Abstract
BACKGROUND
Yes-associated protein (YAP), a downstream target of the Hippo signaling pathway, was recently linked to hepatocarcinogenesis in a mouse hepatocellular carcinoma (HCC) model. The objective of the current study was to investigate the clinical significance of YAP in HCC and its prognostic values in predicting survival and tumor recurrence.
METHODS
The authors collected 177 pairs of tumor and adjacent nontumor tissue from HCC patients with definitive clinicopathologic and follow-up data. YAP expression was determined by immunohistochemistry, Western blot analysis, and quantitative polymerase chain reaction. Association of YAP with each clinicopathologic feature was analyzed by Pearson chi-square test, and HCC-specific disease-free survival and overall survival by Kaplan-Meier curves and log-rank test. Multivariate Cox regression analyses of YAP in HCC were also performed.
RESULTS
YAP was expressed in the majority of HCC cases (approximately 62%) and mainly accumulated in the tumor nucleus. Overexpression of YAP in HCC was significantly associated with poorer tumor differentiation (Edmonson grade; P = .021) and high serum α-fetoprotein (AFP) level (P < .001). Kaplan-Meier and Cox regression data indicated that YAP was an independent predictor for HCC-specific disease-free survival (hazards ratio [HR], 1.653; 95% confidence interval [95% CI], 1.081-2.528 [P = .02]) and overall survival (HR, 2.148; 95% CI, 1.255-3.677 [P = .005]).
CONCLUSIONS
YAP is an independent prognostic marker for overall survival and disease-free survival times of HCC patients and clinicopathologically associated with tumor differentiation and serum AFP level. It is a potential therapeutic target for this aggressive malignancy.
Keywords: hepatocellular carcinoma, hippo signaling, prognostic marker, tumor recurrence, Yes-associated protein
Hepatocellular carcinoma (HCC) is among the most lethal and aggressive neoplasms.1 It is the fifth most frequent cancer worldwide and is the second leading cause of cancer death in China, in which hepatitis B virus (HBV), the most significant risk factor for HCC, is endemic.2,3 The 5-year survival rates for HCC patients are <10%,2,4,5 and >50% of patients develop disease recurrence within 3 years after curative surgery.6 The poor prognosis of HCC patients is largely attributed to the aggressive nature of this malignancy, characterized by multicentric development with a high metastatic potential.7,8 The pathogenesis of HCC is usually associated with liver damage caused by chronic hepatitis, heavy alcohol intake, or nonalcoholic steatohepatitis,3,9 and subsequent development of cirrhosis, dysplastic lesions, and eventually invasive carcinoma. These etiologic factors are believed to give rise to chronic inflammation and recurrent regeneration in parenchyma, thereby potentially activating the hepatic progenitor cells (HPCs) that lead to cellular proliferation and malignant transformation.10
By using an integrative oncogenomic approach, we previously identified a focal lesion of chromosome 9qA1 in a mouse model of liver cancer initiated from HPCs harboring defined cancer-predisposing lesions, syntenic to human chromosome 11q22, which contains the Yes-associated protein (YAP) and baculoviral inhibitor of apoptosis protein repeat-containing 2 (BIRC2) genes.11 Genetic profiling analysis revealed YAP as a novel candidate oncogene in this amplicon, and HPC transduced with the YAP gene demonstrated accelerated liver carcinoma formation when orthotropically engrafted in a mouse model.11 The tumorigenic properties of YAP were later demonstrated in breast cancer. Overexpression of YAP in MCF-10 cells induced epithelial-to-mesenchymal transition, suppression of apoptosis, and anchorage-independent growth in soft agar.12 Furthermore, a recent study demonstrated the oncogenic effects of YAP by overcoming cell contact inhibition and inducing cellular dedifferentiation during dysplastic progression.13
YAP is the mammalian ortholog of Drosophila Yorkie (Yki), which is a negatively regulated downstream target of the Hippo signaling pathway, and functions as a transcriptional coactivator involved in the regulation of cell growth, proliferation, and apoptosis.14 Having an essential role in cellular growth, knockout of the YAP gene in mice could lead to early embryonic lethality.15 The cytoplasmic sequestration and inactivation of Yki in Drosophila is regulated by phosphorylation at its primary Hippo-responsive phosphorylation site, serine residue S-168.16 Inactivation of the Hippo pathway results in nuclear accumulation of Yki, thereby transactivating an array of target genes responsible for cell survival and proliferation such as CycE, diap1/thread, and bantam. The Hippo (size-control) signaling pathway is well conserved from Drosophila to humans, and dysfunction of this pathway can lead to uncontrolled growth of organ size, as demonstrated in Drosophila17-20 and in mouse liver.16 Liver has the virtue of organ regeneration after injury or partial hepatectomy, and this process is tightly controlled, perhaps through the Hippo signaling and other related pathways. Recent observations have suggested that dysregulation of the Hippo/YAP signaling circuit could result in cancer cell proliferation and invasion. Despite these molecular insights, to our knowledge the clinical values of YAP in human HCC and other cancer types have not been explored yet. Thus, the purpose of this study is to investigate the expression of YAP and its associated clinical significance in liver malignancy and to determine whether it is an independent prognostic marker for predicting clinical outcomes.
