not considered suitable for this study.
Fig. 1.
Prevalence of anti-CHO-HCP reactivity in normal human serum (HS) samples. A validated ELISA was used to detect the reactivity to the drug process-specific CHO-HCP in 83 serum samples collected from individuals with no known prior exposure to therapeutic biologics. a Frequency of normal individual serum samples with anti-CHO-HCP reactivity. b Anti-CHO-HCP reactivity distribution. Mean negative control OD = 0.0762. Plate cut point ODs equal to two times the plate mean negative control ODs
Confirmation of the Specificity of the Positive Anti-CHO-HCP Reactivity in Normal Human Serum Samples
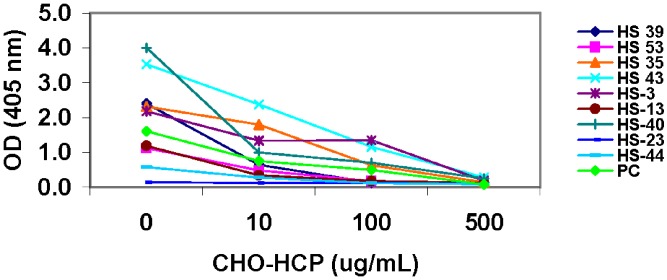
To confirm that the positive anti-CHO-HCP reactivity detected in the normal human serum samples is specific for CHO-HCP, samples and the assay positive control were tested in the competitive inhibition assays and/or on the uncoated plates. In the competitive inhibition assays, individual normal serum samples or the positive control (sheep anti-CHO-HCP anti-serum) were pretreated with CHO-HCP to compete with the plate-bound CHO-HCP for binding to the anti-CHO-HCP antibodies present in the positive control and normal serum samples. As shown in Table I, the maximum OD and the log titer of the assay positive control were decreased gradually with the increasing concentrations of the spiked CHO-HCP. The signal generated by the anti-CHO-HCP-positive control fell below the assay cut point value in the presence of 500 μg/ml of CHO-HCP, indicating that the spiked CHO-HCP had specifically inhibited the anti-CHO-HCP reactivity of the positive control in the assay. Sample HS-23, which previously tested negative for the anti-CHO-HCP reactivity based on the calculated cut point in the screening assay, was used as negative control in the specificity testing. After being spiked with the purified CHO-HCP, sample HS-23 still remained negative for the anti-CHO-HCP reactivity, with OD values below the cut point. In the positive anti-CHO-HCP samples, addition of the CHO-HCP inhibited the anti-CHO-HCP reactivity in a dose-dependent manner. The extent of signal inhibition in the anti-CHO-HCP-positive serum samples was similar to that in the positive control (Fig. 2), indicating that the positive reactivity detected in normal human individual serum samples was specific to CHO-HCP.
Table I.
Confirmation of Anti-CHO-HCP Reactivity in the Assay Positive Control (Sheep Anti-CHO-HCP Anti-sera)
| CHO-HCP | ||||
|---|---|---|---|---|
| 0 (μg/mL) | 10 (μg/mL) | 100 (μg/mL) | 500 (μg/mL) | |
| PC Log Titer | 3.97 | 3.39 | 1.87 | <1.7 |
| PC Max. OD | 1.600 | 0.675 | 0.218 | 0.077 |
Positive control was pre-incubated with purified CHO-HCP at concentrations of 0, 10, 100, and 500 μg/ml, respectively, prior to being tested in the anti-CHO-HCP ELISA
PC positive control
Fig. 2.
Confirmation of the positive reactivity to CHO-HCP in normal human serum (HS) samples—competitive inhibition specificity testing. Normal human serum samples were pre-incubated with purified CHO-HCP at concentrations of 0, 10, 100, and 500 μg/ml, respectively, prior to being tested in the anti-CHO-HCP ELISA. The anti-CHO-HCP-negative sample HS-23 was used as negative control for the testing. Observation of the dose-dependent readout signal inhibition in the tested samples indicates specific reactivity to CHO-HCP
The specificity of the anti-CHO-HCP reactivity was additionally verified using the uncoated plate method. The anti-CHO-HCP-positive and -negative samples were tested on the coated and uncoated plates in parallel. The OD values of the samples with negative reactivity to CHO-HCP were similar on the coated and uncoated plates. The OD values of the samples with positive reactivity to CHO-HCP decreased to below the cut point OD on the uncoated plate (Table II). The test results obtained from the competitive inhibition assay and the uncoated plate method were comparable. Both confirm that the antibodies detected in normal human serum samples are specific for anti-CHO-HCP reactivity.
Table II.
