Abstract
An encapsulated chloride in a thiophene-based cryptand is bridged with the two in-in protons of tertiary amines with a N…Cl− distance of 3.048(3) Å, similar to that observed in the chloride complex of Park and Simmons’ katapinand.
Nearly forty years ago, anion coordination chemistry emerged with the discovery by Park and Simmons of diazabicyclic compounds, known as katapinands.1 These compounds were shown to form inclusion complexes with halide anions by hydrogen bonding interactions in acidic solution. Time-dependent NMR studies suggested that the protons on the nitrogen atoms of the ligand H2[L1]2+ changed their direction from out-out to in-in conformation in the presence of chloride anion. Seven years later of this discovery, Bell et al. isolated crystals of L1 in the presence of hydrochloric acid in water, thereby confirming by X-ray structure analysis that one chloride was encapsulated in the cavity.2 The anion was located along the axis of two apical NH groups with N+…Cl− distance of 3.10(1) Å, where the two protons are oriented with in-in conformation.
During the period of last thirty years, substantial progress has been made on anion binding with polyammonium-based receptors,3 particularly with the Schiff based derived4 biclyclic cryptands containing rigid spacers, e.g., m-xylyl5 and p-xylyl,6 furan7 and pyridine8 in both solution and solid states. This type of ligands provides a well defined cavity which is often suitable for hosting a spherical halide anion. For example, Bowman-James et al. reported that an hexaprotonated azacryptand containing m-xylyl was capable to encapsulate one fluoride and one water molecule in its cavity.5c In a recent work of Ghosh et al., this ligand was shown to form a monotopic complex with iodide, and a ditopic complex with chloride.5d The p-xylyl analogue with a larger cavity was found to form a tritopic (cascade) complex with two fluorides bridging with a water molecule,6a but it was shown to complex chloride and bromide with both ditopic6b and monotopic6c binding modes in the solid phase. The internal anionic guest described above, however, binds to the secondary amino groups in the linking arms of macrocycles. To the best of our knowledge, there has been no structural report that an encapsulated anion is bridged with only protonated bridgehead nitrogens in the presence of secondary amines, unless the protonation ability of the latter sites is blocked by a protecting group, e.g., tosyl9 or imidazole group.10 Herein we report the structural evidence of an encapsulated chloride that is coordinated with only two in-in protons on bridgehead nitrogens in an unprotected octaazacryptand, showing a similar in-in conformation observed in the chloride complex of Park and Simmons’ katapinand.
The cryptand L was first synthesized by Nelson as dicopper and disilver complexes11 and later by Fabbrizzi as dinuclear copper complex.12 To our knowledge, the free ligand has not been previously investigated for anion binding. In an effort to examine the influence of linking spacers on anion binding, we prepared the ligand L13 from high dilution condensation of tren and 2,5-thiophenedicarboxaldehyde followed by reduction using the method that was employed to synthesize other cryptands.4 The chloride complex was obtained by dissolving L (20 mg) in methanol (2 mL) and adding 6N HCl (100 µL). The white precipitate which formed was redissolved in methanol with a few drops of water. Crystals suitable for X-ray analysis were obtained from slow diffusion of methanol.