MATERIALS AND METHODS
Patients and Clinical Samples
A retrospective cohort of 177 HCC patients who underwent hepatectomy at the Department of Surgery, Queen Mary Hospital (Pokfulam, Hong Kong) between 1997 and 2007, with a median follow-up time of 32 months, were included in this study. The demographic features and clinicopathologic data are summarized in Table 1. In brief, the median age of the patients was 54 years (range, 7-82 years), and the median tumor size was 5.8 cm (range, 1.5-28 cm). All patients were diagnosed with primary HCC, and none had received prior radiotherapy or chemotherapy before the surgery. The surgical specimens (both tumor and adjacent nontumor tissue; the latter was dissected by at least 1-cm distance from the tumor edge as described21) were processed immediately after the operation, and then subdivided into 3 major portions for histopathologic analysis, snap frozen in liquid nitrogen, or fixation in buffered paraformaldehyde.22 The study protocol was approved by the internal review board of the Joint Ethics Committee of the University of Hong Kong and the Queen Mary Hospital (Pokfulam, Hong Kong), and all HCC patients gave written informed consent on the use of clinical specimens for medical research.
Table 1.
Clinical Correlation of YAP Expression in HCC
| Clinicopathologic Features | Total No. of Patients, N=177 | No. of Patients | P | |
|---|---|---|---|---|
| YAP Positive | YAP Negative | |||
| Age (mean ± SD), y | 53.9 ± 12.2 | 52.8 ± 12.7 | 55.8 ± 11.2 | .111 |
| Sex | ||||
| Men | 143 | 90 | 53 | |
| Women | 34 | 20 | 14 | .657 |
| Hepatitis | ||||
| Negative | 62 | 41 | 21 | |
| HBV/HCV | 115 | 69 | 46 | .516 |
| No. of tumor nodules | ||||
| 1 | 136 | 86 | 50 | |
| ≥2 | 41 | 24 | 17 | .587 |
| Tumor size (mean±SD), cm* | 7.3 ± 4.6 | 6.9 ± 4.4 | 8.0 ± 4.8 | .142 |
| Serum AFP level, ng/mL | ||||
| ≤400 | 107 | 53 | 54 | |
| >400 | 70 | 57 | 13 | <.001† |
| Venous infiltration‡ | ||||
| Absent | 85 | 50 | 35 | |
| Present | 90 | 59 | 31 | .358 |
| Cellular differentiation§ | ||||
| Well differentiated (1-2) | 33 | 13 | 20 | |
| Moderately differentiated (3) | 89 | 57 | 32 | |
| Poorly differentiated (4) | 30 | 21 | 9 | .021† |
| Adjacent nontumor liver status | ||||
| Noncirrhotic | 18 | 8 | 10 | |
| Chronic hepatitis | 58 | 36 | 22 | |
| Cirrhotic | 101 | 66 | 35 | .242 |
| AJCC tumor stage | ||||
| I | 71 | 42 | 29 | |
| II | 54 | 34 | 20 | |
| III | 52 | 34 | 18 | .772 |
YAP indicates Yes-associated protein; HCC, hepatocellular carcinoma; SD, standard deviation; HBV, hepatitis B virus; HCV, hepatitis C virus; AFP, α-fetoprotein; AJCC, American Joint Commission on Cancer.
Tumor size was measured by the length of the largest tumor nodule.
Statistically significant.
Venous infiltration was defined based findings on final pathologic analysis (microscopic and major).
Scoring was based on Edmonson grade. Partial data are not available, and statistics were based on available data.
Immunohistochemistry
Paraffin-embedded tissue blocks were sectioned (4 μm) for immunohistochemical staining.23 After antigen retrieval and peroxidase blocking, the sections were incubated with rabbit polyclonal antibody against human YAP (H-125; Santa Cruz Biotechnology, Santa Cruz, Calif) (1:500 dilution) at 4°C overnight. After 3 washings in sterile phosphate-buffered saline, sections were incubated with a horseradish peroxidase (HRP)-conjugated antibody against rabbit or mouse immunoglobulin G (IgG) (Invitrogen, Carlsbad, Calif) (1 : 1000 dilution). Isotype-matched IgG control was used in each experiment. After color development using the DAB substrate kit (Invitrogen) and Mayer hematoxylin, the sections were examined and scored in a blinded fashion by a board-certified liver pathologist (I.O.L.N.) according to a semiquantitative scoring method as previously reported with some minor modifications.23 The extents of cytosolic and nuclear staining were considered in the scoring, and the percentage of immunoreactivity in tumor cells or hepatocytes was graded as: 0 (<10%); 1 (10%-30%); 2 (30%-50%); 3 (>50%).
TaqMan Real-Time Quantitative Polymerase Chain Reaction
Total RNA was extracted from cell lines and frozen tissue using the TRIzol reagent (Invitrogen) according to the manufacturer's instructions. An aliquot (500 ng) of purified RNA from each sample was reverse transcribed to cDNA using TaqMan reverse transcription reagents (Applied Biosystems Ltd, Foster City, Calif), followed by real-time quantitative polymerase chain reaction (qPCR) in an ABI PRISM 7900 sequence detection system (Applied Biosystems Ltd).24,25 TaqMan probe (Hs00371735_m1; Applied Biosystems) for human YAP mRNA was used for the gene expression assay. Ribosomal 18S RNA (4319413E, Applied Biosystems) was used as an internal control for normalization.
