Abstract
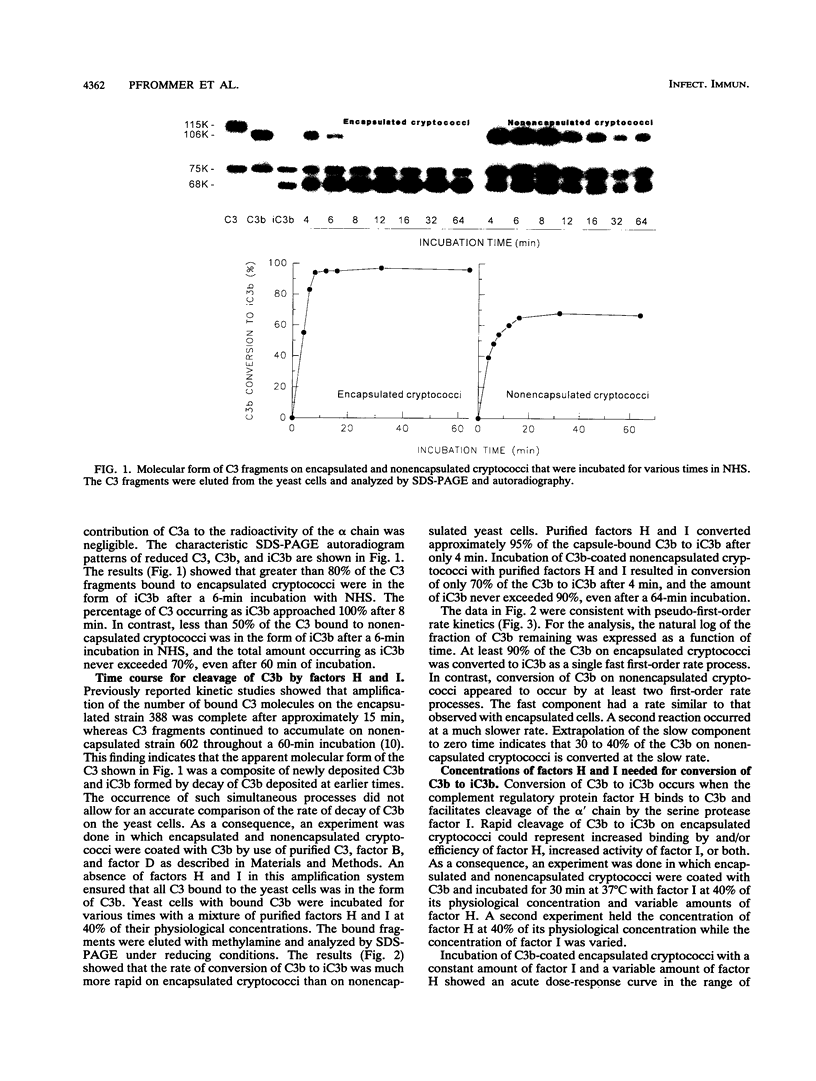
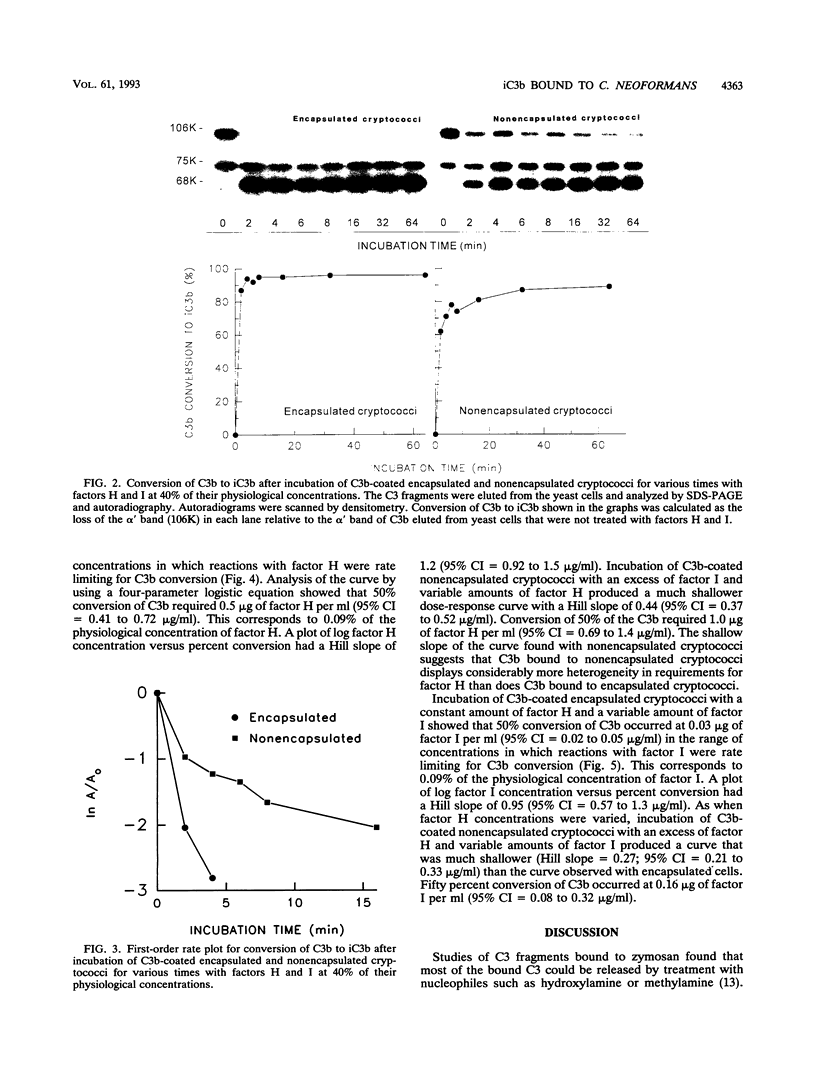
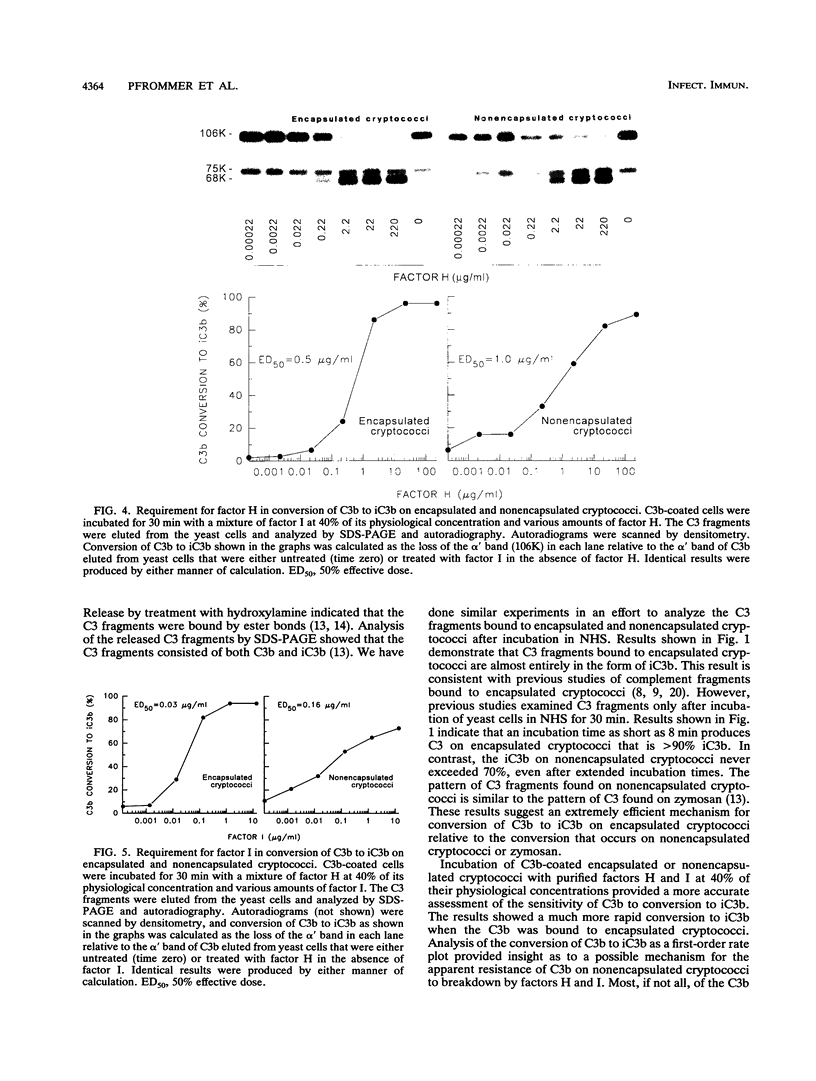
Incubation of encapsulated and nonencapsulated Cryptococcus neoformans in normal human serum (NHS) leads to activation and binding of potentially opsonic fragments of complement component C3 to the yeast cells. Analysis of the molecular forms of C3 after incubation of encapsulated cryptococci in NHS showed that the percentage of bound C3 occurring as iC3b approached 100% after 8 min. The percentage of bound C3 occurring as iC3b on nonencapsulated cryptococci never exceeded 70%, even after 60 min of incubation in NHS. Conversion of C3b to iC3b was assessed further by incubating C3b-coated cryptococci for various times with a mixture of complement factors H and I at 40% of their respective physiological concentrations. Most, if not all, of the C3b on encapsulated cryptococci was converted to iC3b at a single fast rate. Conversion of C3b to iC3b on nonencapsulated cryptococci did not follow a single rate constant and appeared to have a fast and a slow component. Studies of the requirements for factors H and I in cleavage of C3b to iC3b showed steep dose-response curves for both factors in the case of encapsulated cryptococci and shallow curves with C3b bound to nonencapsulated cryptococci. Taken together, our results indicate that C3b molecules bound to encapsulated cryptococci have a uniformly high susceptibility to conversion to iC3b by factors H and I. In contrast, a significant portion of the C3b bound to nonencapsulated cryptococci is very resistant to conversion to iC3b by factors H and I.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chaskes S., Edberg S. C., Singer J. M. A DL-DOPA drop test for the identification of Cryptococcus neoformans. Mycopathologia. 1981 Jun 5;74(3):143–148. doi: 10.1007/BF00437156. [DOI] [PubMed] [Google Scholar]
