Abstract
Purpose
To evaluate the efficacy and safety of lateral shelf acetabuloplasty in Legg–Calvé–Perthes (LCP) hips classically associated with poor prognosis.
Methods
A retrospective study was conducted on 30 consecutive pediatric patients (average age 8.6 years) presenting with a severe and progressive form of LCP disease, with (16 hips) or without (14 hips) femoral varus osteotomy (FVO), and treated by lateral shelf acetabuloplasty. Shelf was done on hips presenting an aspherical incongruency with flattening, subluxation, and lack of femoral head coverage, as demonstrated on pre-operative radiographs and arthrography. All patients were reexamined at an average follow-up of 9.5 years (range 5.2–12 years). Clinical, radiological, and computed tomography scan evaluations were undertaken. Stulberg and Mose classifications were applied as radiological indicators of prognosis. Statistical analysis was performed using Student’s t test and the Pearson correlation test with variance analysis for repetitive measures.
Results
At the last follow-up, all patients were pain free and had normal or almost normal hip motion. Twenty-seven patients are now able to walk normally or with a slight limp. Eighteen hips are classified as Stulberg 1 or 2, and 18 hips are classified as Mose 1 or 2. The average neck-shaft angle is 127°. A statistically significant improvement in the majority of radiographic parameters was found. There was no statistically significant worsening of leg length discrepancy following the procedure. The scanographic study found neither offset nor migration of the shelf in any of the hips. The average coronal and anteroposterior length of the shelf was 9.9 and 25 mm, respectively. A borderline positive correlation was found between Wiberg angle improvement and young age at the time of surgery. There was no statistically significant difference between hips that underwent shelf procedures alone and those in which it was combined with FVO.
Conclusion
Lateral shelf acetabuloplasty improves the outcome of hips with severe LCP. The combined procedure insures a better and lasting coverage and remodeling of the femoral head, while preserving acetabular roof growth.
Keywords: Acetabuloplasty, Lateral shelf, Legg–Calvé–Perthes disease, Perthes disease
Introduction
There is still a lot of controversy concerning the treatment of Legg–Calvé–Perthes (LCP) disease. Surgery is indicated for severely involved hips in older children with femoral head deformation and subluxation. The choice of surgery depends on many factors, including surgeon’s preference, shape and concentricity of the femoral head, and congruency of the hip joint [1]. Redirection osteotomies of the pelvis are performed for hips with mild or moderate deformity of the femoral head. Varus osteotomy of the femur, with or without derotation, is based on the concept—at least theoretical concept—of decreasing pressure on the affected femoral head as well as improving blood flow to the proximal femoral epiphysis. There is currently sufficient evidence to support the fact that pelvic redirection and proximal femoral varus osteotomies give comparable results [1].
Chiari’s osteotomy and shelf acetabuloplasty (SA) are indicated for cases where redirection osteotomy is deemed insufficient to produce enough displacement of the acetabular fragment to insure optimal coverage of the extruded femoral head, due to lack of either concentricity or congruency, or both [2–4].
The orthopedic literature of the last 30 years contains contradictory information on the efficacy and complications of shelf procedures for Perthes’ disease, especially in young children (<8 years of age) [2–5]. The purpose of the study reported here was to evaluate the risks and benefits of lateral SA in children with LCP disease and severely deformed femoral head.
Materials and methods
A retrospective review was conducted on 33 consecutive children who underwent lateral SA for a severe form of Perthes disease in the period between October 1996 and June 2002. Three uncompleted medical records reduced the patient cohort to 30 patients. A total of 139 patients with LCP of various severities and ages presented to our department during the same period; of these, 63 were treated nonoperatively, and 43 were treated with pelvic redirection osteotomy.
Shelf acetabuloplasty surgical technique
The reflected tendon of the rectus femoris is divided from the direct portion and dissected posteriorly. A curvilinear slot is produced in the subchondral bone of the anterolateral aspect of the acetabular roof, 3–3.5 cm in length, 2–3 mm in height, and 1–1.5 cm in depth, in an ascending direction from lateral to medial and from distal to proximal. One or two cortical grafts are harvested from the iliac wing, tightly inserted in the slot, with an extrusion of 1–1.5 cm lying on the hip capsule after its exposure, and secured with the reflected head of the rectus femoris. This is sutured back to its origin on the direct head, keeping 5–6 mm of the most lateral aspect of the graft, exposed in such a way as to be in contact with the cancellous and cortical graft harvested from the same iliac wing, and put on top of it.
