Abstract
During its assembly, human HIV-1 selectively packages the tRNALys isoacceptors, including tRNALys3, the primer for the reverse transcriptase. However, other low molecular weight RNA species are also seen in the virus. We profiled the tRNAs packaged into HIV-1 using microarray analysis and validated our results by two-dimensional gel electrophoresis and RT-PCR. In addition to tRNALys isoacceptors, tRNAAsn and the rare isoacceptor of tRNAIle are also selectively packaged. In Gag viral-like particles missing the GagPol protein, overall tRNA incorporation is reduced by >80%. This reduction is significantly greater than can be accounted for by the reduction in tRNALys isoacceptors, tRNAAsn and tRNAIle, suggesting that incorporation of other tRNAs may also require the GagPol protein. These results demonstrate selective incorporation of non-lysyl tRNAs into HIV-1 and highlight the application of microarrays as a novel method to study tRNA incorporation into viruses.
Keywords: HIV, microarray, tRNA, viral packaging
INTRODUCTION
During HIV-1 assembly, the tRNALys isoacceptors, tRNALys1,2 and tRNALys3, are selectively incorporated into the virus (Jiang et al. 1993). tRNALys3 anneals to the primer binding site (PBS) in the 5′ regions of the viral RNA genome, and serves as the primer for initiating reverse transcription of minus strand strong stop DNA. tRNALys1,2, which differs from tRNALys3 by 14 or 16 bases, is the isoacceptor of tRNALys3 and differs by 1 base pair (bp) in the anticodon stem (Raba et al. 1979). While tRNALys1,2 does not function as a primer in HIV-1, it may play a role in the import of the pre-integration complex into the nucleus of the infected cell (Zaitseva et al. 2006).
Previous work has indicated that the nucleoprotein complex involved in promoting the selective incorporation of tRNALys includes the viral precursor proteins Gag and GagPol, viral genomic RNA, lysyl-tRNA synthetase (LysRS), and tRNALys. The formation of this complex involves a specific interaction of Gag with both LysRS (Javanbakht et al. 2003) and GagPol. tRNALys is bound to LysRS and interacts with the reverse transcriptase segment within GagPol, thus stabilizing the complex (Khorchid et al. 2000). Thus, viral-like particles (VLPs) composed only of Gag have been reported to package only LysRS, and not tRNALys (Cen et al. 2001).
While resolution of HIV-1 low molecular weight RNA by two-dimensional (2D) PAGE shows the tRNALys isoacceptors to be major components of this population, other low molecular weight species can also be seen. In this work, we have identified some of the other tRNAs present in HIV-1 through hybridization of labeled viral RNA to human tRNA microarrays. The microarray contains 40 DNA probes representing all >270 human nuclear-encoded tRNA genes as closely related families (Dittmar et al. 2006; Pavon-Eternod et al. 2009). We validated our microarray results by 2D polyacrylamide gel electrophoresis (2D PAGE) and RT/PCR. We found that in addition to the tRNALys isoacceptors, tRNAAsn and the rare isoacceptor of tRNAIle are also selectively packaged into HIV-1. Like tRNALys, the selective packaging of non-lysyl tRNAs also depends on the GagPol protein of HIV.
RESULTS AND DISCUSSION
Microarray analysis of viral tRNAs
Viruses were produced from 293T cells transfected with BH10, a plasmid coding for the BH10 strain of HIV-1. Gag viral-like particles (VLPs) were produced from 293T cells transfected with GagFS(−), a plasmid coding for all HIV-1 proteins except GagPol (the frameshift in viral RNA responsible for GagPol production has been eliminated in this plasmid). To determine the identity and abundance of tRNAs packaged into HIV-1 and GagVLPs, we applied a previously described microarray method, as applied in studies of tissue-specific expression of human tRNAs (Dittmar et al. 2006) and tRNA expression in cancer tissues and cell lines (Pavon-Eternod et al. 2009; Zhou et al. 2009).