Confirmation of Anti-CHO-HCP Reactivity in Normal Human Serum Samples—Uncoated Plate Specificity Testing
| Human serum (HS) | Mean OD | Reported as | |
|---|---|---|---|
| Coated | Uncoated | ||
| HS-23a | 0.119 | 0.099 | Negative |
| HS-36a | 0.121 | 0.087 | Negative |
| HS-48a | 0.117 | 0.121 | Negative |
| HS-15a | 0.115 | 0.115 | Negative |
| HS-39 | 2.426 | 0.086 | Positive/Specific |
| HS-44 | 0.564 | 0.079 | Positive/Specific |
| HS-53 | 0.816 | 0.087 | Positive/Specific |
| HS-3 | 2.500 | 0.113 | Positive/Specific |
| HS-13 | 1.133 | 0.098 | Positive/Specific |
| HS-35 | 1.634 | 0.080 | Positive/Specific |
| HS-40 | 4.000 | 0.107 | Positive/Specific |
| HS-43 | 2.778 | 0.105 | Positive/Specific |
| HS-8 | 0.394 | 0.162 | Positive/Specific |
| HS-27 | 1.990 | 0.087 | Positive/Specific |
cutpoint OD is defined as two times mean of the negative sample OD
aTested negative for anti-CHO-HCP reactivity
Specificity of the Antibody Isotyping Detector Reagents
The isotypes of the anti-CHO-HCP antibodies found in the normal human serum samples were identified using specific anti-human Ig isotype antibody detectors. The cross-reactivity of the antibody isotyping detector reagents with various purified myeloma Ig isotype proteins (IgG1, IgG2, IgG3, IgG4, IgG, IgM, and IgE) was tested. As shown in Table III, each of the anti-Ig isotype antibody–HRP conjugates only produced strong signals in reaction with the corresponding Ig isotype. No cross-reactivity with other Ig isotypes was observed.
Table III.
Antibody Isotyping Reagents Specificity Testing
| Coat Reagent | OD values | |||||||
|---|---|---|---|---|---|---|---|---|
| Anti-lgG-HRP | Anti-lgG1-HRP | Anti-lgG2-HRP | Anti-lgG3-HRP | Anti-lgG4-HRP | Anti-lgM-HRP | Anti-lgE-HRP | Biotin anti-lgG4 and avidin-D-HRP | |
| lgG1 | 2.694 | 3.264 | 0.125 | 0.080 | 0.079 | 0.073 | 0.069 | 0.141 |
| lgG2 | 3.532 | 0.077 | 1.927 | 0.082 | 0.069 | 0.072 | 0.066 | 0.115 |
| lgG3 | 0.501 | 0.070 | 0.063 | 2.561 | 0.070 | 0.073 | 0.066 | 0.079 |
| lgG4 | 3.191 | 0.154 | 0.084 | 0.104 | 3.864 | 0.084 | 0.073 | 3.039 |
| gM | 0.101 | 0.079 | 0.069 | 0.072 | 0.071 | 3.893 | 0.065 | 0.076 |
| lgE | 0.098 | 0.076 | 0.067 | 0.090 | 0.067 | 0.073 | 2.313 | Not tested |
The reagent specificity of the isotyping detectors including mouse anti-human IgG1-HRP, mouse anti-human IgG2-HRP, mouse anti-human IgG3-HRP, mouse anti-human IgG4-HRP, mouse anti-human IgG-HRP, mouse anti-human IgM-HRP, mouse anti-human IgE-HRP, and biotin mouse anti-human IgG4 were confirmed by coating the microtiter plate with various purified antibody isotypes (IgG1, IgG2, IgG3, IgG4, IgM, and IgE) at 1.0 μg/ml and detecting with the corresponding isotyping detectors, respectively. Avidin-D-HRP was used in conjunction with biotin-mouse anti-human IgG4
In addition, the IgG4 type anti-CHO-HCP reactivity was identified using the biotin mouse anti-human IgG4 antibody detector. The specificity of biotin mouse anti-human IgG4 was tested in conjunction with avidin-D-HRP conjugate. The biotinylated mouse anti-human IgG4 antibody only bound to the coated IgG4 isotype protein; no cross-reactivity with other Ig isotypes was observed. Therefore, all the isotyping detector reagents were confirmed specific for the corresponding Igs.
Isotyping of the Anti-CHO-HCP Antibodies in Normal Human Serum Samples
Normal human individual serum samples with various levels of anti-CHO-HCP reactivity were further tested to identify the anti-CHO-HCP antibody isotypes present. Due to the lack of isotype-specific anti-CHO-HCP-positive controls, for example the IgG1 type anti-CHO-HCP antibody, sample readout signals were reported as OD values instead of antibody titers or concentrations. Samples that scored below the cut point during the anti-CHO-HCP reactivity screening were used as negative controls in the antibody isotyping assays. The negative control sample OD values measured with each antibody isotyping reagent are shown in Table IV. Cut point values for each of the isotyping assays (isotypic cut points) were calculated by multiplying the negative sample (NS) mean OD value by the multiplication factor of 2. Samples with OD values greater than the corresponding isotypic cut point value were considered positive for the presence of anti-CHO-HCP antibody of that isotype. Samples with OD values lower than the corresponding isotypic cut point OD were considered negative for the presence of anti-CHO-HCP antibody of that isotype. Of the 15 anti-CHO-HCP-positive individual samples tested (Table V), all samples exhibited IgG class anti-CHO-HCP reactivity, while 40% (6/15) of the samples exhibited specific IgM class reactivity in addition to IgG. Presence of anti-CHO-HCP-specific IgE was not detected. In further isotyping for the IgG subclasses, 66.6% (10/15) of samples tested positive for IgG1, 60% (9/15) of samples tested positive for IgG2, and 33.3% (5/15) of samples tested positive for IgG3. The magnitude of the isotypic reactivity detected from each sample varied from low (cut point <OD < 0.5), medium (0.5 < OD < 1.0), to high (OD > 1.0). Some samples exhibited single isotypic reactivity (e.g., IgG2 type antibody for samples HS-35 and HS-27); others hosted a mixture of multiple IgG subclasses (e.g. IgG1, IgG2, and IgG3 type antibodies for sample HS-40). IgG1 and IgG2 appeared to be the predominant antibody isotypes that mediate the anti-CHO-HCP reactivity in human serum.