In the 30 cases reported here, surgery involved the left hip in 19 patients and the right hip in 11 patients and was performed during the fragmentation or fragmentation/reossification stage in all cases. The decision to perform a shelf rather than a redirection osteotomy was based on the presence of a flat extruded and uncovered femoral head on anteroposterior (AP) and Von Rosen views as well as the evidence of hinge abduction under anesthesia, as demonstrated by plain radiographs and preoperative arthrography (Fig. 1). None of the children treated nonoperatively had the same radiographic characteristics as the ones selected for acetabuloplasty. In the former group, involvement was mild to moderate (Herring classification A and B; Caterall groups I and II), or there was no severe flattening or extrusion even there was severe involvement, mainly if the patients were younger than 5 years of age.
Fig. 1.
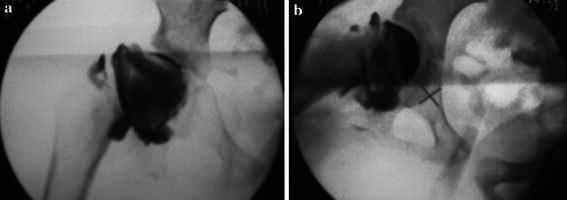
a An example of preoperative arthrography showing hinge abduction, b the femoral head does not penetrate the acetabulum in abduction, which becomes rather filled by a large amount of dye
Percutaneous adductor tenotomy was performed as a first surgical step in all cases. Shelf acetabuloplasty was performed alone in 14 cases. A proximal femoral varus osteotomy (FVO) of 10–15° was undertaken in combination with and prior to acetabuloplasty (during the same setting) in 16 patients with the worst subluxation, hinge abduction, and deformity of the femoral head. The rationale for such an osteotomy was to better distribute and decrease axial loading across the hip joint, to improve blood flow to the proximal femoral epiphysis, and to obtain a better surgical positioning of the shelf. The number of hips involved in the study is too small to be able to define a more precise cutoff for this decision. A spica cast was applied postoperatively for 4 weeks; this procedure is not necessary in older children, and we are gradually shifting away from this step.
There were 22 boys and eight girls. The surgery was the first treatment undertaken in 28 cases; in two cases, surgery followed the failure of a previous innominate redirection osteotomy (Fig. 2). The average age of our patient cohort at diagnosis was 7.5 years (range 6.2–11), and the average age at the time of surgery was 8.6 years (range 7.1–13). Eleven patients younger than 8 years at surgery. Pain (28/30) and various degrees of limping (30/30) were the two major chief complaints. Moderate or severe limitation of hip motion was present in seven and 23 cases, respectively. Discrepancy in leg length was found in nine patients, ranging from 1.5 to 3.5 cm (average 1.9). Based on radiographic assessment, 19 hips were graded Caterall group 4 and 11 hips as Caterall group 3. Twenty-five hips were graded Herring group C and five as Herring group B. Femoral head “at risk signs” were present in all cases ranging in number from one to four (one risk sign, 5%; two, 10%; three, 40%; four, 45%).
Fig. 2.