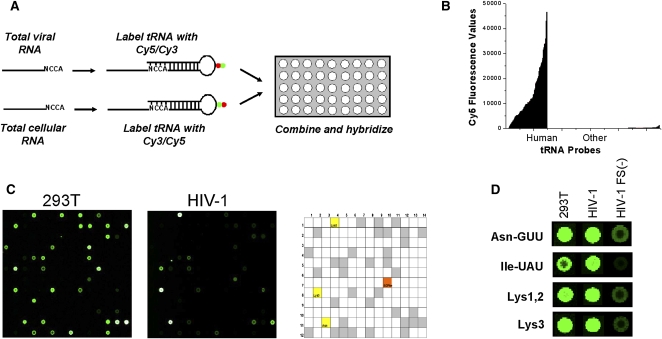
Arrays used for this study contain 40 probes for human nuclear-encoded tRNAs, which represent all >270 different human tRNA sequences as closely related families, and 22 probes for all 22 human mitochondrial-encoded tRNAs. The array also includes 42 probes for bacterial tRNAs and 275 probes complementary to short regions in yeast and human rRNA, which serve as hybridization and specificity controls. Each probe is repeated 21 times in the array. After isolation of total RNA from 293T cells, HIV-1, and Gag VLPs, tRNAs in the sample were labeled by selective ligation to a fluorophore-containing oligonucleotide. The labeled samples were hybridized directly onto the array (Fig. 1A). The 293T cellular RNA sample was included as a reference in all array hybridizations to correct for variations in fluorescence labeling and array manufacturing. Since tRNA constitutes up to 30% of the total RNA, the method requires no amplification and only 1–2 μg of total RNA were used per array. The specificity of the array is illustrated by the fluorescence signals derived from human tRNA probes compared to the nonhuman tRNA probes (Fig. 1B,C). As expected, tRNALys1,2(CUU) and tRNALys3(UUU) are present in large quantities in the wild-type HIV sample (Fig. 1D).
FIGURE 1.
Detection of tRNAs by microarray analysis. (A) Array scheme. Total cellular or viral RNA were deacylated, and directly labeled with a Cy3 or Cy5 containing oligonucleotide using T4 DNA ligase. The labeling samples with the opposite fluorophores were combined and hybridized together on the array. (B) Array specificity. Fluorescence values for the total RNA of HEK293T using human tRNA probes and other probes (Caulobacter tRNA, human rRNA). (C) Array images of one block in the human tRNA microarray hybridized with 293T or HIV-1. Only Cy3 spots are shown for better visualization. The entire array contains 48 blocks, and each probe is repeated 21 times on the array. The particular block shown contains two probes for human tRNALys3, one for tRNAAsn, and one for a yeast tRNAPhe standard (SCPhe). Both images have the same contrast for the SCPhe standard. In the array key grid, gray squares indicate probe locations for human tRNAs and open squares indicate probe locations for nonhuman tRNA or rRNA. (D) Selected spots from Cy3-labeled 293T total RNA, wild-type HIV-1, or GagVLP, indicating that significantly lower amounts of tRNA are present in GagVLPs.
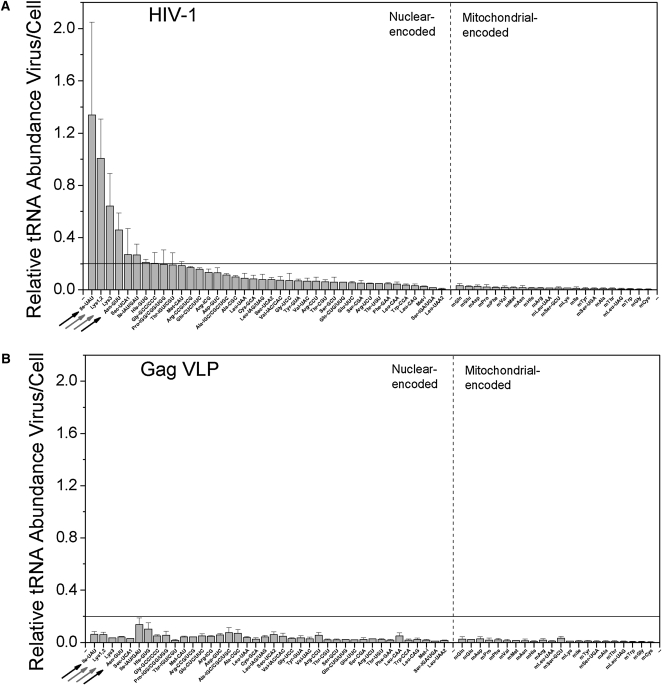
As is typically done in two-color microarray analysis, we first analyzed the relative abundance for the nuclear- and mitochondrial-encoded tRNAs in HIV-1 and GagVLPs compared to the total RNA in HEK293T (Fig. 2). Each bar indicates the ratio of viral:cellular RNA hybridization signals for a specific tRNA, such that a ratio of 1 would indicate similar abundance in both samples. A single ratio value is not by itself reflective of selective packaging since the nature of