Western Blot Analysis
Cultured cells or frozen tissue samples were lysed in the RIPA buffer (50 mM Tris/HCl [pH 7.5], 1 mM ethylenediamine tetraacetic acid, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1/% sodium dodecyl sulfate [SDS]).26 Protein lysates were obtained after ultra-centrifugation, and protein concentration was measured by NanoDrop (Thermo Scientific, Waltham, Mass). For SDS–polyacrylamide gel electrophoresis, 20 μg of protein was electrophoresed in a 10% SDS polyacrylamide gel, and then electrotransferred to polyvinylidene difluoride membrane (0.4 μm; Millipore, Billerica, Mass) using a semidry blotting system (Bio-Rad Laboratories, Hercules, Calif).27 After blocking, the membrane was probed with rabbit polyclonal antibody against human YAP (H-125, Santa Cruz Biotechnology) at 1:1000 dilution and with mouse monoclonal antibody against human beta-actin (Sigma Chemical Company, Saint Louis, Mo) at 1 : 5000 dilution. HRP-conjugated antibodies against rabbit, goat, or mouse IgG (Invitrogen) (1:10,000 dilution) were used as the secondary antibodies. Immunoreactivity signals were amplified using the ECL detection reagents (GE Healthcare Bioscience, Hong Kong). The amount of YAP protein expression was measured semiquantitatively using the Image J software version 1.38 (National Institutes of Health, Bethesda, Md) by analyzing the peak area of the YAP protein band relative to the beta-actin within the same sample on the same membrane.
Statistical Analysis
The SPSS statistical package for Window version 13 (SPSS, Chicago, Ill) was used for data analysis. YAP expression levels in tumor tissue and matched adjacent nontumor tissue were compared using the Wilcoxon signed rank test on the presence or absence of YAP expression. YAP protein levels and mRNA transcriptional levels in the tumor and nontumor tissue pairs were compared by the Student t test for paired data. Clinicopathologic features in YAP-positive patients and YAP-negative patients, or nucleus-positive and nucleus-negative patients, were compared using the Pearson chi-square tests for categoric variables, and the Student t test for continuous data. Kaplan-Meier plots and log-rank tests were used for survival analysis. Disease-free survival (DFS) times were calculated from the date of curative surgery to HCC recurrence, death, or the last follow-up date; overall survival time was calculated from the date of surgery to death or last follow-up date. Cox regression was used in the univariate survival analysis to determine the association of individual clinicopathologic variables with DFS or overall survival. All variables with P < .1 in addition to age and gender and the identified prognostic factors for this cohort (ie, tumor size, venous infiltration, tumor stage [American Joint Committee on Cancer (AJCC)], and α-fetoprotein [AFP] >400 ng/mL),28-30 were subsequently subjected to the multivariate Cox regression analysis to determine the hazards ratios (HRs) and the independence of effects. Because of the exploratory nature of the study, all P values were not adjusted for multiple comparisons.
RESULTS
Clinical Significance of YAP Overexpression in HCC Specimens
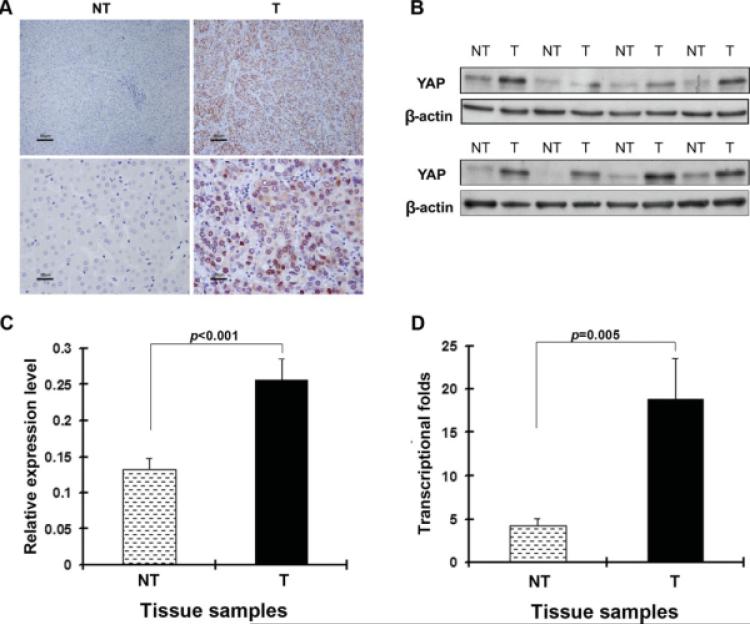
To determine the prevalence and clinical significance of YAP in liver cancer, we determined the expression of YAP protein by immunohistochemistry in a retrospective cohort of 177 pairs of tumor and matched adjacent nontumor tissue samples from HCC patients after liver resection. YAP immunoreactivity was graded as negative (score 0) and positive (scores 1 to 3) according to the previously reported procedures and summarized in Table 2. Expression level of YAP was significantly elevated in tumor tissue samples compared with the nontumor counterparts (P < .0001). In the 177 cases examined, YAP expression was detected in 110 (62.1%) HCC specimens, whereas only 16 (9.0%) of the nontumor specimens yielded positive YAP signal. As shown in Figure 1A, YAP was predominantly present in the nuclei of tumor cells, and was also present to a lesser extent in the cytoplasm. In contrast, YAP staining was seldom detected in corresponding nontumor tissue, and generally not observed in the nuclei of nontumor cells. The immunostaining data were further confirmed by Western blot analysis (Figs. 1B and C) and qPCR (Fig. 1D) assays. The protein and mRNA level of YAP were approximately 2 to 4 times higher in tumors than in matched nontumor tissue. Next, clinical association analysis by the Pearson chi-square test revealed that YAP expression in HCC tumors was significantly associated with poor cellular differentiation (Edmonson grade; P = .021) and high serum AFP levels (>400 ng/mL; P < .001) (Table 1).