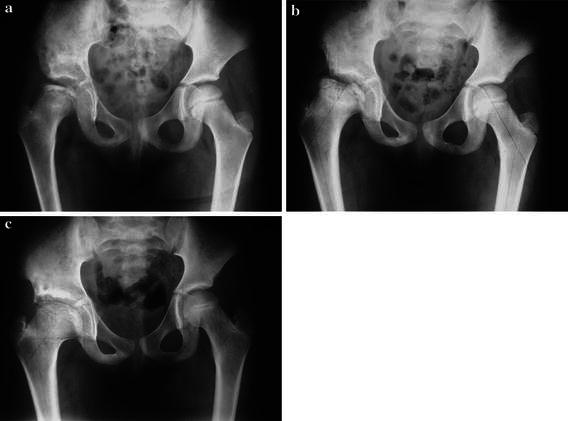
a Five-year-old girl with a Herring group C Perthes disease 6 months following a Salter osteotomy performed at another institution. There is persistent flattening and subluxation of the femoral epiphysis; the spherical acetabulum was unable to fully rotate over the flattened femoral head despite a good lateral displacement. b 1.5 year following surgery, there is worsening of the femoral head deformity and subluxation with an obvious adduction contracture of the hip joint. c 3.5 years following adductor tenotomy and Staheli’s shelf acetabuloplasty (SA). There is a good and lasting coverage with a satisfactory remodeling of the femoral head
Both clinical and radiographic evaluations were performed at regular intervals and at a final mean follow-up of 9.5 years (range 5.2–12). Hip passive range of motion was assessed using an articulated double arm goniometer and was considered normal when it was either equal to or maximally 10° less than the opposite side for all sectors. The Iowa Hip Score was used to determine the clinical outcome at final follow-up. Radiographic parameters included femoral head subluxation ratio (s), femoral head size ratio between operated and non-operated hips (H), the sharp angle, percentage of acetabular coverage (A/B), Wiberg center-edge angle (CE), anterior coverage angle (VCA) (only at last follow-up), and neck-shaft angle (NSA) (Fig. 3; Table 1). Preoperative values, recent postoperative values (6 weeks following cast removal), and last follow-up values were statistically compared. Major reported shelf complications, i.e., graft resorption, proximal migration, or growth disturbance of the lateral aspect of the acetabulum, were looked for and recorded. Despite the fact that only 21 of our patients had reached skeletal maturity at the time of review, we used the Stulberg [6] and Mose classifications as radiographic prognostic indicators at the last follow-up. This extrapolation was based on several previous reports that found a significant correlation between results given before the end of growth and those found in early adulthood [7–10]. At the last follow-up, a computed tomography (CT) scan with two- and three-dimensional (2D and 3D, respectively) reconstructions was performed on 25 patients in order to assess the shape and AP and coronal length of the shelf as well as its remodeling and incorporation based on for any offset between the healed graft and the original acetabular roof. An offset >1 mm on more than one CT cut was considered to be a bad result, based on the assumption that this degree of offset may increase the risk of progressive joint degeneration. The need for additional procedures was recorded without being necessarily being considered as a treatment failure.
Fig. 3.
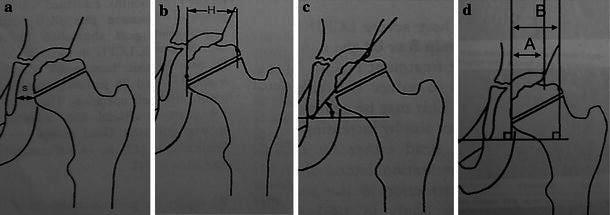
a Subluxation femoral ratio (s), b femoral head size ratio between the involved and the normal hip (H), c sharp angle, d femoral head coverage ratio (A/B). In the majority of the cases, A becomes greater than B following the shelf procedure
Table 1.
Summary of preoperative and postoperative data
| Patient no. | Age at diagnosis (years) | Age at surgery (years) | Type of surgery | Lateral pillar class | Stulberg classification [6] at last follow-up | Additional surgery |
|---|---|---|---|---|---|---|
| 1 | 6.2 | 7.1 | SA | C | 1 | |
| 2 | 6.2 | 7.3 | SA | B | 1 | EGT |
| 3 | 6.4 | 7.4 | SA | C | 2 | |
| 4 | 8.1 | 9.0 | SA | C | 4 | FL |
| 5 | 6.3 | 7.2 | SA | B | 2 | |
| 6 | 7.3 | 7.7 | SA | C | 3 | |
| 7 | 6.3 | 7.5 | SA | C | 2 | |
| 8 | 8.9 | 11.1 | SA | C | 3 | DTGT |
| 9 | 6.6 | 7.8 | SA | B | 2 | |
| 10 | 6.3 | 7.2 | SA | C | 2 | |
| 11 | 7.7 | 9.8 | SA | C | 4 | |
| 12 | 6.6 | 7.8 | SA | C | 1 | |
| 13 | 6.2 | 7.4 | SA | B | 2 | |
| 14 | 9.4 | 10.2 | SA | C | 3 | DTGT |
| 15 | 6.3 | 8.8 | SA + FVO | C | 1 | |
| 16 | 7.7 | 8.2 | SA + FVO | C | 2 | |
| 17 | 7.7 | 8.3 | SA + FVO | C | 4 | |
| 18 | 6.4 | 7.7 | SA + FVO | C | 2 | |
| 19 | 8.5 | 9.4 | SA + FVO | C | 3 | |
| 20 | 8.2 | 8.3 | SA + FVO | C | 2 | |
| 21 | 6.7 | 8.9 | SA + FVO | C | 2 | |
| 22 | 6.6 | 7.4 | SA + FVO | C | 2 | EGT |
| 23 | 7.8 | 8.6 | SA + FVO | C | 2 | |
| 24 | 9.2 | 9.4 | SA + FVO | C | 4 | DTGT |
| 25 | 7.7 | 9.3 | SA + FVO | C | 2 | |
| 26 | 9.4 | 9.7 | SA + FVO | C | 3 | |
| 27 | 7.3 | 8.2 | SA + FVO | C | 2 | |
| 28 | 6.3 | 7.9 | SA + FVO | B | 1 | |
| 29 | 10.0 | 10.1 | SA + FVO | C | 3 | |
| 30 | 11.0 | 13 | SA + FVO | C | 4 | DTGT |
SA, Shelf acetabuloplasty; FVO, femoral varus osteotomy; EGT, epiphysiodesis of the greater trochanter; FL, femoral lengthening; DTGT, distal transfer of greater trochanter
Gender, involved side, age at diagnosis or treatment, number of “head at risk” signs, Catterall and Herring stages of the disease, and absence or presence of associated FVO were correlated to the end result.