the cellular and viral RNA samples are different. For HEK293T, the total RNA is primarily rRNA and tRNA, whereas for HIV-1 and GagVLP, the total RNA is primarily the HIV genomic RNA and tRNA. However, a comparative analysis across different tRNA species indicates which tRNAs are selectively packaged into virions. For HIV-1 (Fig. 2A), a majority of the nuclear-encoded tRNAs show ratios between 0.01 and 0.2, while four tRNAs show significantly higher ratios than 0.2. For Gag VLPs, all nuclear-encoded tRNAs show ratios below 0.2 (Fig. 2B). These results also indicate that the amount of tRNAs packaged into GagVLPs is significantly reduced as compared to HIV-1. All mitochondrial-encoded tRNAs in both HIV-1 and GagVLPs show values below 0.05, indicating they are not incorporated into virions. Assuming tRNAs present in GagVLP samples represent nonselective incorporation, we use the value of 0.2 as a cutoff for selective packaging. By this criterion, four tRNAs are selectively incorporated into HIV-1 virions by a factor of twofold or more: tRNAIle(UAU) (1.4), tRNALys1,2(CUU) (1.0), tRNALys3(UUU) (0.65), and tRNAAsn(GTT) (0.4).
FIGURE 2.
Selective packaging of tRNAs into HIV-1. Each bar represents the HIV-1:cell ratio for a specific tRNA. DNA probes on the microarray are complementary to either nuclear-encoded or mitochondrial-encoded tRNAs. (A) Ratios using tRNA in wild-type HIV-1. (B) Ratios using tRNA present in Gag VLPs lacking GagPol. tRNALys1,2 and tRNALys3 probes are indicated by gray arrows, and tRNAAsn and tRNAIle(UAU) probes are indicated by black arrows.
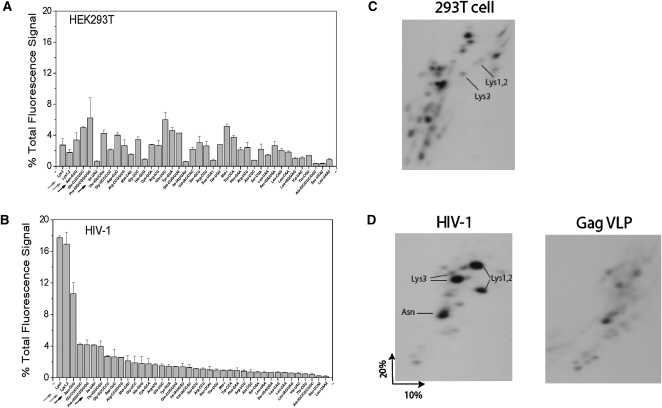
We next calculated from our microarray data the concentration of each tRNA relative to the total tRNA population, as determined by the percent of the total fluorescence signal obtained from each labeled tRNA (Fig. 3A,B). Although this type of analysis is uncommon for two-color microarray studies, we believe this analysis can be justified for this tRNA study: (1) our fluorescence labeling scheme relies only on the 3′NCCA, which is single-stranded and universal in all tRNAs; (2) our labeling scheme does not require reverse transcription, and is therefore less sensitive to tRNA structure or modifications; (3) the array probes have a nearly uniform melting temperature; and (4) there is a very good agreement between the percent tRNAs determined by the array and 2D gel electrophoresis methods, as will be shown in Figure 3, C and D.
FIGURE 3.
Abundance of tRNAs in HIV-1. tRNA abundance is expressed as percent of total tRNA fluorescence signal in HEK293 tRNA (A) and HIV-1 tRNA (B). Only nuclear-encoded tRNAs are shown. (C,D) Analysis of low molecular weight RNA in HEK293T cells, HIV-1, and Gag VLPs by 2D PAGE. Total RNA was 3′ labeled with 32pCp. For purposes of quantitative comparison, all samples used equal amounts of viral genomic RNA, were electrophoresed in the same chamber, and exposed to either film or to phosphorimaging at the same time.