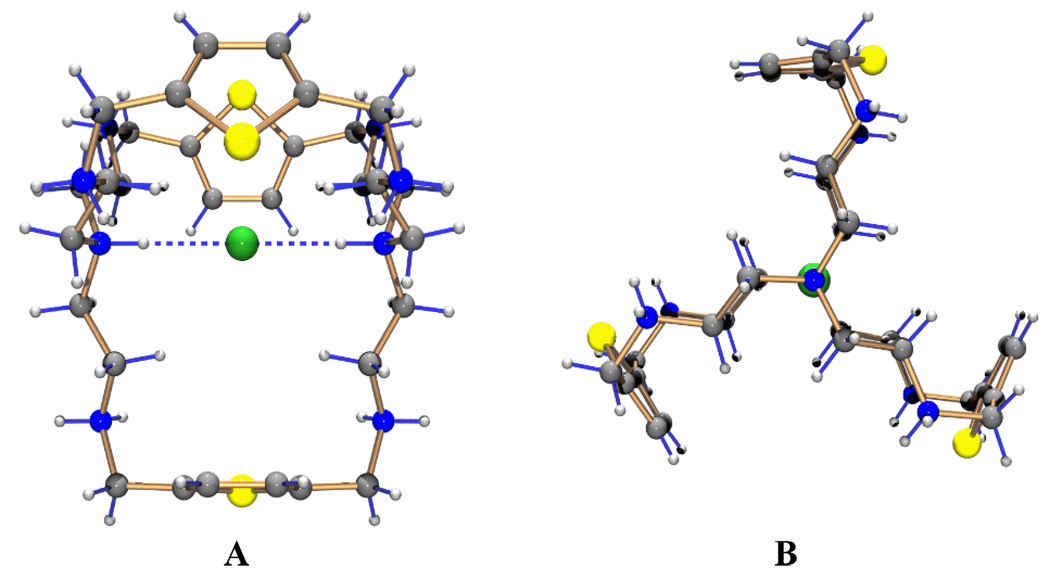
Single crystal analysis reveals that the crystal is hexagonal, and the molecule has its full C3h symmetry in the crystal.‡ In the complex, all eight nitrogen atoms are protonated and the charges are balanced by eight chloride anions. Fig. 1 shows the perspective views looking into the cavity (A) and down the three-fold axis (B). The macrocyclic moiety contains a 3-fold rotation axis and exhibits a remarkable anion chelating geometry in which the protons on the terminal nitrogen atoms are pointed toward the cavity center with in-in conformation. As shown in the crystal views, one chloride is linearly coordinated to both axial NH groups, and located at the midpoint of the two bridgehead nitrogen atoms with N…Cl− distances of 3.048(3) Å, half the N…N distance, 6.096(4) Å. The N…Cl− distance is much shorter than the sum of the Van der Waals radii of nitrogen and chloride atoms, 3.30 Å14 indicating strong hydrogen bonding interactions.
Fig. 1.
Perspective views of [H8L(Cl)]7+: (A) Side view. (B) 3-fold axis view (seven external chloride anions and water molecules of crystallization are omitted for clarity).
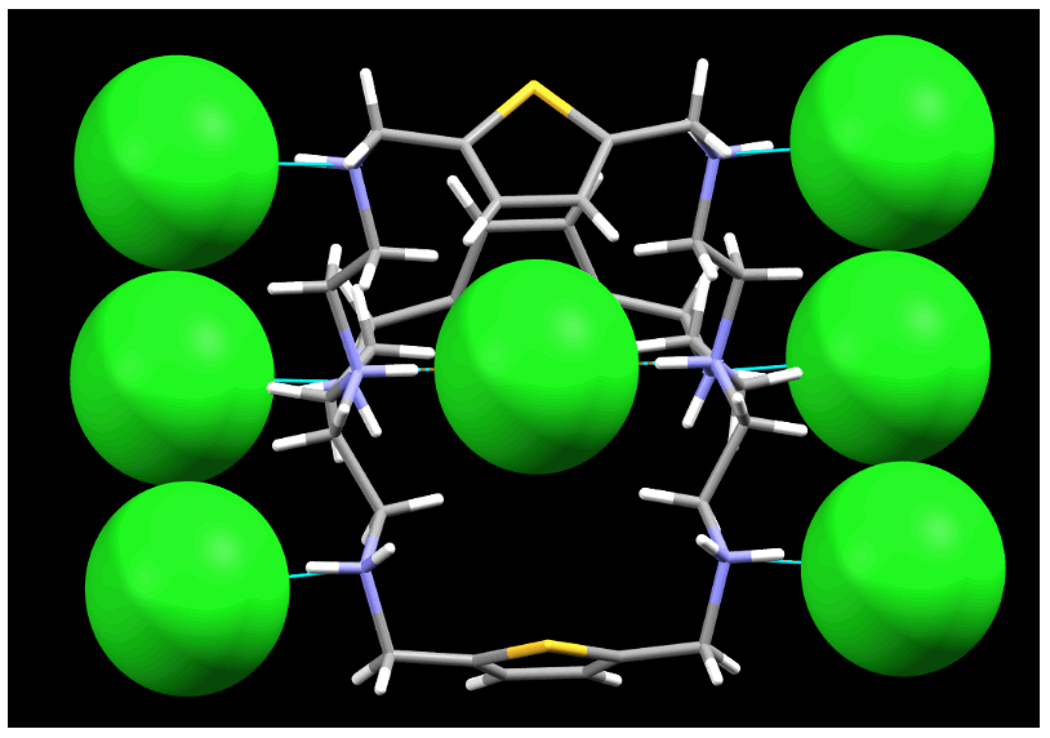
Although the encapsulated chloride in [H8L]8+ is encrypted by a highly positively charged cage, surprisingly the coordination pattern is similar to that observed in the katapinand L1, where the chloride was bound at the center of the two axial nitrogens with N…N = 6.2 Å as compared with N…N = 6.096(4) Å in [H8L(Cl)]7+. The encapsulated chloride in [H8L]8+ does not form a hydrogen bond with the secondary amine protons, and is equidistant (4.886(3) Å) from the six nitrogen atoms. Through the hydrogen bonding interactions with axial protons, the internal chloride strongly pulls the terminal nitrogen groups towards the cavity center. As a result, all the secondary nitrogen groups move away from the central anion. However, six out of seven extracavity chlorides are singly coordinated with the secondary nitrogen atoms with N…Cl distance of 3.0425(17) Å (Fig. 2). The remaining chloride does not interact with the macrocycle and is located at 5.551 Å from the nearest nitrogen.