Statistical analysis was performed using the Student’s t test and the Pearson correlation test with variance analysis for repetitive measures. A P value < 0.05 was considered to be significant. Analysis was undertaken using SPSS ver. 13 software (SPSS, Chicago, IL).
Results
Clinical evaluation
We found a significant improvement in all clinical parameters (P values between 0.042 and 0.000) with the exception of leg length discrepancy (P = 0.054). All patients had normal (24 patients) or almost normal (six patients) and painless hip joint mobility at last follow-up. Three patients (10%) had an obvious Trendelenbourg gait, 11 (35%) had a moderate limp, and 16 (55%) had a normal gait pattern, without any statistically significant difference between the isolated SA group and the combined procedure group (SA + FVO) (P = 0.09). The mean Iowa hip score at the time of final evaluation for the whole series was 88.6 ± 5.4, with no significant difference (P = 0.58) between the isolated SA group (88.2 ± 5.2) and the SA + FVO group (88.9 ± 5.7). A leg length discrepancy (LLD) ranging from 1 to 3.5 cm (average 1.72 cm) was found in eight patients: three in the isolated SA group and five in the SA + FVO group. Seven patients (23%) required additional surgery in the form of distal transfer (four cases) or epiphysiodesis (two cases) of the greater trochanter, and femoral lengthening (one case) (Table 1).
Radiographic evaluation
Statistical analysis of the radiographic evaluation (Table 2) showed a significant improvement in all radiographic parameters (P values between 0.049 and 0.000) with the exception of the femoral head subluxation ratio and femoral head size ratio (P = 0.09 and 0.12, respectively). No significant difference was found between post-operative and last follow-up values (P = 0.4). At last follow-up, five hips (17%) were classified as Stulberg grade 1, 14 (47%) as grade 2, six (20%) as grade 3, and five (16%) as grade 4. Eight hips (27%) were classified as Mose grade 1, 11 (37%) as grade 2, and 11 (36%) as grade 3. The CT 2D and 3D reconstructions did not show proximal migration of the shelf in any case, as evidenced by the absence of offset between the shelf and the original acetabular roof (Fig. 4). Dysplastic changes of the acetabular roof were observed on radiographs and confirmed on the CT scans in two cases (Fig. 5) without any major negative clinical or radiological impact. These changes are possibly explained by a growth disturbance due to surgical aggression of the limbus. There was, however, a good incorporation of the shelf into the adjacent bone in all cases. The average coronal and AP lengths of the shelf were 9.9 mm (5.3–14.8 mm) and 25 mm (18.4–29.2 mm), respectively (Fig. 4). There was no statistically significant difference at final follow-up for all radiographic parameters between the isolated SA group and the combined procedure group (SA + FVO)—even for the NSA (P values ranging from 0.08 to 0.32).
Table 2.