As expected, the tRNALys isoacceptors represent the majority of the total tRNA population in HIV-1 (Fig. 3A,B). tRNALys isoacceptors together represent 35% of total tRNA in HIV-1 versus only 4.5% in 293T cells, indicating an eightfold selective packaging. tRNAAsn(GTT) represents 11% of total tRNA in HIV-1 versus 3.4% in 293T cells, indicating a threefold selective packaging. Finally, tRNAIle(UAU) represents 4% of total tRNA in HIV-1 versus 0.6% in 293T cells, indicating a sevenfold selective packaging. Though tRNAIle(UAU) has a high selection relative to cellular RNA, it is one of the less abundant tRNAs present in HIV-1. This reflects the fact that tRNAIle(UAU) is one of the rare tRNAs in 293T cells. The human genomic codon usage for isoleucine has frequencies of 47% (AUC), 36% (AUU), and 17% (AUA). In contrast, the HIV-1 genomic codon usage for isoleucine has frequencies of 18% (AUC), 29% (AUU), and 54% (AUA) (Nakamura et al. 2000). The tRNAIle(UAU) selectively packaged in HIV only reads the AUA codon, which is the rare Ile codon in human, but the most common Ile codon in HIV-1. As for GagVLPs, the total amount of tRNA packaged is reduced by 80% compared to the wild-type HIV-1, assuming that the total RNA contains similar amounts of viral RNA in HIV and GagVLP.
Analysis of major viral tRNA species using 2D PAGE
Total viral or Gag VLP RNA was 3′-end-labeled with 32pCp, and labeled tRNAs were resolved by 2D PAGE, which only lets in low molecular weight RNAs into the gel. For wild-type HIV-1 (Fig. 3D), the identity of the major tRNALys isoacceptors has been previously reported (Jiang et al. 1993), including a minor tRNALys3 isoacceptor (Gabor et al. 2002). The species marked tRNAAsn was identified by (1) amplifying the RNA in the spot using RT/PCR with primers specific for this tRNA, and (2) allowing radioactivity in a Northern blot of the gel to decay, and then hybridizing the blot with a labeled DNA probe specific for tRNAAsn (data not shown). Because the tRNAIle spot does not stand out among the remaining spots, consistent with the microarray abundance data, we did not attempt the validation of tRNAIle using these methods.
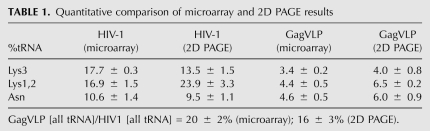
We also analyzed the tRNA pattern in GagVLPs (Fig. 3D). To compare the results quantitatively, the gels for the wild-type and GagVLP samples were loaded with equal amounts of viral genomic RNA (representing equal numbers of viral particles), electrophoretically resolved at the same time in the same chamber, and exposed afterward to film for the same length of time (Table 1). Measurements of total radioactive tRNA species in the gel indicate that in GagVLPs, the amount of tRNA incorporated is reduced by 84% compared to HIV-1, a result very similar to that obtained using microarray analysis (80%). In HIV-1, the percentage of total radioactivity present in the tRNALys (37.4%) isoacceptors and tRNAAsn (9.5%) is very similar to that determined by microarray analysis (34.6% and 10.6%, respectively) (Table 1). Maintenance of the pattern of selection of tRNALys isoacceptors does not appear to be due to a preference for labeling tRNALys isoacceptors with 32pCp, as indicated by the 2D PAGE pattern for 293T cellular low molecular weight RNA (Fig. 3C).
TABLE 1.
Quantitative comparison of microarray and 2D PAGE results
These data support a previous observation that in HIV-1 containing a deletion in reverse transcriptase and integrase sequences, there was an eightfold decrease in tRNALys3 in the virion (Mak et al. 1994). The severe reduction or elimination of selective packaging of tRNALys isoacceptors and tRNAAsn in Gag VLPs suggests that other tRNAs incorporated into virions may also depend upon interaction with GagPol for their incorporation.