Table IV.
Anti-CHO-HCP Antibody Isotypic Cutpoint Determination
| Anti-CHO-HCP negative samples | Anti-CHO-HCP isotypic reactivity (OD) | Anti-CHO-HCP (OD) | ||||||
|---|---|---|---|---|---|---|---|---|
| lgG1 | lgG2 | lgG3 | lgG4 | lgG | lgM | lgE | ||
| HS-6 | 0.074 | 0.071 | 0.074 | 0.090 | 0.104 | 0.158 | 0.126 | 0.079 |
| HS-7 | 0.091 | 0.068 | 0.071 | 0.066 | 0.091 | 0.110 | 0.068 | 0.074 |
| HS-17 | 0.071 | 0.067 | 0.071 | 0.078 | 0.110 | 0.171 | 0.068 | 0.076 |
| HS-23 | 0.077 | 0.071 | 0.081 | 0.091 | 0.095 | 0.282 | 0.070 | 0.095 |
| HS-B12 | 0.073 | 0.110 | 0.088 | 0.090 | 0.146 | 0.270 | 0.071 | 0.146 |
| HS-B14 | 0.090 | 0.076 | 0.088 | 0.110 | 0.134 | 0.202 | 0.074 | 0.156 |
| NS mean OD | 0.079 | 0.077 | 0.079 | 0.087 | 0.113 | 0.199 | 0.079 | 0.104 |
| SD | 0.009 | 0.016 | 0.008 | 0.015 | 0.022 | 0.067 | 0.023 | 0.037 |
| cutpoint OD | 0.158 | 0.154 | 0.157 | 0.175 | 0.226 | 0.397 | 0.158 | 0.208 |
Six human serum samples with negative reactivity to CHO-HCP were tested as negative controls of the assays. The cutpoint ODs for each of the isotyping assays were defined as two times the mean of the anti-CHO-HCP negative sample (NS) ODs obtained in each of the isotypic assays
NS negative samples
Table V.
Isotyping the Anti-CHO-HCP Antibodies in Normal Human Serum (HS) Samples with Positive Reactivity to CHO-HCP
| Anti-CHO-HCP-positive samples | Anti-CHO-HCP isotypic reactivity | Anti-CHO-HCP | ||||||
|---|---|---|---|---|---|---|---|---|
| lgG1 | lgG2 | lgG3 | lgG4 | lgG | lgM | lgE | ||
| HS-39 | +++ | − | − | − | +++ | − | − | +++ |
| HS-44 | − | − | − | − | + | +++ | − | + |
| HS-53 | + | ++ | − | − | +++ | − | − | ++ |
| HS-13 | +++ | − | − | − | +++ | − | − | ++ |
| HS-35 | − | + | − | − | +++ | − | − | +++ |
| HS-40 | +++ | +++ | + | − | +++ | − | − | +++ |
| HS-43 | + | +++ | − | +b | +++ | + | − | +++ |
| HS-8 | − | − | +++ | +++b | +++ | + | − | + |
| HS-14 | − | − | − | − | ++ | − | − | + |
| HS-B3 | +++ | + | − | − | +++ | + | − | +++ |
| HS-B2 | ++ | +++ | + | − | +++ | − | − | +++ |
| HS-B8 | + | + | + | − | +++ | ++ | − | +++ |
| HS-B17 | ++ | − | + | − | +++ | + | − | +++ |
| HS-27 | − | + | − | − | +++ | − | − | +++ |
| HS-3 | +++ | + | − | − | +++ | +++a | − | +++ |
The anti-CHO-HCP antibodies were isotyped using various mouse anti-human Ig isotype antibody–HRP conjugates. Samples with OD values greater than the corresponding isotypic cutpoint were considered positive for the isotype tested. Samples with OD values lower than the corresponding isotypic cutpoint were considered negative for the isotype tested
+++: OD > 1.0; ++: 1.0 > OD > 0.5; +: 0.5 > OD> cutpoint; −: OD below cutpoint
aTested non-specific for anti-CHO-HCP reactivity
bTested positive using anti-human lgG4-biotin and avidin-D-HRP (Reference Figs. 3, 5); tested negative using anti-human lgG4-HRP
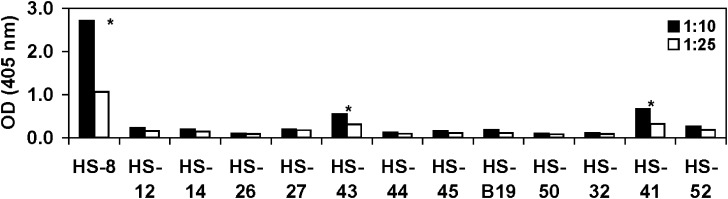
IgG4 type anti-CHO-HCP reactivity was not detected in the tested human serum samples when using mouse anti-human IgG4-HRP as a detector. Modifications of the assay conditions, such as reduction of the assay MRD and increase of the concentrations of the capture material and the detector reagent did not result in detectable IgG4 type antibody signal generation. To further assess the presence of the IgG4 reactivity, an assay based on the use of biotinylated mouse anti-human IgG4 in conjunction with the avidin-D-HRP was developed. Similar to the assay strategy described above, the anti-CHO-HCP-negative samples were used as assay negative controls in order to calculate IgG4-specific isotypic cut point value. Samples were tested at 1:10 and 1:25 dilutions. The cut point values were calculated by multiplying the corresponding negative sample mean OD values by the multiplication factor of 2. The cut point values were calculated as 0.274 (1:10 dilution) and 0.198 (1:25 dilution; Table VI). Of the 13 individual samples that tested anti-CHO-HCP-positive, three samples generated OD values above the IgG4 isotypic cut point and were identified as positive for the presence of anti-CHO-HCP IgG4 antibody (Fig. 3). Sample HS-41 was further confirmed non-specific for anti-CHO-HCP reactivity in the confirmation assays described below. Overall, a low level of IgG4 type anti-CHO-HCP reactivity was detected in the normal human serum samples when compared with the presence of other IgG subclasses or IgM class reactivity.