Average of radiographic data for the entire patient series
| Radiographic evaluationa | s | H | Sharp angle (°) | A/B | CE (°) | VCA (°) | NSA (°) |
|---|---|---|---|---|---|---|---|
| I | 14/10 | 38/33.5 | 45 | 26.5/37.5 | 12.5 | – | 130 |
| II | 15/9.5 | 34.5/32.5 | 32.5 | 41.5/38 | 47.5 | – | 121 |
| III | 13/8.5 | 48.5/43 | 35 | 53/48.5 | 44 | 49.5 | 127 |
s, Femoral head subluxation ratio; H, femoral head size ratio between operated and non-operated hips; A/B, percentage of acetabular coverage; CE, Wiberg center-edge angle; VCA, anterior coverage angle; NSA, Neck-shaft angle
aRadiographic data for the entire patient series: I, average preoperative values; II, average postoperative (6 weeks following cast removal) values; III, last follow-up values
Fig. 4.
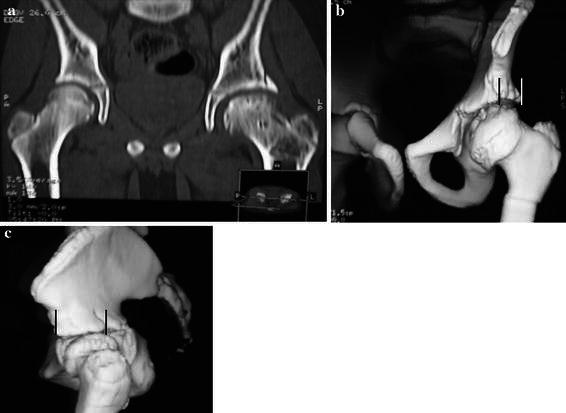
Computed tomography scan with two-dimensional (2D) and 3D reconstructions. a 2D reconstruction showing a good incorporation of the shelf with no offset, b lateral (coronal) coverage, c anteroposterior (AP) coverage
Fig. 5.
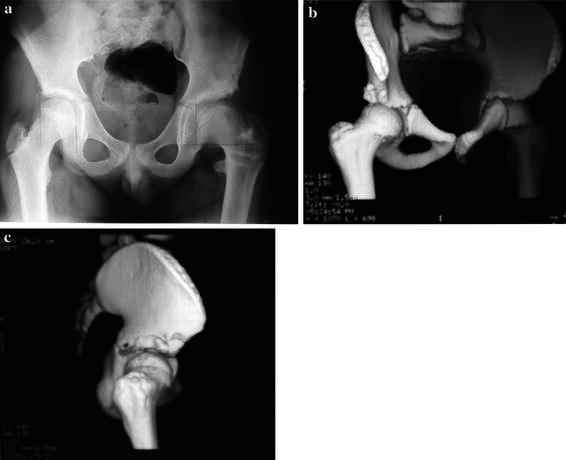
A case of growth disturbance of the acetabular roof 5.3 years following a shelf procedure. a AP of the pelvis shows acetabular dysplasia, b, c coronal and AP appearance of the shelf and the lateral aspect of the acetabulum on CT scan 3D reconstructions
Gender, involved side, age at diagnosis or treatment, number of “head at risk” signs, and Catterall and Herring stages of the disease did not have any significant influence on the final clinical and/or radiographic result (P ranging from 0.07 to 0.23). However, we did find a borderline positive correlation (P = 0.05) between Wiberg angle improvement and young age at surgery (younger than 8 years of age).
Discussion
This study shows that lateral SA is a safe and effective procedure in the management of severely involved hips with LCP disease associated with severe femoral head flattening, subluxation, and hinge abduction. Age at surgery, severity of femoral head involvement, and the presence or absence of “head at risk” signs do not appear to influence the final outcome.
The “containment” principle along with the biologic plasticity of osteo-cartilaginous structures in children allows satisfactory reconstruction and remodeling of the femoral head with time, even in older children. Similar results were reported by Willet et al. [11] in 20 LCP cases treated using a SA and by Villet et al. [12], who showed good results following the same procedure in 18 patients presenting with a severe LCP disease. Kruse et al. [9] compared 20 patients with LCP disease who underwent SA with 18 patients treated by conservative methods, reviewed at an average follow-up of 19 years. These researchers found an improvement of the VCE angle and the Mose stage over the years in the group treated surgically and concluded that an improved coverage of the femoral head in LCP disease leads—with time—to an improvement in congruency and decreases the risk of early joint degeneration. Our midterm results support Kruse et al.’s findings. The procedure improves femoral head coverage, thereby possibly contributing to remodeling improvement of the hip joint. Subluxation, as measured by the distance between the teardrop and the medial epiphysis or metaphysis, seems to remain unchanged.