- Ezzell J. L., Parker C. J. Cell-surface regulation of the human alternative pathway of complement. Sheep but not rabbit erythrocytes express factor I-dependent cofactor activity. Scand J Immunol. 1992 Jul;36(1):79–87. doi: 10.1111/j.1365-3083.1992.tb02943.x. [DOI] [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F. Activation of the alternative complement pathway with rabbit erythrocytes by circumvention of the regulatory action of endogenous control proteins. J Exp Med. 1977 Jul 1;146(1):22–33. doi: 10.1084/jem.146.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Horstmann R. D., Pangburn M. K., Müller-Eberhard H. J. Species specificity of recognition by the alternative pathway of complement. J Immunol. 1985 Feb;134(2):1101–1104. [PubMed] [Google Scholar]
- Kozel T. R., Cazin J. Nonencapsulated Variant of Cryptococcus neoformans I. Virulence Studies and Characterization of Soluble Polysaccharide. Infect Immun. 1971 Feb;3(2):287–294. doi: 10.1128/iai.3.2.287-294.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Highison B., Stratton C. J. Localization on encapsulated Cryptococcus neoformans of serum components opsonic for phagocytosis by macrophages and neutrophils. Infect Immun. 1984 Feb;43(2):574–579. doi: 10.1128/iai.43.2.574-579.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Pfrommer G. S. Activation of the complement system by Cryptococcus neoformans leads to binding of iC3b to the yeast. Infect Immun. 1986 Apr;52(1):1–5. doi: 10.1128/iai.52.1.1-5.