Table VI.
IgG4 Cutpoint Determination in the Assays Using the Biotin Mouse Anti-human IgG4 Detector
| Anti-CHO-HCP negative samples | lgG4 (OD) | |
|---|---|---|
| 1:10 | 1:25 | |
| HS-6 | 0.181 | 0.140 |
| HS-23 | 0.077 | 0.074 |
| HS-B14 | 0.086 | 0.078 |
| HS-63 | 0.163 | 0.119 |
| HS-B13 | 0.249 | 0.094 |
| HS-B20 | 0.131 | 0.094 |
| HS-B12 | 0.120 | 0.107 |
| HS-B16 | 0.094 | 0.084 |
| Mean NS OD | 0.137 | 0.099 |
| SD | 0.058 | 0.022 |
| cutpoint OD | 0.274 | 0.198 |
The cutpoint OD was calculated by multiplying the mean of the anti-CHO-HCP negative sample (NS) ODs obtained in the IgG4 assay by a multiplication factor of 2. Samples were tested at dilutions of 1:10 and 1:25
NS negative sample
Fig. 3.
Identification of the IgG4 type reactivity in anti-CHO-HCP-positive serum samples using the biotin mouse anti-human IgG4 detector. IgG4 isotypic cut point OD is defined as two times the mean of the negative sample OD. Samples with OD values greater than the IgG4 cut point were considered positive; samples with OD values lower than the IgG4 cut point were considered negative. Samples were tested at dilutions of 1:10 and 1:25. *Identified positive for IgG4 reactivity
The IgE type reactivity was assessed using two sets of detector reagents, mouse anti-human IgE-HRP, and the biotin mouse anti-human IgE in conjunction with avidin-D-HRP. Various assay conditions were evaluated. No IgE type anti-CHO-HCP reactivity was detected in the human serum samples tested in this study.
Confirmation of the Specificity of Anti-CHO-HCP Antibody Isotypes in Normal Human Serum Samples
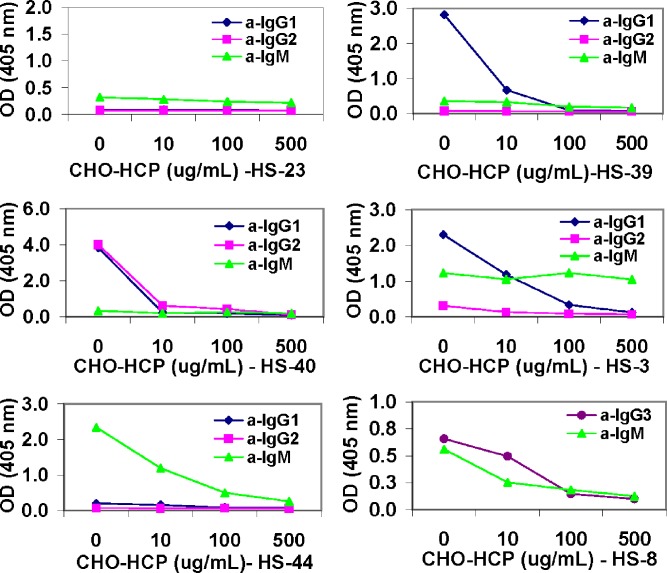
Specificity of the isotypic anti-CHO-HCP reactivity in the normal human individual serum samples was verified using the uncoated plate and the competitive inhibition methods. In the competitive inhibition assays, purified CHO-HCP was spiked into individual serum samples at varying concentrations to compete for binding to the anti-CHO-HCP antibodies. The plate-bound anti-CHO-HCP antibodies were detected with the antibody isotyping detector reagents mouse anti-human IgG1-HRP, mouse anti-human IgG2-HRP, mouse anti-human IgG3-HRP, mouse anti-human IgM-HRP, and/or biotin mouse anti-human IgG4 in conjunction with avidin-D-HRP, respectively. As shown in Fig. 4, the OD values of the anti-CHO-HCP-negative sample HS-23 remained below the isotypic cut points in the presence of the spiked protein as expected. The isotypic reactivity of the majority of the anti-CHO-HCP-positive samples was inhibited by the spiked CHO-HCP in a dose-dependent manner. Different samples required different amounts of the competing protein to achieve substantial signal reduction. All of the detected IgG1, IgG2, and IgG3 type antibody-containing samples were confirmed specific for interaction with CHO-HCP. The specificity for the IgM antibody-based reactivity was not confirmed in the sample HS-3 because the assay readout signal was not inhibited by the spiked CHO-HCP up to 500 μg/ml. The CHO-HCP specificity of the IgM antibody-based reactivity was confirmed in other IgM-positive samples (HS-8 and HS-44).