Concluding remarks
At this time, we do not know why non-lysyl tRNAs are selectively packaged into HIV-1. We cannot rule out the possibility that non-lysyl tRNAs are packaged as by-products of tRNALys packaging and play no relevant role in retroviral biology. Like tRNALys1,2, non-lysyl tRNAs may also facilitate the import of the pre-integration complex into the nucleus (Zaitseva et al. 2006). Since the lysyl-tRNA (LysRS) and asparagyl-tRNA (AsnRS) synthetases belong to the same structural group (class IIB) (Delarue and Moras 1993), it is possible that tRNAAsn incorporation is due to a secondary interaction with LysRS. However, the human tRNAAsn and tRNALys sequences differ by over 30 nucleotides (nt), suggesting that they are indeed different species and that any function played by tRNAAsn and tRNALys is not interchangeable. Interestingly, tRNAIle(UAU) is rare in human cells, but reads the most common Ile codon in the HIV-1 genome. Selective incorporation of tRNAIle(UAU) into HIV-1 may facilitate translation of key HIV-1 proteins.
Regardless, our result has a practical bottom line: tRNA profiling by microarray is a very useful and convenient tool that can be easily applied to the studies of other viruses or other biological processes for which unknown tRNA species need to be identified. Aside from retro-viruses, early reports show that (−)sense single-stranded RNA viruses also contain multiple, unidentified tRNA species in the virion (Kolakofsky 1972; Isaac and Keene 1981). Our tRNA microarray method can be readily applied to determine whether selective packaging also occurs in other viruses.
MATERIALS AND METHODS
Microarray analysis
The tRNA microarray experiment , described in detail by Dittmar et al. (2006) and Pavon-Eternod et al. (2009), consists of four steps starting from total RNA: (1) deacylation to remove remaining amino acids attached to the tRNA; (2) selective Cy3/Cy5 labeling of tRNA; (3) hybridization on commercially printed arrays; and (4) data analysis.
Deacylation
Total viral (0.25 μg/μL) or cellular RNA was spiked with three tRNA standards (Escherichia coli tRNALys, E. coli tRNATyr, and yeast tRNAPhe) at 0.67 pmol each/μg total RNA. The mixture was incubated in 100 mM Tris-HCl (pH 9.0) at 37°C for 30 min. The solution was neutralized by the addition of an equal volume of 100 mM Na-acetate/acetic acid (pH 4.8) plus 100 mM NaCl, followed by ethanol precipitation. Deacylated total RNA was dissolved in water, and its integrity verified by agarose gel electrophoresis.
Cy3/Cy5 labeling
tRNA in the total RNA mixture was selectively labeled with either Cy3 or Cy5 fluorophore using an enzymatic ligation method previously described (Dittmar et al. 2006). The ligation reaction relies on an 8-bp RNA:DNA hybrid helix containing a Cy3 or Cy5 fluorophore pre-attached to the loop and an overhang complementary to the universally conserved 3′CCA nucleotides present in all tRNAs (Fig. 1A). The ligation reaction was carried out overnight (∼16 h) at 16°C with 0.13 μg/μL total RNA in 1X T4 DNA ligase buffer, 0.5 U/μL T4 DNA ligase (USB Corporation, 70042X), 15% DMSO, and 4.5 μM labeling oligonucleotide.
Hybridization
Hybridization was performed at 60°C overnight (∼16 h) with 1–2 μg each of Cy3- or Cy5-labeled total RNA as previously described (Dittmar et al. 2004). Multiple arrays were run using the 293T cell reference sample labeled with either Cy3 or Cy5.
Data analysis
Arrays were scanned using a GenePix 4000b scanner (Axon Instruments) to obtain fluorescence intensities and the Cy5/Cy3 ratio per pixel at each probe spot. For both Cy3 and Cy5 wavelengths, PMT gain was set at 600 and power at 100%. These settings were chosen to provide optimal signal without saturation. Array images were generated and analyzed using GenePix 6.0 software. The median Cy5/Cy3 ratio per pixel at each probe spot was normalized to an average value of the three tRNA standards prior to subsequent analysis.