Fig. 2.
Space filling views of the coordinated internal and external chlorides in [H8L(Cl)](Cl)6+ motif. The cryptand is shown in capped sticks.
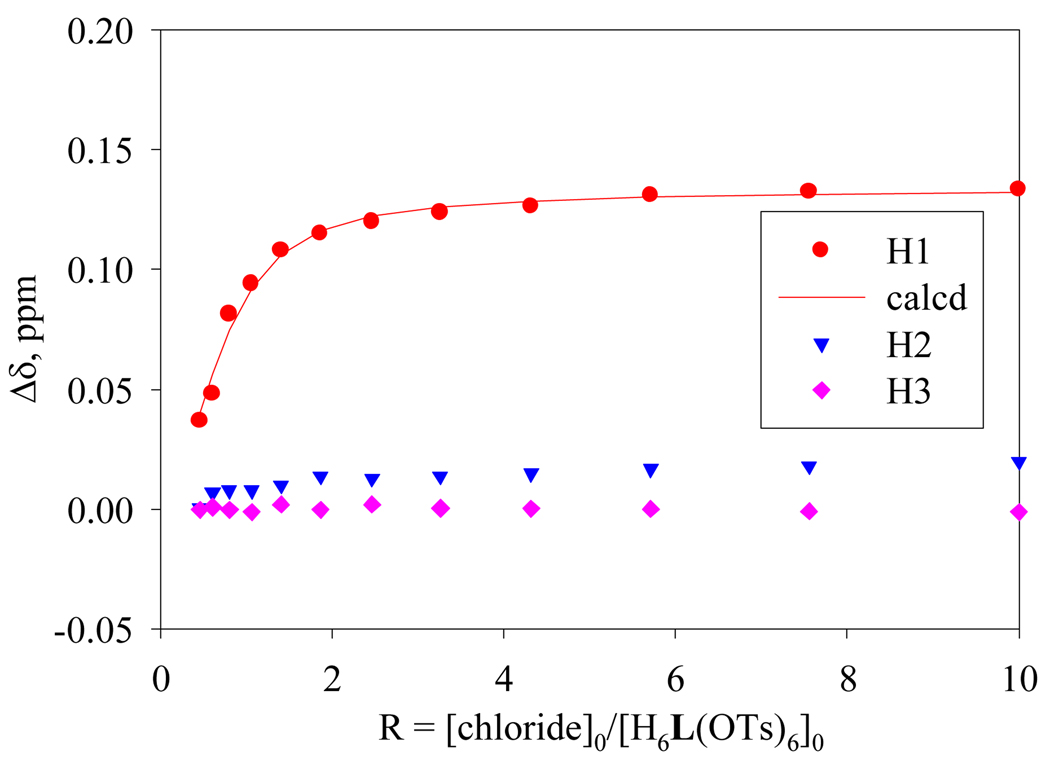
The structural results were confirmed by 1H NMR studies in D2O at pH 2, in which hexatosylated salt of L15 (2 mM) was titrated with an increasing amount of NaCl solution (20 mM), providing the best fit for a 1:1 binding model (Fig. 3), yielding a log K 3.60.16 The 1:1 stoichiometry of the complex was further confirmed by the Job plot, showing a maximum at the 0.5 mole fraction of L in D2O at pH 2 (see supplementary information). The addition of chloride anion to the ligand solution at pH 4.5, however, did not result in an appreciable change in proton signals. While measured at pH 2, the methylene protons (3.17 ppm) adjacent to tertiary amines were shifted downfield by 0.10 ppm in the presence of one equivalent of anion. Very little change was observed in the chemical shift of other protons under the same conditions. These results suggest that the binding occurs when the tertiary amines are protonated, as expected at the low pH. A similar binding pattern was reported previously for a tiny cryptand in chloride binding at pH 2.5.17 The solution studies thus indicate a direct bond between the NH of the tertiary amines and the encapsulated species, as seen in the X-ray structure. The calculated binding constant in L is comparable to that observed in the bigger p-xylyl analogue with a log K of 3.37, which was measured at pH 5.6b
Fig. 3.
1H NMR titration curves for chloride binding with H6L(OTs)6 in D2O at pH = 2. Net changes in the chemical shifts of NCH2 (H1), NCH2CH2 (H2) and ArCH2 (H3) are shown against the anion concentration.