Table 2.
IHC of Tumor and Nontumor Tissue of 177 HCC Patients
| No. of Sections | IHC Scores of Tumor Tissue (No. of Sections) | Total | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||
| IHC scores of nontumor tissue (No. of sections) | 0 | 62 | 47 | 25 | 27 | 161 |
| 1 | 4 | 1 | 6 | 4 | 15 | |
| 2 | 1 | 0 | 0 | 0 | 1 | |
| Total | 67 | 48 | 31 | 31 | 177 | |
IHC indicates immunohistochemistry; HCC, hepatocellular carcinoma.
FIGURE 1.
Overexpression of Yes-associated protein (YAP) in clinical samples of hepatocellular carcinoma (HCC) is shown. (A) Immunohistochemical staining of anti-YAP antibody in paired tumor (T) and adjacent nontumor (NT) tissue is shown. A total of 177 tumor/nontumor tissue pairs were tested, and representative photomicrographs are shown. Strong YAP immunoreactivity was found in both the cytoplasm and nucleus in tumor tissue, but not in the corresponding nontumor tissue (upper panels: scale bar, 80 μm; lower panels: scale bar, 20 μm). (B) Representative Western blot analysis of YAP expression in tumors and self-paired adjacent nontumor tissue is shown. (C) Semiquantitative Western blot analysis of lysates of 44 paired tissue samples is shown. β-Actin was used as an internal control. The means (n = 44) ± the standard error of the mean mean (SEM) are shown, and P values are given. The protein expression level was significantly increased in tumors (0.26) compared with nontumor tissue (0.13; P < .001). (D) TaqMan real-time polymerase chain reaction assay of YAP in HCC is shown. 18S rRNA levels were used as internal controls. The transcriptional fold change was calculated using liver samples from 3 healthy donors as a reference. The means (n = 20) ± SEM are shown, and P values are given. The mean fold change was 18.89 in tumors and 4.25 in nontumor tissue (P = .005).
YAP Expression Associated With Short Overall Survival
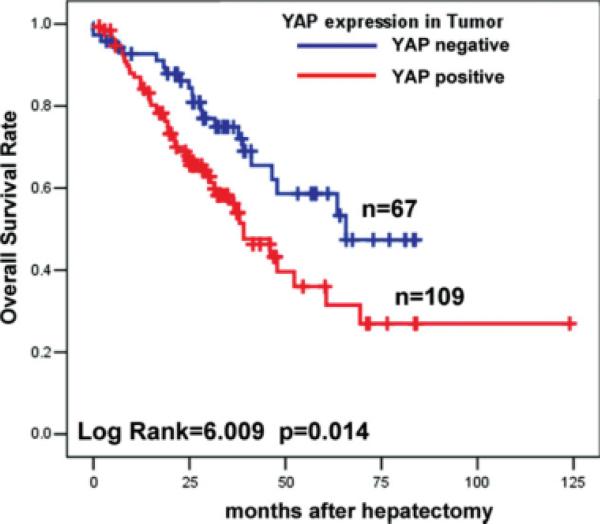
To determine the prognostic significance of YAP expression for HCC patients, we attempted to relate the YAP signal to the clinical outcomes. For overall survival analysis, 176 HCC patients with sufficient and valid follow-up data were included, and 1 subject without follow-up data was excluded. As shown in Figure 2, YAP overexpression (grades 1-3) in HCC was significantly associated with short overall survival time (log-rank = 6.009; P = .014); the 5-year survival rate decreased from 58% in YAP-negative HCC to 36% in patients with YAP-positive tumors. Multivariate Cox regression indicated that tumor size (P = .056) and tumor stage (AJCC stage III; P = .07) were marginally associated with overall survival; moreover, in addition to age (P = .032), YAP expression was found to be an independent prognostic marker for the overall survival of HCC patients (HR, 2.148; 95% confidence interval [95% CI], 1.255-3.677 [P = .005]) (Table 3).
FIGURE 2.
Yes-associated protein (YAP) expression in hepatocellular carcinoma was associated with poor overall survival in 176 patients based on YAP immunoreactivity by immunohistochemistry.
Table 3.