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Pfrommer G. S., Guerlain A. S., Highison B. A., Highison G. J. Strain variation in phagocytosis of Cryptococcus neoformans: dissociation of susceptibility to phagocytosis from activation and binding of opsonic fragments of C3. Infect Immun. 1988 Nov;56(11):2794–2800. doi: 10.1128/iai.56.11.2794-2800.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Wilson M. A., Murphy J. W. Early events in initiation of alternative complement pathway activation by the capsule of Cryptococcus neoformans. Infect Immun. 1991 Sep;59(9):3101–3110. doi: 10.1128/iai.59.9.3101-3110.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Wilson M. A., Pfrommer G. S., Schlageter A. M. Activation and binding of opsonic fragments of C3 on encapsulated Cryptococcus neoformans by using an alternative complement pathway reconstituted from six isolated proteins. Infect Immun. 1989 Jul;57(7):1922–1927. doi: 10.1128/iai.57.7.1922-1927.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Polacheck I., Bennett J. E. Improved diagnostic medium for separation of Cryptococcus neoformans var. neoformans (serotypes A and D) and Cryptococcus neoformans var. gattii (serotypes B and C). J Clin Microbiol. 1982 Mar;15(3):535–537. doi: 10.1128/jcm.15.3.535-537.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law S. K., Levine R. P. Interaction between the third complement protein and cell surface macromolecules. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2701–2705. doi: 10.1073/pnas.74.7.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law S. K., Lichtenberg N. A., Levine R. P. Evidence for an ester linkage between the labile binding site of C3b and receptive surfaces. J Immunol. 1979 Sep;123(3):1388–1394. [PubMed] [Google Scholar]