In conclusion, by X-ray crystallography we have shown that an octaprotonated ligand L encapsulates a single chloride in its cavity though hydrogen bonding interactions with two NH protons on tertiary amines, adopting an ‘in-in’ conformation that was seen in the first synthetic anion receptors ‘katapinands’ in the chloride binding. In the chloride complex of H8L(Cl)]7+, the encapsulated chloride anion is linearly coordinated with the two bridgehead protons, not to the protons on secondary amines. The binding pattern of this ligand differs from its analogues, e.g. m-xylyl cryptand in nitrate5a,b or fluoride binding,5c p-xylyl cryptand in halide binding6b,c and furan cryptand in perchlorate binding,7 in which the secondary NH donor groups are involved in binding process. But in the present structure, the protons on the secondary amines point outside the cavity and are involved in coordinating external chlorides. We are currently investigating the binding mechanism, and selectivity aspects of this and related ligands which will be reported elsewhere.
Supplementary Material
Acknowledgments
The project described was supported by Grant Number G12RR013459 from the National Center for Research Resources. This material is based upon work supported by the National Science Foundation under CHE-0821357. Purchase of the diffractometer was made possible by grant No. LEQSF (1999–2000)-ENH-TR-13, administered by the Louisiana Board of Regents.
Footnotes
Electronic Supplementary Information (ESI) available: 1H NMR titration spectra and details of the Job plot in pdf format. See DOI: 10.1039/b000000x/
Crystal data: For [H8L(Cl)]Cl7·2.5H2O: C30H61N8S3Cl8O2.5, M = 953.65, crystal size 0.30 × 0.25 × 0.20 mm3, hexagonal, P63/m, a = 12.7445 (10), c = 19.393 (2) Å, V = 2727.9 (4) Å3, Z = 2, dcalc = 1.161 g cm−3, T = 100.0 (5) K, Nonius KappaCCD diffractmeter, µ(Mο−K α) = 0 . 5 6 mm−1, 2241 independent reflections (1864 observed), 77 parameters, Rint = 0.028, R[F2>2σ(F2)] = 0.039, wR(F2) = 0.100. CCDC 710863.