Cox Regression Analysis of Overall Survival (n = 174)*
| Univariate Analysis,†P | Multivariate Analysis | |||
|---|---|---|---|---|
| P | Hazards Ratio | 95% CI | ||
| Age | .064 | .032‡ | 1.023 | 1.002-1.044 |
| Sex | .931 | .928 | 1.030 | 0.542-1.959 |
| Tumor size | .007 | .056 | 1.054 | 0.999-1.112 |
| Venous infiltration | .003 | .660 | 1.191 | 0.547-2.593 |
| AJCC tumor stage | ||||
| I | 1 | |||
| II | .020 | .267 | 1.701 | 0.666-4.342 |
| III | <.001 | .070 | 2.230 | 0.937-5.308 |
| Serum AFP level | .157 | .532 | 1.174 | 0.709-1.943 |
| YAP expression in tumor | .014 | .005‡ | 2.148 | 1.255-3.677 |
95% CI indicates 95% confidence interval; AJCC, American Joint Committee on Cancer; AFP, α-fetoprotein; YAP, Yes-associated protein.
Venous infiltration data were not determined in 2 patients.
Univariate analysis was performed using Cox regression.
Statistically significant.
Association of YAP With Tumor Recurrence and Disease-Free Survival
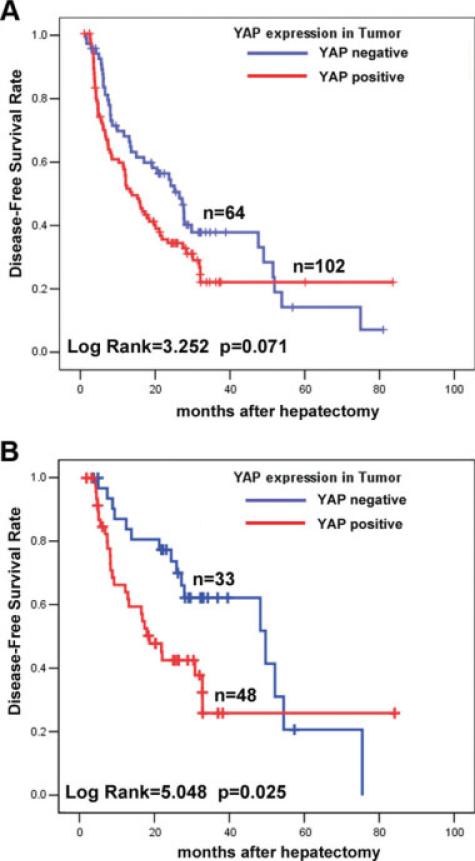
Then we examined whether YAP expression was associated with tumor recurrence and DFS of HCC. Of the 166 patients who received curative surgery for HCC and with sufficient follow-up data, the median DFS times in the YAP-negative (n = 64) and YAP-positive (n = 102) subgroups of HCC patients were 27.1 months (95% CI, 19.5 months-34.7 months) and 14.5 months (95% CI, 10.8 months-18.2 months), respectively. Kaplan-Meier analysis also revealed the association of YAP expression with short DFS times (log-rank = 3.252; P = .071) (Fig. 3A). By multivariate Cox regression analysis, YAP was found to be an independent indicator of DFS time (HR, 1.653; 95% CI, 1.081-2.528 [P = .02]) (Table 4), in addition to the number of tumor nodules (P = .019), tumor size (P = .004), and tumor venous infiltration (P = .041). Thus, our data indicated that YAP expression in HCC was indicative of a potential early disease recurrence.
FIGURE 3.
Kaplan-Meier disease-free survival analyses of hepatocellular carcinoma (HCC) patients based on Yes-associated protein (YAP) immunoreactivity by immunohistochemistry are shown. (A) YAP expression in tumors was found to be associated with poor disease-free survival (DFS) in 166 patients who underwent curative surgery. (B) YAP expression in tumors was found to be associated with poor DFS in the subgroup of HCC patients (n = 81) without histopathologic evidence of tumor venous infiltration.
Table 4.
Cox Regression Analysis of HCC-Specific Postoperative Disease-Free Survival
| All Patients (n=164)* | Patients Without Venous Infiltration (n=81), Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Univariate Analysis,†P | Multivariate Analysis | ||||||
| P | Hazard Ratio | 95% CI | P | Hazards Ratio | 95% CI | ||
| Age | .421 | .099 | 1.015 | 0.997-1.034 | .003‡ | 1.055 | 1.019-1.092 |
| Sex | .832 | .236 | 0.728 | 0.431-1.230 | .123 | 0.492 | 0.200-1.211 |
| Hepatitis | .007 | .051 | 0.671 | 0.450-1.001 | .865 | 1.067 | 0.504-2.260 |
| No. of tumor nodules | .009 | .019‡ | 2.040 | 1.126-3.697 | .940 | 1.064 | 0.209-5.421 |
| Tumor size | .001 | .004‡ | 1.070 | 1.021-1.120 | .015‡ | 1.104 | 1.019-1.196 |
| Venous infiltration | <.001 | .041‡ | 2.049 | 1.029-4.080 | NA | NA | NA |
| AJCC tumor stage | |||||||
| I | 1 | 1 | |||||
| II | .011 | .505 | 0.762 | 0.343-1.694 | .366 | 2.332 | 0.372-14.636 |
| III | <.001 | .553 | 0.773 | 0.328-1.820 | .998 | 1.002 | 0.204-4.908 |
| Serum AFP level | .046 | .474 | 1.163 | 0.769-1.759 | .974 | 1.013 | 0.460-2.234 |
| YAP expression in tumor | .073 | .020‡ | 1.653 | 1.081-2.528 | .001‡ | 3.547 | 1.644-7.653 |
HCC indicates hepatocellular carcinoma; 95% CI, 95% confidence interval; NA, not applicable; AJCC, American Joint Committee on Cancer; AFP, α-fetoprotein; YAP, Yes-associated protein.