Fig. 4.
Confirmation of the specificity of the anti-CHO-HCP antibody isotypes in normal human serum (HS) samples—competitive inhibition specificity testing. Serum samples were pre-incubated with purified CHO-HCP at concentrations of 0, 10/ml, 100, and 500 μg/ml, respectively, prior to being analyzed in the antibody isotyping assays using the mouse anti-human Ig isotype antibody–HRP conjugates detectors
In the uncoated specificity plate method, the same set of samples that was tested in the competitive inhibition assays was analyzed on the CHO-HCP-uncoated and -coated plates in parallel. Observing very low signal or no signal on an uncoated plate compared to a coated plate would indicate the anti-CHO-HCP specificity of the tested antibody isotypes. As shown in Table VII, the OD values of the anti-CHO-HCP-negative sample HS-23 remained below isotypic cut points on the coated and uncoated plates as expected. The OD values produced by the IgG1, IgG2, and IgG3 type anti-CHO-HCP reactivity-containing samples were substantially decreased on the uncoated plate, suggesting that these identified antibody isotypes are specific for CHO-HCP reactivity. In concert with the results obtained from the competitive inhibition assays, the IgM antibody reactivity was confirmed non-specific to CHO-HCP in sample HS-3 because similar IgM type OD values were observed on the coated and uncoated plates. The OD values produced by other IgM type reactivity-containing samples (HS-8 and HS-44) fell below the IgM isotypic cut point values on the uncoated plate; therefore, the IgM type reactivity present in samples HS-8 and HS-44 was confirmed specific for CHO-HCP.
Table VII.
Confirmation of the Specificity of the Anti-CHO-HCP Antibody Isotypes in Normal Human Serum Samples—Uncoated Plate Specificity Testing
| Human serum | lg Isotypes | Mean OD | Reported as | |
|---|---|---|---|---|
| Coated | Uncoated | |||
| HS-23a | a-lgG1 | 0.080 | 0.099 | Negative |
| a-lgG2 | 0.076 | 0.066 | Negative | |
| a-lgG3 | 0.084 | 0.119 | Negative | |
| a-lgM | 0.276 | 0.183 | Negative | |
| HS-39 | lgG1 | 1.791 | 0.092 | Positive/Specific |
| HS-40 | lgG1 | 2.721 | 0.100 | Positive/Specific |
| lgG2 | 3.043 | 0.076 | Positive/Specific | |
| HS-3 | lgG1 | 2.069 | 0.088 | Positive/Specific |
| lgG2 | 0.367 | 0.069 | Positive/Specific | |
| lgM | 1.037 | 1.248 | Positive/Non-specific | |
| HS-8 | lgG3 | 1.211 | 0.144 | Positive/Specific |
| lgM | 0.476 | 0.084 | Positive/Specific | |
| HS-44 | lgM | 1.378 | 0.132 | Positive/Specific |
Samples were analyzed on the CHO-HCP-uncoated and -coated plates using the mouse anti-human Ig isotype antibody-HRP detectors
aTested negative for anti-CHO-HCP reactivity
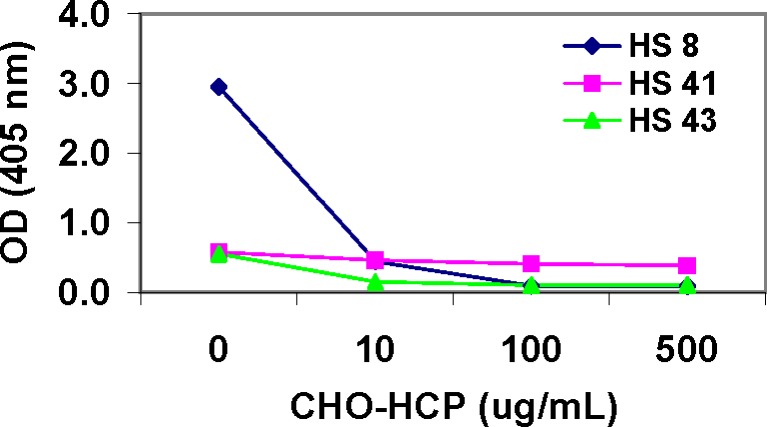
The specificity of the IgG4 type anti-CHO-HCP reactivity identified using the biotinylated mouse anti-human IgG4 antibody detector was evaluated. The IgG4 type reactivity present in sample HS-41 was confirmed non-specific for CHO-HCP due to the lack of signal inhibition upon sample pretreatment with CHO-HCP in the competitive inhibition assay and observation of similar high OD readings on the uncoated and coated plates. On the contrary, the IgG4 type reactivity present in samples HS-8 and HS-43 was confirmed specific for CHO-HCP in both methods (Fig. 5 and Table VIII).
Fig. 5.