Notes and references
- 1.Park CH, Simmons HE. J. Am. Chem. Soc. 1968;90:2431. [Google Scholar]
- 2.Bell RA, Christoph GG, Fronczek FR, Marsh RE. Science. 1975;190:151. [Google Scholar]
- 3.(a) Bianchi A, García-España E, Bowman-James K. Supramolecular Chemistry of Anions. New York: Wiley-VCH; 1997. [Google Scholar]; (b) Beer PD, Wheeler JW, Moore C. In: Supramolecular Chemistry. Balzani V, De Cola L, editors. Dordrecht: Kluwer Academic Publishers; 1992. p. 105. [Google Scholar]; (c) Katz HE. In: Inclusion Compounds. Atwood JL, Davies JED, MacNichol DD, editors. Oxford: Oxford University Press; 1991. p. 391. [Google Scholar]; (d) Seel C, Galan A, deMendoza J. Top. Curr. Chem. 1995;175:101. [Google Scholar]; (e) Gale PA, editor. Coord. Chem. Rev. Vol. 240 2003. 35 years of Synthetic Anion Receptor Chemistry. [Google Scholar]; (f) Llinares JM, Powell D, Bowman-James K. Coord. Chem. Rev. 2003;240:57. [Google Scholar]; (g) García-España E, Díaz P, Llinares JM, Bianchi A. Coord. Chem. Rev. 2006;250:2952. [Google Scholar]; (h) Illioudis CA, Steed JW. Organ. Biomol. Chem. 2005;3:2935. doi: 10.1039/b506828b. [DOI] [PubMed] [Google Scholar]; (i) Gale PA. Acc. Chem. Res. 2006;39:465. doi: 10.1021/ar040237q. [DOI] [PubMed] [Google Scholar]; (j) Sessler L, Gale PA, Cho WS. In: Anion Receptor Chemistry, (Monographs in Supramolecular Chemistry) Stoddart JF, editor. Cambridge: Royal Society of Chemistry; 2006. [Google Scholar]; (k) Hossain MA. Curr. Org. Chem. 2008;12:1231. [Google Scholar]
- 4.Chen D, Martell AE. Tetrahedron. 1991;47:6900. [Google Scholar]
- 5.(a) Mason S, Seib L, Bowman-James K. J. Am. Chem. Soc. 1998;120:8899. [Google Scholar]; (b) Hynes MJ, Maubert B, McKee V, Town RM, Nelson J. J. Dalton Trans. 2000;2853 [Google Scholar]; (c) Mason S, Llinares JM, Morton M, Clifford T, Bowman-James K. J. Am. Chem. Soc. 2000;122:1814. [Google Scholar]; (d) Ravikumar I, Lakshminarayanan PS, Suresh SE, Ghosh P. Inorg. Chem. 2008;47:7992. doi: 10.1021/ic702056y. [DOI] [PubMed] [Google Scholar]
- 6.(a) Hossain MA, Llinares JM, Mason S, Morehouse P, Powell D, Bowman-James K. Angew. Chem. Int. Ed. Engl. 2002;41:2335. doi: 10.1002/1521-3773(20020703)41:13<2335::AID-ANIE2335>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]; (b) Hossain MA, Morehouse P, Powell D, Bowman-James K. Inorg. Chem. 2005;44:2143. doi: 10.1021/ic048937e. [DOI] [PubMed] [Google Scholar]; (c) Lakshminarayanan PS, Kumar DK, Ghosh P. Inorg. Chem. 2005;44:7540. doi: 10.1021/ic051191f. [DOI] [PubMed] [Google Scholar]
- 7.Morgan G, Nelson J, McKee V. Chem. Commun. 1995:1649. [Google Scholar]
- 8.McKee V, Morgan GG. Acta Cryst. 2003;C59:150. [Google Scholar]
- 9.Morehouse P, Hossain MA, Llinares JM, Powell D, Bowman James K. Inorg. Chem. 2003;42:8131. doi: 10.1021/ic034972u. [DOI] [PubMed] [Google Scholar]
- 10.Zhang B, Cai P, Duan C, Miao R, Zhu L, Niitsu T, Inoue H. Chem. Commun. 2004:2206. doi: 10.1039/b407434c. [DOI] [PubMed] [Google Scholar]
- 11.Drew MGB, Harding CJ, Howarth OW, Lu Q, Marrs DJ, Morgan GG, McKee V, Nelson J. J. Chem. Soc., Dalton Trans. 1996:3021. [Google Scholar]