Plasmids
BH10 is a simian virus 40-based vector that contains full-length wild-type HIV-1 proviral DNA. BH10.P− is a simian virus 40-based vector that contains full-length wild-type HIV-1 proviral DNA with a single point mutation at position 25 of the protease region, converting Asp25 to Arg25. Transfection of BH10.P− produces noninfectious viral particles containing wild-type genomic RNA and unprocessed precursor proteins Gag and GagPol (Gottlinger et al. 1989).
Cell culture and transfection
HEK-293T cells were grown in complete Dulbecco's modified Eagle's medium (DMEM) plus 10% fetal calf serum (FCS), 100 U of penicillin, and 100 μg of streptomycin/mL. For the production of viruses or viral-like particles (VLPs), HEK-293T cells were transfected with BH10 or BH10.FS− using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
Virus isolation
Forty-eight hours after transfection of 293T cells with plasmids coding for wild-type or mutant HIV-1, the virus-containing culture supernatants were collected. Viruses were pelleted from culture medium by centrifugation in a Beckman 45 Ti rotor at 35,000 rpm for 1 h. The viral pellets were then purified by centrifugation in a Beckman SW41 rotor at 26,500 rpm for 1 h through 15% sucrose onto a 65% sucrose cushion. The band of purified virus was removed and pelleted in 1X TNE in a Beckman 45 Ti rotor at 40,000 rpm for 1 h (Cen et al. 2001).
RNA purification, 2D PAGE, and RNA analysis
Total cellular or viral RNA was extracted from cell or viral pellets by the guanidinium isothiocyanate procedure, and dissolved in 5 mM Tris buffer, pH 7.5 (Cen et al. 2001). To determine the packaging pattern of tRNAs in the viruses, 2D PAGE of 32pCp-3′-end-labeled total viral RNA was performed as described previously (Jiang et al. 1993). Hybridization to dot blots of viral RNA and tRNAs was carried out with 32P-5′-end-labeled DNA probes complementary to genomic RNA nucleotides 791-807 (5′-CTGACGCTCTCGCACCC-3′), and to the 3′ terminal 18 nucleotides of tRNALys3 (5′-TGGCGCCCGAACAGGGAC-3′) and tRNAAsn (5′-TGGCGTCCCTGGGTGGGC-3′). Phosphorimaging was used to quantitate the relative amount of HIV-1 genomic RNA per sample and the relative amount of tRNAs.
Identification of tRNAAsn by sequencing
The 32pCp-labeled RNA species in spot 4 was excised from a fresh 2D PAGE gel, eluted overnight, and ethanol precipitated, as previously described (Wei et al. 2005). RT/PCR was performed upon the purified RNA from spot 4 using ThermoScript RT/PCR system with Platinum Taq (Invitrogen Life Technologies). The primer for reverse transcript to synthesize cDNA of tRNA was 5′-TGGCGTCCCTGGGTGGGCTC-3′. PCR was carried out by using a pair of internal primers (forward primer: 5′-GTGGCGCAATGCGTT-3′; reverse primer: 5′-TGGGTGGGCTCGAA-3′). The PCR products were inserted into the pCR4-TOPO vector (Invitrogen Life Technologies) and individual clones were sequenced.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (USA) (to L.K. and to T.P.) and the Canadian Institutes for Health Research (Canada) (to L.K.). M.P.E. was supported by a Ruth Kirshstein Pre-doctoral Fellowship from the NIH (1F31CA139968). M.P.-E. and M.W. contributed equally to this work. M.P.E. carried out the microarray studies; M.W. carried out the 2D PAGE studies; and L.K. conceived of the study and participated in its design and coordination with T.P.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.1928110.