Confirmation of the specificity of the IgG4 type anti-CHO-HCP antibody in normal human serum (HS) samples—competitive inhibition specificity testing. Serum samples were pre-incubated with purified CHO-HCP at concentrations of 0, 10, 100, and 500 μg/ml, respectively, prior to being analyzed in the antibody isotyping assays using the biotin mouse anti-human IgG4 antibody detector in conjunction with avidin-D-HRP
Table VIII.
Confirmation of the Specificity of the IgG4 Type Anti-CHO-HCP Antibody in Normal Human Serum (HS) Samples—Uncoated Plate Specificity Testing
| Human serum | Mean OD | Reported as | |
|---|---|---|---|
| Coated | Uncoated | ||
| HS-8 | 2.517 | 0.121 | Positive/Specific |
| HS-41 | 0.573 | 0.598 | Positive/Non-specific |
| HS-43 | 0.477 | 0.144 | Positive/Specific |
| HS-6a | 0.127 | 0.147 | Negative |
| HS-23a | 0.080 | 0.071 | Negative |
| HS-63a | 0.119 | 0.124 | Negative |
| HS-B13a | 0.094 | 0.084 | Negative |
| HS-B14a | 0.079 | 0.081 | Negative |
Samples were analyzed on the CHO-HCP-coated and uncoated plates using the biotin mouse anti-human IgG4 antibody detector in conjunction with avidin-D-HRP
aTested negative for anti-CHD-HCP reactivity
Taken together, the specificity testing results obtained in the competitive inhibition assay and the uncoated plate testing were consistent. The specificity of the various anti-CHO-HCP antibody isotypes present in human serum samples was confirmed by both methods.
DISCUSSION
Assessment of unwanted immunogenicity of therapeutic biologics in humans is an important aspect of drug safety evaluation (12). A risk-based immunogenicity assessment approach is recommended and generally accepted by the industry. Besides the detection of neutralizing antibodies and identification of anti-drug antibody (ADA) binding epitopes for the high-risk category drugs, additional characterization of the ADA isotypes in humans may assist in evaluating the immune response progression and provide mechanistic insight for the immunogenicity response development (8,13). For example, an IgA anti-drug antibody response might be observed after the administration of some inhaled therapeutic proteins (14). Repeated, long-term antigenic stimulation with T cell-dependent antigens may lead to a marked IgG4 antibody response (15). The IgG responses to a variety of allergens are predominated by IgG1 and IgG4. With several allergens, during the antibody response to desensitization/immunotherapy, initially mainly IgG1 was formed, whereas IgG4 became more prominent after 1–2 years (16,17). IgG2 is predominantly produced in response to polysaccharide antigens (18).
Circulating human antibodies reactive with animal proteins have been observed in normal humans. The prevalence of people positive for anti-mouse antibodies (HAMA) and antibodies to other species varies widely ranging from 0.3% to 80% (19). The causes of anti-animal antibodies include blood transfusion (20), vaccination against infectious diseases (21,22), maternal transfer across the placenta to the unborn child (23,24), keeping of animals as pets (25), and so on. The human anti-animal antibodies can be of the IgG, IgA, IgM, or, rarely, the IgE class (19,26–28).
The presence of antibody reactivity to CHO-HCP was observed in high prevalence (54%) in normal individuals with no known exposure to therapeutic biologics. Although the origin of such anti-CHO-HCP antibody reactivity in humans is unclear, characterization of the antibody isotypes may provide insight for the composition of the anti-CHO-HCP reactivity. The anti-CHO-HCP antibody isotyping and specificity confirmation experiments were performed sequentially. The specificity of the isotyping reagents, mouse anti-human Ig isotype antibody–HRP conjugates, and the biotinylated mouse anti-human Ig isotype was confirmed upon the reagent specificity testing. No cross-reactivity with other than the designated Ig isotype was observed.
The presence of IgG type anti-CHO-HCP reactivity suggested the maturity of the anti-CHO-HCP antibodies in the human individual serum samples tested in this study. IgG is predominantly involved in secondary immune response, and IgM is the first antibody to appear in response to an initial exposure to an antigen. The anti-CHO-HCP reactivity was not isotypically restricted. Some serum samples exhibited single isotypic anti-CHO-HCP reactivity, while others contained a mixture of multiple anti-CHO-HCP antibody isotypes.
The use of the biotinylated mouse anti-human IgG4 antibody in conjunction with the avidin-D-HRP detector system provided output signal amplification so as to increase the IgG4 detection sensitivity. For this reason, two out of the 15 anti-CHO-HCP-positive human serum samples tested positive for IgG4 reactivity when using the biotinylated mouse anti-human IgG4 and avidin-D-HRP detector system, but negative when using the mouse anti-human IgG4-HRP one step detection. Overall, IgG4 reactivity was detected in low abundance compared to other IgG subclasses. Low binding affinity and the potential monovalency of IgG4 may have reduced the chance of the IgG4 type anti-CHO-HCP reactivity detection (15,29). The IgG4 detection reagents (anti-human IgG4-HRP and biotin-anti-human IgG4) were not considered accounting for the low level of IgG4 detection because both detectors produced strong IgG4-binding signals, comparable with those from the detection of other IgG subclasses in the reagent specificity testing.
Despite the multiple assay conditions evaluated, IgE type anti-CHO-HCP reactivity was not detected in the tested human serum samples using the one-step mouse anti-human IgE-HRP detection nor the two-step detection with biotinylated mouse anti-human IgE. IgA was not tested in this study.