- Levitz S. M., Tabuni A. Binding of Cryptococcus neoformans by human cultured macrophages. Requirements for multiple complement receptors and actin. J Clin Invest. 1991 Feb;87(2):528–535. doi: 10.1172/JCI115027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Müller-Eberhard H. J. Kinetic and thermodynamic analysis of the control of C3b by the complement regulatory proteins factors H and I. Biochemistry. 1983 Jan 4;22(1):178–185. doi: 10.1021/bi00270a026. [DOI] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution. J Exp Med. 1977 Jul 1;146(1):257–270. doi: 10.1084/jem.146.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Trombold J. S., Müller-Eberhard H. J. Paroxysmal nocturnal hemoglobinuria: deficiency in factor H-like functions of the abnormal erythrocytes. J Exp Med. 1983 Jun 1;157(6):1971–1980. doi: 10.1084/jem.157.6.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn R. G., Bryant-Varela B. J., Julian N. C., Bennett J. E. Differences in Cryptococcus neoformans capsular polysaccharide structure influence assembly of alternative complement pathway C3 convertase on fungal surfaces. Mol Immunol. 1991 Apr-May;28(4-5):465–470. doi: 10.1016/0161-5890(91)90160-l. [DOI] [PubMed] [Google Scholar]
- Wilson M. A., Kozel T. R. Contribution of antibody in normal human serum to early deposition of C3 onto encapsulated and nonencapsulated Cryptococcus neoformans. Infect Immun. 1992 Mar;60(3):754–761. doi: 10.1128/iai.60.3.754-761.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B. J., Kozel T. R. Effects of strain variation, serotype, and structural modification on kinetics for activation and binding of C3 to Cryptococcus neoformans. Infect Immun. 1993 Jul;61(7):2966–2972. doi: 10.1128/iai.61.7.2966-2972.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]