Venous infiltration data were not determined in 2 patients.
Univariate analysis was performed using Cox regression.
Statistically significant.
We next investigated whether YAP could be indicative of tumor recurrence in HCC patients without histopathologic evidence of tumor vascular invasion, which is a well-documented prognostic indicator for HCC recurrence and short DFS times.31 In the present cohort, the median DFS times of HCC patients with and without venous infiltration were 12.7 months (n = 83; 95% CI, 7.8 months-17.6 months) and 30.7 months (n = 81; 95% CI, 23.2 months-38.1 months), respectively.
To examine the clinical utility of YAP as a predictive DFS marker independent of tumor vascular invasion, we selected those 81 HCC patients without venous infiltration for further analysis. A significant difference was found in median DFS times between the 2 subgroups (log-rank value = 5.048; P = .025) (Fig. 3B): 49.5 months (95% CI, 22.0 months-76.9 months) in the YAP-negative subgroup (n = 33) and 18.6 months (95% CI, 13.0 months-24.2 months) in the YAP-positive subgroup (n = 48). In addition, multivariate Cox regression also showed that YAP was an independent predictor of short DFS time for HCC patients without venous infiltration (HR, 3.547; 95% CI, 1.644-7.653 [P = .001]) (Table 4) in addition to age (P = .003) and tumor size (P = .015).
DISCUSSION
To date, there is evidence linking YAP oncogene to tumorigenicity of several solid cancer types, including prostate, colon, and liver.13,32 YAP is the downstream target of the Hippo signaling pathway, and in cooperation with TEAD transcription factor, overexpression of YAP could aberrantly activate an array of target genes (eg, CTGF, CCND1, ITGB2, and BCL2L1) responsible for cell proliferation, antiapoptosis, survival, and migration.14,16,33 To further characterize the clinical presentation and implication of YAP in HCC, we examined its expression level in an HCC cohort in China, in which hepatitis B is endemic, with a high prevalence of liver malignancy. Both the YAP protein and mRNA transcription levels were significantly elevated in the majority of HCC tumor tissue when compared with adjacent nontumor tissue by 2-fold to 4-fold, respectively. Immunochemistry study revealed accumulation of YAP oncoprotein in tumor cell nuclei, which was barely observed in the adjacent parenchymal tissue. These findings were consistent with a previous report that 54% of 115 HCC patient showed overexpression of YAP in a tissue microarray study in which the majority of normal liver demonstrated very weak staining.13 A similar observation was also made in prostate cancer tissue. These data suggest that YAP was important for tumorigenesis in different solid cancer types.
Clinically, in our cohort of 177 patients, YAP expression in HCC tumors was significantly associated with poor differentiation of tumor cells (P = .021) and high serum AFP level (P < .001). Cellular dedifferentiation in HCC is regarded as a sign of tumor progression and increased malignant potential.34 In accord with our clinical findings, Camargo et al reported that YAP could induce loss of differentiation and expansion of multipotent undifferentiated progenitor cells in the small intestine.32 Conversely, a recent cDNA microarray study demonstrated that AFP was 1 of the YAP-induced gene targets in murine liver.16 AFP, which has been used as a conventional serum biomarker for liver malignancy, is a developmental gene product highly expressed in embryonic yolk sac and fetal liver, but its biosynthesis is greatly reduced in the adult liver. Our study clearly indicated that YAP expression in HCC tumors was significantly associated with high serum AFP level in the patients. Indeed, YAP could also transduce normal hepatocyte cell line MIHA to produce AFP protein in vitro (unpublished data), which has an important role in driving hepatic oncogenesis. Although statistically insignificant, there was a trend toward increasing YAP expression in the adjacent nontumor tissue from noncirrhotics to chronic hepatitis; this was even higher in cirrhosis (Table 1).