It is understandable that IgG1 type reactivity (66.6%) prevails in the normal individual serum samples tested, and 33.3% of the samples tested had specific IgG3 type reactivity. IgG1 and IgG3 are the most frequently presented isotypes in the antibody response to protein antigens. The remarkable observation is the high prevalence (60%) of the specific IgG2 type anti-CHO-HCP reactivity. The IgG2 subclass is generally, if not exclusively, restricted to the anti-carbohydrate antibodies evoked by the polysaccharide antigens in a T cell-independent manner (18). It is postulated that the individuals that exhibit IgG2 type anti-CHO-HCP reactivity must have prior exposure to the carbohydrate components of glycoproteins or carbohydrates from other sources that are homologous to the CHO-HCP. The competitive inhibition assays conducted by pre-incubating samples with the soluble CHO-HCP confirmed the IgG2 isotypic specificity to CHO-HCP in the tested samples given that the dose-dependent signal was substantially reduced. This does not rule out the possibility of the anti-carbohydrate reactivity having originated from exposure to glycoproteins or carbohydrates unrelated to CHO-HCP.
In general, manufacturing processes for therapeutic drug products are developed to minimize the level of CHO-HCP that patients receive during drug administration. As the CHO proteins are composed of a broad spectrum of proteins, the exposure to any individual protein is much lower. It is considered a low-risk event for the trace amount of CHO-HCP associated with a drug product to induce anti-CHO-HCP immune response post-drug administration. In a study reported by Ingerslev et al. (30), anti-CHO cell lysate antibodies were detected in hemophilia A patients prior to and post the recombinant human factor VIII (Recombinate) treatment, though the source of CHO-HCP and other reagents, assay formats, assay conditions, and the data analysis method were different from what were used in our study. No adverse events associated with the development or presence of anti-CHO cell lysate antibodies was reported in hemophilia patients. The presence of anti-CHO-HCP antibodies may become a practical issue when the level of an individual CHO-HCP associated with a drug product is above levels associated with induction of immune responses or levels expected to cause significant reactivity with these pre existing antibodies. In this regard, understanding the prevalence and the isotypic complexity of the endogenous anti-CHO-HCP antibodies in normal humans may aid in evaluating the potential impact of these antibodies after administration of a CHO cell-derived therapeutic protein.
CONCLUSIONS
The antibody reactivity to CHO-HCP has been characterized and confirmed in normal human serum samples. Data presented in this study suggest the prevalence and the isotypic complexity of the anti-CHO-HCP reactivity present in normal humans. Understanding of the prevalence and isotypic complexity of the anti-CHO-HCP reactivity in normal humans may be beneficial to characterizing the immunogenicity response to CHO cell-derived therapeutic biologics.
Acknowledgments
The authors would like to thank Dr. Bonita Rup for reviewing the manuscript and providing important critiques and suggestions. Thanks to Dr. Michel Awwad for suggestions on the manuscript. We thank Melissa Jezuit, Nicole Duriga, Shobha Purushothama, and Natalie Jacob in the bioanalytical group of Wyeth Research for providing the purified myeloma immunoglobulin isotype proteins and the negative control for the anti-CHO-HCP antibody detection assay. We thank Parul Rangwalla for technical assistance.
References
- 1.Schirrmann T, Al-Halabi L, Dubel S, Hust M. Production systems for recombinant antibodies. Front Biosci. 2008;13:4576–94. doi: 10.2741/3024. [DOI] [PubMed] [Google Scholar]
- 2.Jones D, Kroos N, Anema R. High-level expression of recombinant IgG in the human cell line per.c6. Biotechnol Prog. 2003;9(1):163–8. doi: 10.1021/bp025574h. [DOI] [PubMed] [Google Scholar]
- 3.Werner RG, Noe W, Kopp K, Schluter M. Appropriate mammalian expression systems for biopharmaceuticals. Arzneimittelforschung. 1998;48(8):870–80. [PubMed] [Google Scholar]
- 4.Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol. 2004;22(11):1393–8. doi: 10.1038/nbt1026. [DOI] [PubMed] [Google Scholar]
- 5.Jayapal KP, Wlaschin KF, Hu W. Recombinant protein therapeutics from CHO cells—20 years and counting. CHO Consortium SBE special section. 2007;40–7.