REFERENCES
- Cen S, Khorchid A, Javanbakht H, Gabor J, Stello T, Shiba K, Musier-Forsyth K, Kleiman L. Incorporation of lysyl-tRNA synthetase into human immunodeficiency virus type 1. J Virol. 2001;75:5043–5048. doi: 10.1128/JVI.75.11.5043-5048.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delarue M, Moras D. The aminoacyl-tRNA synthetase family: Modules at work. Bioessays. 1993;15:675–687. doi: 10.1002/bies.950151007. [DOI] [PubMed] [Google Scholar]
- Dittmar KA, Mobley EM, Radek AJ, Pan T. Exploring the regulation of tRNA distribution on the genomic scale. J Mol Biol. 2004;337:31–47. doi: 10.1016/j.jmb.2004.01.024. [DOI] [PubMed] [Google Scholar]
- Dittmar KA, Goodenbour JM, Pan T. Tissue-specific differences in human transfer RNA expression. PLoS Genet. 2006;2:e221. doi: 10.1371/journal.pgen.0020221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabor J, Cen S, Javanbakht H, Niu M, Kleiman L. Effect of altering the . tRNA3Lys concentration in human immunodeficiency virus type 1 upon its annealing to viral RNA, GagPol incorporation, and viral infectivity. J Virol. 2002;76:9096–9102. doi: 10.1128/JVI.76.18.9096-9102.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlinger HG, Sodroski JG, Haseltine WA. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci. 1989;86:5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac CL, Keene JD. Transfer RNAs associated with vesicular stomatitis virus. J Gen Virol. 1981;56:141–151. doi: 10.1099/0022-1317-56-1-141. [DOI] [PubMed] [Google Scholar]
- Javanbakht H, Halwani R, Cen S, Saadatmand J, Musier-Forsyth K, Gottlinger H, Kleiman L. The interaction between HIV-1 Gag and human lysyl-tRNA synthetase during viral assembly. J Biol Chem. 2003;278:27644–27651. doi: 10.1074/jbc.M301840200. [DOI] [PubMed] [Google Scholar]
- Jiang M, Mak J, Ladha A, Cohen E, Klein M, Rovinski B, Kleiman L. Identification of tRNAs incorporated into wild-type and mutant human immunodeficiency virus type 1. J Virol. 1993;67:3246–3253. doi: 10.1128/jvi.67.6.3246-3253.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorchid A, Javanbakht H, Wise S, Halwani R, Parniak MA, Wainberg MA, Kleiman L. Sequences within Pr160gag-pol affecting the selective packaging of primer tRNA3Lys into HIV-1. J Mol Biol. 2000;299:17–26. doi: 10.1006/jmbi.2000.3709. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D. Transfer ribonucleic acid nucleotidyltransferase and transfer ribonucleic acid in Sendai virions. J Virol. 1972;10:555–559. doi: 10.1128/jvi.10.3.555-559.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak J, Jiang M, Wainberg MA, Hammarskjold ML, Rekosh D, Kleiman L. Role of Pr160gag-pol in mediating the selective incorporation of tRNALys into human immunodeficiency virus type 1 particles. J Virol. 1994;68:2065–2072. doi: 10.1128/jvi.68.4.2065-2072.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Gojobori T, Ikemura T. Codon usage tabulated from international DNA sequence databases: Status for the year 2000. Nucleic Acids Res. 2000;28:292. doi: 10.1093/nar/28.1.292. url: http://nar.oxfordjournals.org/cgi/content/full/28/1/292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavon-Eternod M, Gomes S, Geslain R, Dai Q, Rosner MR, Pan T. tRNA over-expression in breast cancer and functional consequences. Nucleic Acids Res. 2009 doi: 10.1093/nar/gkp787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raba M, Limburg K, Burghagen M, Katze JR, Simsek M, Heckman JE, Rajbhandary UL, Gross HJ. Nucleotide sequence of three isoaccepting lysine tRNAs from rabbit liver and SV40-transformed mouse fibroblasts. Eur J Biochem. 1979;97:305–318. doi: 10.1111/j.1432-1033.1979.tb13115.x. [DOI] [PubMed] [Google Scholar]
- Wei M, Cen S, Niu M, Guo F, Kleiman L. Defective replication in human immunodeficiency virus type 1 when nonprimers are used for reverse transcription. J Virol. 2005;79:9081–9087. doi: 10.1128/JVI.79.14.9081-9087.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitseva L, Myers R, Fassati A. tRNAs promote nuclear import of HIV-1 intracellular reverse transcription complexes. PLoS Biol. 2006;4:e332. doi: 10.1371/journal.pbio.0040332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Goodenbour JM, Godley LA, Wickrema A, Pan T. High levels of tRNA abundance and alteration of tRNA charging by bortezomib in multiple myeloma. Biochem Biophys Res Commun. 2009;385:160–164. doi: 10.1016/j.bbrc.2009.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]