Because of the aggressive nature of HCC, hepatic surgery is the most effective curative therapy and provides better survival outcomes. Unfortunately, approximately 33% of HCC patients die within the first year even after curative surgery, mainly because of tumor recurrence and spread. The most commonly accepted survival indices are still based on tumor staging and histopathologic observation such as tumor size, number of nodules, and vascular invasion.6,7,35-37 However, we found that although patients have modest tumor presentation, the prediction for the patients’ overall survival and DFS can be variable and inaccurate. To identify a new cancer biomarker in addition to the common clinicopathological risk factors that would assist the follow-up management after surgery, we examined and characterized the clinical significance of YAP as an independent prognostic factor in determining the outcomes of HCC patients. Using multivariate Cox regression analyses, YAP expression in HCC tumors was shown to associate with a decreased overall survival rate and an increased likelihood of tumor recurrence. In line with the previous studies, age is an independent factor for the overall survival, whereas the number of tumor nodules, tumor size, and vascular invasion are associated with DFS rate.6,31,35,37,38 Venous infiltration is defined as local resident tumor infiltrating through all vessel structures including the endothelial layer, an indication of free tumor entry into blood circulation. Obviously, any sign of tumor venous infiltration is an important risk factor for early disease recurrence and poor DFS.27 However, there is a subgroup of HCC patients who develop early tumor recurrence despite the absence of any pathologic finding of venous infiltration in the tumor specimens. In the present cohort, there were patients without venous infiltration having short DFS of 4 months. Thus, we analyzed the HCC subgroup with no histological findings of tumor venous infiltration, and found that YAP expression in tumor tissue can be used to distinguish a set of patients with increased risk of poor DFS rate independent of venous infiltration. The median DFS time was 14.5 months (95% CI, 10.8 months-18.2 months) in total patients and 18.6 months (95% CI, 13.0 months-24.2 months) in the venous infiltration-negative subgroup, respectively. These data indicated most of the YAP-positive HCC patients would likely suffer from tumor recurrence within the first 2 years after curative therapies. Therefore, assessment of YAP expression level in the resected HCC tissue would provide a valuable indication for effective follow-up management, especially for those patients with modest tumor staging or histopathologic features.
Taken together, we believe that the current study provides the first clinical evidence that YAP is an independent prognostic marker for overall survival and DFS in HCC patients. Expression of YAP in tumors is strongly correlated with poor cellular differentiation, high serum AFP levels, and tumor recurrence independent of venous infiltration. These results, which are based on a Chinese cohort (all surgical patients and mostly associated with HBV infections) in Hong Kong, should be further confirmed in other populations of patients with HCC. Our findings suggest that YAP is highly associated with aggressive liver cancer, is a new biomarker for this disease, and is a potential therapeutic target.
Conflict of Interest Disclosures
Supported by Grants 772109M and 771607M from the Research Grants Council of Hong Kong, ITS120/07 from the Innovation and Technology Commission of the Hong Kong Government, and the Sun Chieh Yeh Research Foundation for Hepatobiliary and Pancreatic Surgery. IOL Ng is Loke Yew Professor in Pathology, and SW Lowe is Howard Hughes Medical Institute Investigator.
We thank Ashley Wong, Kit Mak, Banny Lam, and W. Y. Yu of the Hepatobiliary and Pancreatic Surgery team for excellent technical assistance. We also thank Dr. Arieh Bomzon (Cambridge, United Kingdom) for critical reading of the article and Professor S. T. Fan (Queen Mary Hospital, Hong Kong) for providing insightful advice and comments on this study.
Footnotes
Published online June 23, 2009 in Wiley InterScience (www.interscience.wiley.com)
References
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across 5 continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 3.Yu MC, Yuan JM. Environmental factors and risk for hepatocellular carcinoma. Gastroenterology. 2004;127(5 suppl 1):S72–S78. doi: 10.1016/j.gastro.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002;35(5 suppl 2):S72–S781. doi: 10.1097/00004836-200211002-00002. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 6.Shah SA, Cleary SP, Wei AC, et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery. 2007;141:330–339. doi: 10.1016/j.surg.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 7.Adachi E, Maehara S, Tsujita E, et al. Clinicopathologic risk factors for recurrence after a curative hepatic resection for hepatocellular carcinoma. Surgery. 2002;131(1 suppl):S148–S152. doi: 10.1067/msy.2002.119496. [DOI] [PubMed] [Google Scholar]