- 6.Koren E, Zuckerman LA, Mire-Sluis AR. Immune responses to therapeutic proteins in humans—clinical significance, assessment and prediction. Curr Pharm Biotechnol. 2002;3(4):349–60. doi: 10.2174/1389201023378175. [DOI] [PubMed] [Google Scholar]
- 7.Schellekens H. Factors influencing the immunogenicity of therapeutic proteins. Nephrol Dial Transplant. 2005;20(Suppl 6):3–9. doi: 10.1093/ndt/gfh1092. [DOI] [PubMed] [Google Scholar]
- 8.Shankar G, Pendley C, Stein KE. A risk-based bioanalytical strategy for the assessment of antibody immune responses against biological drugs. Nat Biotechnol. 2007;25(5):555–61. doi: 10.1038/nbt1303. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman K. Strategies for host cell protein analysis. BioPharm. 2000;13:38–45. [Google Scholar]
- 10.Van Cleave VH. Validation of immunoassays for anti-drug antibodies. In: Brown F, Mire-Sluis AR, editors. Immunogenicity of therapeutic biological products. Dev Biol. Basel: Karger; 2003;112:107–12. [PubMed]
- 11.Shankar G, Devanarayan V, Amaravadi L, et al. Recommendations for the validation of immunoassays used for detection of host antibodies against biotechnology products. J Pharm Biomed Anal. 2008;48(5):1267–81. doi: 10.1016/j.jpba.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Wadhwa M, Bird C, Dilger P, et al. Strategies for detection, measurement and characterization of unwanted antibodies induced by therapeutic biologicals. J Immunol Methods. 2003;278(1–2):1–17. doi: 10.1016/S0022-1759(03)00206-0. [DOI] [PubMed] [Google Scholar]
- 13.Koren E, Smith HW, Shores E, et al. Recommendations on risk-based strategies for detection and characterization of antibodies against biotechnology products. J Immunol Methods. 2008;333(1–2):1–9. doi: 10.1016/j.jim.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Thippawong J. Inhaled cytokines and cytokine antagonists. Adv Drug Deliv Rev. 2006;58(9–10):1089–105. doi: 10.1016/j.addr.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Aalberse RC, van der Gagg R, van Leeuwen J. Serologic aspects of IgG4 antibodies. I. Prolonged immunisation results in an IgG4-restricted response. J Immunol. 1983;130(2):722–6. [PubMed] [Google Scholar]
- 16.Urbanek R. IgG subclasses and subclass distribution in allergic disorders. Monogr Allergy. 1988;23:33–40. [PubMed] [Google Scholar]
- 17.Lucas AH. IgG subclass restricted immune responses to allergens. Springer Semin Immunopathol. 1990;2(4):385–400. doi: 10.1007/BF00225325. [DOI] [PubMed] [Google Scholar]
- 18.Siber GR, Schur PH, Aisenber AC, Weitzmann SA, Schiffman G. Correlation between serum IgG2 concentrations and the antibody response to bacterial polysaccharide antigens. New Engl J Med. 1980;303(4):178–82. doi: 10.1056/NEJM198007243030402. [DOI] [PubMed] [Google Scholar]
- 19.Kricka LJ. Human anti-animal antibody interferences in immunological assays. Clin Chem. 1999;45(7):942–56. [PubMed] [Google Scholar]
- 20.Hawkins BR, Saueracker GC, Dawkins RL, et al. Population study of heterophile antibodies. Vox Sang. 1980;39:339–42. doi: 10.1111/j.1423-0410.1980.tb02973.x. [DOI] [PubMed] [Google Scholar]
- 21.Schaison G, Thomopoulos P, et al. False hyperthyrotropinemia induced by heterophilic antibodies against rabbit serum. J Clin Endocrinol Metab. 1981;53(1):200–2. doi: 10.1210/jcem-53-1-200. [DOI] [PubMed] [Google Scholar]
- 22.Padova G, Briguglia G, Tita P, et al. Hypergonadotropinemia not associated to ovarian failure and induced by factors interfering in radioimmunoassay. Fertil Steril. 1991;55(3):637–9. doi: 10.1016/s0015-0282(16)54201-0. [DOI] [PubMed] [Google Scholar]
- 23.Czernichow P, JL Van dalem, Hennen G. Transient neonatal hyperthyrotropinemia: a factitious syndrome due to the presence of heterophilic antibodies in the plasma of infants and their mothers. J Clin Endocrinol Metab. 1981;53(2):387–93. doi: 10.1210/jcem-53-2-387. [DOI] [PubMed] [Google Scholar]
- 24.Larsson A, Hedenborg G, Carlstrom A. Placental transfer of maternal anti-rabbit IgG causing falsely elevated TSH levels in neonates. Acta Paediatr Scand. 1981;70(5):699–703. doi: 10.1111/j.1651-2227.1981.tb05771.x. [DOI] [PubMed] [Google Scholar]
- 25.Berglund L, Holmberg NG. Heterophilic antibodies against rabbit serum causing falsely elevated gonadotropin levels. Acta Obstet Gynecol Scand. 1989;68(4):377–8. doi: 10.3109/00016348909028676. [DOI] [PubMed] [Google Scholar]
- 26.Chatenoud L, Baudrihaye MF, Chkoff N, et al. Restriction of the human in vivo immune response against the mouse monoclonal antibody OKT3. J Immunol. 1986;137(3):830–8. [PubMed] [Google Scholar]
- 27.McCarthy RC. Interference in immunoenzymometric assays caused by IgM anti-mouse IgG antibodies. Arch Pathol Lab Med. 1988;112(9):901–7. [PubMed] [Google Scholar]
- 28.Frodin JE, Lefvert AK, Mellstedt H. Clinical significance of HAMA in patients treated with mouse monoclonal antibodies. Cell Biophys. 1992;21(1–3):153–65. doi: 10.1007/BF02789485. [DOI] [PubMed] [Google Scholar]
- 29.van der Neut Kolfschoten M, Schuurman J, Losen M, et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science. 2007;317(5844):1554–7. doi: 10.1126/science.1144603. [DOI] [PubMed] [Google Scholar]
- 30.Ingerslev J, Christiansen K, Ravn HB, et al. Antibodies to heterologous proteins in hemophilia A patients receiving recombinant factor VIII (Recombinate) Thromb Haemost. 2002;87(4):626–34. [PubMed] [Google Scholar]