Growth disturbance of the lateral aspect of the acetabulum is uncommon, even when surgery is undertaken in young children. In a study on 43 patients treated by a SA for LCP disease with an average follow-up of 3.7 years, Jacobs et al. [8] showed an improvement in the prognosis in children >5 years of age with a persistence of growth of the lateral aspect of the acetabulum. Daly et al. [7] reviewed 26 children >8 years of age treated by SA with a long-term follow-up. These researchers demonstrated that the acetabulum continues to grow following surgery, thereby adequately covering the femoral head throughout the patient’s childhood and adolescence. They concluded by rejecting the theory that the procedure may jeopardize acetabular growth. Our results are in accordance with the findings of these two previous studies: we found a dysplastic acetabular roof at the final follow-up on radiographs and CT scans of two of the 30 hips, without any negative clinical impact or secondary lateral and proximal migration of the femoral epiphysis. These findings are thought to be related to some degree of growth disturbance of the acetabular roof.
Proximal migration of the shelf is another reported complication of this procedure. When proper measures are taken to insure an optimal position of the shelf, i.e., in continuity with the subchondral bone of the acetabular roof (Fig. 3), along with its stabilization using the reflected head of the rectus femoris tendon, proximal migration of the shelf becomes extremely rare, even in younger children. None of our patients showed proximal offset of the graft, and the shelf was well incorporated into the original acetabular roof in all of them. One or two millimeters of proximal offset may be acceptable; however, the surgeon should insure a proper positioning of the graft from the very beginning, prolonging the subchondral bone of the acetabular roof. Association of a proximal femoral VO in severely “subluxed” and proximally displaced femoral heads may lower the flattened epiphysis to a level where proper placement of the shelf becomes easier.
Kuwajima et al. [10] reviewed 90 cases of LCP disease treated by either innominate osteotomy or SA and showed that there was a better and long-lasting coverage of the femoral head in the group of children who underwent SA. These findings provide yet further evidence that this procedure does not produce any growth disturbance of the lateral aspect of the acetabulum and that graft resorption is uncommon. However, the authors did not underline the difference in patient selection between innominate redirection osteotomies and SA. In our practice, the former is indicated in cases where there is an optimal spherical congruency of the hip joint despite the lack of coverage of the femoral head, allowing a free rotation of the acetabular fragment over the femoral head; The latter, i.e., SA, remains a salvage procedure for those cases with aspherical congruency or incongruency with hinge abduction, as documented by intraoperative dynamic arthrography. The less coverage produced by innominate osteotomy in Kuwajima et al.’s study may have been simply a lack of rotation of the acetabular fragment due to a severely flattened femoral head and uncongruent hip joint.
In our series, the senior surgeon made the conscious decision to perform a combined femoral varus osteotomy of 10–15° in cases associated with the worst subluxation and hinge abduction. This procedure may have had a positive effect on the overall surgical result; in fact, although it was performed for the most severely deformed hips, the overall treatment outcome for these hips was statistically similar to that observed in relatively less involved hips that underwent SA alone. Our results support the hypothesis that the combined approach (FOV + SA) produces a better load redistribution over the affected femoral head, decreases the pressure across the hip joint, and increases the proximal femoral vascular flow, at least theoretically. In order to avoid the known complications of femoral osteotomy, i.e., limb shortening, decreased hip abduction, and long-lasting Trendelenbourg gait, we chose to undertake minimal angular correction (10–15°). The results of this approach have been rewarding. Although it is difficult to discern the input of femoral osteotomy specifically based on our results, we have shown that a combined femoral varus osteotomy of 10–15° does not have any significant negative effect on NSA, limb length, and hip joint motion.
We did not find in information in the literature indicating that SA with or without FVO produces impingement problems later in life. Although this complication remains theoretically possible, we personally feel that it is unlikely to occur if the shelf’s shape and size are appropriate.
In the absence of a control group—i.e., patients for which SA is indicated according to our criteria but not performed—we cannot be completely sure of the efficacy of the procedure. However, a prospective randomized study on the outcome of severe LCP cases with flattened femoral heads and with subluxed femoral heads with hinge abduction is difficult, if not impossible, to perform in terms of the ethical issues involved. In fact, one of the very rare consensual points on the management of this disease is to treat severe cases with femoral extrusion surgically. In our institution, a similar study would never get the approval of our institutional review board.