- 12.Amendola V, Fabbrizzi L, Mangano C, Pallavicini P, Zema M. Inorg. Chim. Acta. 2002;337:70. [Google Scholar]
- 13.The ligand L was prepared from high dilution condensation of tren and 2,5-thiophenedicarboxaldehyde followed by diborane reduction using the literature method4. A solution of tris(2-aminoethyl)amine (1.00 g, 6.84 × 10−3 mol) in 200 mL of CH3OH and 2,5-thiophenedicarboxaldehyde (1.44 g, 10.26 × 10−3 mol) in 200 mL of CH3OH were added dropwise to 400 mL of CH3OH over 3 h. The resulting mixture was stirred at room temperature for 24 h and the solvent was evaporated under reduced pressure. The oily Schiff base product was redissolved in 100 mL of CH3OH and NaBH4 (1.73 g, 45.7 mmol) was added. After stirring at room temperature for 24 h, the solvent was removed in vacuo. The resulting yellowish residue was dissolved in 1 M a q N a O H solution (100 mL), and the aqueous phase was extracted by CH2Cl2 (3×50 mL). The combined organic layers were dried (MgSO4) and concentrated to give a light yellowish oil. The crude product was purified by column chromatography (neutral alumina, 2% CH3OH in CH2Cl2). Yield: 0.85 g, 40%. FAB-MS: m/z 617 [HL]+. 1H NMR (300 MHz, CDCl3, TMS): 2.61 (t, 12H, NCH2), 2.72 (t, 12H, NCH2CH2), 3.85 (s, 12H, ArCH2), 6.62 (d, 6H, ArH). Anal. Calcd. for (C30H45N8S3): C, 58.40; H, 7.84; N, 18.16. Found: C, 58.53; H, 7.97; N, 18.61.
- 14.Alcock N. Bonding and Structure. West Sussex, England: Ellis Horwood; 1990. p. 316. [Google Scholar]
- 15.The protonated ligand, H6L·6Ts, was prepared by reacting 100 mg L with 8-fold p-toluenesulfonic acid in methanol. The addition of diethyl ether resulted in a white microcrystalline product that was filtered and washed by diethyl ether. The ligand was found to be hexaproptonated as supported by NMR and elemental analysis. Yield: 85 mg, 40 %. 1H NMR (300 MHz, D2O, TSP): δ 2.32 (s, 18H, CH3,), 2.69 (t, 12H, NCH2,), 3.05 (t, 12H, NCH2CH2), 4.31 (s, 12H, ArCH2), 7.10 (d, 6H, ArH), 7.29 (d, 12H, TsCH2,), 7.60 (d, 12H, TsCH2,). Anal. Calcd. for (C72H90N8S9O18): C, 52.60; H, 5.52; N, 6.82. Found.: C, 52.90; H, 5.45; N, 6.55.
- 16.Binding constants were obtained by 1H NMR (300 MHz Bruker) titrations of H6L · 6Ts with NaCl in D2O at pH 2. Initial concentrations were [ligand]0 = 2 mM, and [anion]0 = 20 mM. Sodium salt of 3-(Trimethylsilyl)propionic-2,2,3,3,-d4 acid (TSP) in D2O was used as an external reference in a capillary tube. The pH was adjusted with a concentrated solution of TsOH and NaOH in D2O. Each titration was performed by 12 measurements at room temperature. The association constant K was calculated by fitting of several independent NMR signals with a 1:1 association model using Sigma Plot software, from the following equations: Δδ = ([A]0 + [L]0 + 1/K − (([A]0 + [L]0 + 1/K)2 − 4[L]0[A]0)1/2) Δδmax/2[L]0 (where L = ligand and A = chloride). Error limit in K was less that 15%.
- 17.Hossain MA, Llinares JM, Miller C, Seib L, Bowman-James K. Chem. Commun. 2000:2269. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.