- 8.Park JH, Koh KC, Choi MS, et al. Analysis of risk factors associated with early multinodular recurrences after hepatic resection for hepatocellular carcinoma. Am J Surg. 2006;192:29–33. doi: 10.1016/j.amjsurg.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43(2 suppl 1):S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 10.Alison MR, Lovell MJ. Liver cancer: the role of stem cells. Cell Prolif. 2005;38:407–421. doi: 10.1111/j.1365-2184.2005.00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zender L, Spector MS, Xue W, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–1267. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Overholtzer M, Zhang J, Smolen GA, et al. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci USA. 2006;103:12405–12410. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao B, Wei X, Li W, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao B, Ye X, Yu J, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morin-Kensicki EM, Boone BN, Howell M, et al. Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Mol Cell Biol. 2006;26:77–87. doi: 10.1128/MCB.26.1.77-87.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong J, Feldmann G, Huang J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buttitta LA, Edgar BA. How size is controlled: from Hippos to Yorkies. Nat Cell Biol. 2007;9:1225–1227. doi: 10.1038/ncb1107-1225. [DOI] [PubMed] [Google Scholar]
- 18.Cook M, Tyers M. Size control goes global. Curr Opin Biotechnol. 2007;18:341–350. doi: 10.1016/j.copbio.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Pan D. Hippo signaling in organ size control. Genes Dev. 2007;21:886–897. doi: 10.1101/gad.1536007. [DOI] [PubMed] [Google Scholar]
- 20.Yin F, Pan D. Fat flies expanded the hippo pathway: a matter of size control. Sci STKE. 2007;(380):pe12. doi: 10.1126/stke.3802007pe12. [DOI] [PubMed] [Google Scholar]
- 21.Poon RT, Fan ST, Ng IO, Wong J. Significance of resection margin in hepatectomy for hepatocellular carcinoma: a critical reappraisal. Ann Surg. 2000;231:544–551. doi: 10.1097/00000658-200004000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yi X, Luk JM, Lee NP, et al. Association of mortalin (HSPA9) with liver cancer metastasis and prediction for early tumor recurrence. Mol Cell Proteomics. 2008;7:315–325. doi: 10.1074/mcp.M700116-MCP200. [DOI] [PubMed] [Google Scholar]
- 23.Wong BW, Luk JM, Ng IO, Hu MY, Liu KD, Fan ST. Identification of liver-intestine cadherin in hepatocellular carcinoma—a potential disease marker. Biochem Biophys Res Commun. 2003;311:618–624. doi: 10.1016/j.bbrc.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 24.Poon RT, Lau CP, Cheung ST, Yu WC, Fan ST. Quantitative correlation of serum levels and tumor expression of vascular endothelial growth factor in patients with hepatocellular carcinoma. Cancer Res. 2003;63:3121–3126. [PubMed] [Google Scholar]
- 25.Wang XQ, Luk JM, Leung PP, Wong BW, Stanbridge EJ, Fan ST. Alternative mRNA splicing of liver intestine-cadherin in hepatocellular carcinoma. Clin Cancer Res. 2005;11(2 pt 1):483–489. [PubMed] [Google Scholar]
- 26.Wong WS, Luk JM. Signaling mechanisms of pertussis toxin-induced myelomonocytic cell adhesion: role of tyrosine phosphorylation. Biochem Biophys Res Commun. 1997;236:479–482. doi: 10.1006/bbrc.1997.6986. [DOI] [PubMed] [Google Scholar]
- 27.Luk JM, Lam CT, Siu AF, et al. Proteomic profiling of hepatocellular carcinoma in Chinese cohort reveals heat-shock proteins (Hsp27, Hsp70, GRP78) up-regulation and their associated prognostic values. Proteomics. 2006;6:1049–1057. doi: 10.1002/pmic.200500306. [DOI] [PubMed] [Google Scholar]
- 28.Poon RT, Fan ST, Lo CM, Liu CL, Ng IO, Wong J. Long-term prognosis after resection of hepatocellular carcinoma associated with hepatitis B-related cirrhosis. J Clin Oncol. 2000;18:1094–1101. doi: 10.1200/JCO.2000.18.5.1094. [DOI] [PubMed] [Google Scholar]
- 29.Poon RT, Fan ST. Evaluation of the new AJCC/UICC staging system for hepatocellular carcinoma after hepatic resection in Chinese patients. Surg Oncol Clin N Am. 2003;12:35–50, viii. doi: 10.1016/s1055-3207(02)00086-8. [DOI] [PubMed] [Google Scholar]
- 30.Pang RW, Joh JW, Johnson PJ, Monden M, Pawlik TM, Poon RT. Biology of hepatocellular carcinoma. Ann Surg Oncol. 2008;15:962–971. doi: 10.1245/s10434-007-9730-z. [DOI] [PubMed] [Google Scholar]
- 31.Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–207. doi: 10.1016/s0168-8278(02)00360-4. [DOI] [PubMed] [Google Scholar]
- 32.Camargo FD, Gokhale S, Johnnidis JB, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 33.Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem. 2008;283:5496–5509. doi: 10.1074/jbc.M709037200. [DOI] [PubMed] [Google Scholar]
- 34.Kojiro M, Nakashima O. Histopathologic evaluation of hepatocellular carcinoma with special reference to small early stage tumors. Semin Liver Dis. 1999;19:287–296. doi: 10.1055/s-2007-1007118. [DOI] [PubMed] [Google Scholar]
- 35.Benzoni E, Lorenzin D, Favero A, et al. Liver resection for hepatocellular carcinoma: a multivariate analysis of factors associated with improved prognosis. The role of clinical, pathological and surgical related factors. Tumori. 2007;93:264–268. doi: 10.1177/030089160709300306. [DOI] [PubMed] [Google Scholar]
- 36.Chedid A, Ryan LM, Dayal Y, Wolf BC, Falkson G. Morphology and other prognostic factors of hepatocellular carcinoma. Arch Pathol Lab Med. 1999;123:524–528. doi: 10.5858/1999-123-0524-MAOPFO. [DOI] [PubMed] [Google Scholar]
- 37.Kuriyama H, Okada S, Okusaka T, Ueno H, Ikeda M. Prognostic factors in patients with small hepatocellular carcinoma treated by percutaneous ethanol injection. J Gastroenterol Hepatol. 2002;17:1205–1210. doi: 10.1046/j.1440-1746.2002.02807.x. [DOI] [PubMed] [Google Scholar]
- 38.Kee KM, Wang JH, Lee CM, et al. Validation of clinical AJCC/UICC TNM staging system for hepatocellular carcinoma: analysis of 5,613 cases from a medical center in southern Taiwan. Int J Cancer. 2007;120:2650–2655. doi: 10.1002/ijc.22616. [DOI] [PubMed] [Google Scholar]