To the best of our knowledge, this is the first study to assess the results of SA in LCP disease using CT scans. Our results are comparable to those reported by Zimmerman et al. [13] who performed CT scans to assess SA in children with cerebral palsy. Our good clinical and radiographic results were confirmed on 2D and 3D reconstruction images: graft resorption and proximal migration are uncommon; there is long-lasting femoral head coverage, joint congruency improves with growth. The shelf was found to laterally prolong the acetabular roof without being distinguished from it, thereby reflecting good graft incorporation.
In conclusion, lateral shelf acetabuloplasty is technically simple, safe, and effective in severe cases of LCP disease with major deformity and subluxation of the femoral head as well as joint incongruency and impingement. This procedure insures good and long-lasting femoral head coverage, allowing optimal remodeling of the hip joint. However, despite these documented advantages in LCP disease, lateral SA should be kept as a salvage procedure and reserved to well-selected cases, where other more “anatomical” procedures, such as redirection innominate osteotomies, are less efficacious.
References
- 1.Herring JA, Kim HT, Browne R. Legg-Calve-Perthes disease. Part II: Prospective multicenter study of the effect of treatment on outcome. J Bone Joint Surg Am. 2004;86:2121–2134. [PubMed] [Google Scholar]
- 2.Bursal A, Erkula G. Lateral shelf acetabuloplasty in the treatment of Legg-Calve-Perthes disease. J Pediatr Orthop B. 2004;13:150–152. doi: 10.1097/00009957-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell CS, Parisi MT. Pediatric acetabuloplasty procedures: radiologic evaluation. Am J Roentgenol. 1998;170:49–54. doi: 10.2214/ajr.170.1.9423598. [DOI] [PubMed] [Google Scholar]
- 4.Van Der Geest IC, Kooijman MA, Spruit M, et al. Shelf acetabuloplasty for Perthes’ disease: 12-year follow-up. Acta Orthop Belg. 2001;67:126–131. [PubMed] [Google Scholar]
- 5.Van Der Haven I, Kooijman MA, Havinga ME, et al. Teardrop-femoral head distance after shelf acetabuloplasty for Perthes’disease. Acta Orthop Belg. 2003;69:157–161. [PubMed] [Google Scholar]
- 6.Neyt JG. Stulberg classification system for evaluation of Legg-Calve-Perthes disease: intra-rater and inter-rater reliability. J Bone Joint Surg Am. 1999;81:1209–1216. doi: 10.2106/00004623-199909000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Daly K, Bruce C, Catterall A. Lateral shelf acetabuloplasty in Perthes’ disease. A review of the end of growth. J Bone Joint Surg Br. 1999;81:380–384. doi: 10.1302/0301-620X.81B3.9405. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs R, Moens P, Fabry G. Lateral shelf acetabuloplasty in the early stage of Legg-Calve-Perthes disease with special emphasis on the remaining growth of the acetabulum: a preliminary report. J Pediatr Orthop B. 2004;13:21–28. doi: 10.1097/00009957-200401000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Kruse RW, Guille JT, Bowen R. Shelf arthroplasty in patients who have Legg-Calve-Perthes disease. J Bone Joint Surg Am. 1991;73:1338–1347. [PubMed] [Google Scholar]
- 10.Kuwajima SS, Crawford AH, Ishida A, et al. Comparison between Salter’s innominate osteotomy and augmented acetabuloplasty in the treatment of patients with severe Legg-Calve-Perthes disease. Analysis of 90 hips with special reference to roentgenographic sphericity and coverage of the femoral head. J Pediatr Orthop B. 2002;11:15–28. doi: 10.1097/00009957-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Willet K, Hudson I, Catterall A. Lateral shelf acetabuloplasty: an operation for older children with Perthes’ disease. J Pediatr Orthop. 1992;12:563–568. doi: 10.1097/01241398-199209000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Villet L, Laville JM. Shelf acetabuloplasty in Legg-Perthes-Calve disease. Rev Chir Orthop. 2003;89:234–241. [PubMed] [Google Scholar]
- 13.Zimmermann SE, Sturm PF. Computed tomographic assessment of shelf acetabuloplasty. J Pediatr Orthop. 1992;12:581–585. doi: 10.1097/01241398-199209000-00003. [DOI] [PubMed] [Google Scholar]


