Abstract
Taste buds are dependent on innervation for normal morphology and function. Fungiform taste bud degeneration after chorda tympani nerve injury has been well documented in rats, hamsters, and gerbils. The current study examines fungiform taste bud distribution and structure in adult C57BL/6J mice from both intact taste systems and after unilateral chorda-lingual nerve transection. Fungiform taste buds were visualized and measured with the aid of cytokeratin 8. In control mice, taste buds were smaller and more abundant on the anterior tip (<1 mm) of the tongue. By 5 days after nerve transection taste buds were smaller and fewer on the side of the tongue ipsilateral to the transection and continued to decrease in both size and number until 15 days posttransection. Degenerating fungiform taste buds were smaller due to a loss of taste bud cells rather than changes in taste bud morphology. While almost all taste buds disappeared in more posterior fungiform papillae by 15 days posttransection, the anterior tip of the tongue retained nearly half of its taste buds compared to intact mice. Surviving taste buds could not be explained by an apparent innervation from the remaining intact nerves. Contralateral effects of nerve transection were also observed; taste buds were larger due to an increase in the number of taste bud cells. These data are the first to characterize adult mouse fungiform taste buds and subsequent degeneration after unilateral nerve transection. They provide the basis for more mechanistic studies in which genetically engineered mice can be used.
Indexing terms: receptor cells, gustation, axotomy, cyokeratin, neurofilament
The gustatory system provides a prime model to study neural plasticity in adult animals. Whereas in most sensory systems the innervation of the periphery by primary afferent neurons is determined early in development, the gustatory system is unique in that taste bud innervation is plastic throughout adulthood as receptor cells turn over (Farbman, 1980; Hendricks et al., 2004). Therefore, receptive fields rearrange as taste bud cells die and new cells are added. Concomitant with naturally occurring taste bud cell death, taste buds are dependent on the chorda tympani nerve to maintain their functional integrity and structure. Specifically, taste buds degenerate after nerve injury (Farbman, 1969; Hård af Segerstad et al., 1989) and reappear upon reinnervation by regenerating nerves (Cheal et al., 1977; Cheal and Oakley, 1977; Cain et al., 1996).
While taste bud degeneration after injury to the chorda tympani nerve occurs in a variety of species, there is a substantial species-dependent diversity in the time course and degree of fungiform taste bud degeneration (Farbman, 1969; Cheal and Oakley, 1977; Whitehead et al., 1987; Ganchrow and Ganchrow, 1989; Oakley et al., 1990, 1993; Cain et al., 1996). For example, over 90% of taste buds are lost by 9 days posttransection in the gerbil (Cheal and Oakley, 1977), while the rat and hamster lose only 28% and 26% of their taste buds after chorda tympani transection, respectively (Oakley et al., 1993). The abundance of studies done in these species provides a rich foundation for characterizing the cellular and structural changes related to nerve/target interactions; however, identification of the underlying cellular and molecular mechanisms have been elusive.
Oddly, the dynamics of fungiform taste bud degeneration after denervation has not been studied in mice. The anterior tongue provides many distinct advantages in studying taste bud development, degeneration, and regeneration: the ease of accessibility of the anterior tongue along with the discrete grouping of taste buds in fungiform papillae make it possible to perform noninvasive experimental manipulations with single taste buds in live animals (Krimm and Hill, 1998, 2000; Whitehead et al., 1999; Shuler et al., 2004). Most important, studies focused on mouse fungiform taste buds provide another benefit that is not readily available in other rodent species. Due to the ability to experimentally alter genes, the mouse is an excellent animal model to uncover the molecular mechanisms responsible for neural dependence in the peripheral taste system. A similar strategy has been successfully used to answer long-standing problems in gustatory neurobiology. Genetically engineered mouse models have provided insights into the functional identification of neurotransmitters in taste buds (Finger et al., 2005), as well as the cellular/molecular mechanisms of gustatory organization (Zhang et al., 1997; Nosrat et al., 1997, 2004; LeMaster et al., 1999; Liebl et al., 1999; Mistretta et al., 1999; Ringstedt et al., 1999; Krimm et al., 2001; Sun and Oakley, 2002; Yee et al., 2003, 2005; Agerman et al., 2003).
To better design studies that take advantage of the ability to manipulate genes in mice, it is important to first characterize the neural dependence of fungiform taste buds in a background strain, especially given the dramatic species-related diversity. Once these data are obtained they can then be used as a standard to which findings from genetically modified mice can be compared. Therefore, we chose to characterize the topography of taste buds on the anterior tongue and provide the first data describing the effects of axotomy on taste bud structure in wildtype mice. Two unexpected results surfaced in the course of these studies. First, there was a large difference in the amount of taste bud degeneration between the tip of the tongue and the intermediate region of the tongue. Second, changes in taste bud volume occurred on the side of the tongue contralateral to nerve transection.
MATERIALS AND METHODS
Animals
Female C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME) and housed in groups of 1–4 mice per cage in a temperature- and humidity-controlled room with a 12:12 light/dark cycle. Free access to standard rodent chow and tap water were provided. Mice between the ages of 50 and 80 days were assigned to one of five groups: a control group with no surgical manipulation (n = 4) or one of four surgical groups. The surgical groups received unilateral chorda-lingual nerve transection and were allowed 5 (n = 4), 10 (n = 3), 15 (n = 3), or 20 (n = 3) days to recover before being sacrificed. In addition, four mice (10-day posttransection, n = 2; and 20-day posttransection, n = 2) were used for neurofilament staining and 4 mice (10-day posttransection, n = 4) for a neuron-specific ubiquitin carboxyl-terminal hydrolase (PGP 9.5) staining to examine the presence of inner-vation. Finally, six mice (control mice, n = 3; 10-day posttransection, n = 3) were used for taste bud cell counts. All experiments were done with the approval of the University of Virginia Animal Use and Care Committee.
Chorda-lingual nerve transection
Mice were anesthetized with intramuscular (IM) injections of medetomadine hydrochloride (0.4 mg/kg body weight) followed by ketamine hydrochloride (40 mg/kg body weight) (Sun et al., 2003; May and Hill, 2006). The right chorda-lingual nerve was exposed in the neck of the mouse and transected between the anterior belly of the digastric and masseter muscles. Although the proximal and distal stumps of the transected chorda-lingual nerve were separated, no further steps were taken to minimize regeneration. Surgeries were brief (≈10 minutes) and had no complications. After the surgery mice were injected IM with atipamezole (2 mg/kg body weight), an antidote to medetomadine, and allowed to recover on a water-circulating heating pad until fully awake.
Tissue preparation
Mice were sacrificed with urethane (≈4 mg/kg body weight) and tongues were collected for sectioning. The anterior tongue was gently manipulated with blunt forceps, removed, rinsed in a 0.1 M phosphate-buffered saline (PBS) solution (pH = 7.2), and suspended in tissue freezing medium (Triangle Biological Sciences, Durham, NC). The specimen was frozen and stored in a deep freeze at −80°C until coronally sectioned on a cryostat (Leica 3050s, Bannockburn, IL). Sections were collected from the anteriormost 5.0 mm of tongue. Serial sections (12 µm thick) were mounted on glass slides for taste bud counts and volume measurements, while tongues used for neuro-filament immunohistochemistry were sectioned at 14–16 µm. Since most taste buds remaining after chorda-lingual nerve transection were only present in the very anterior portion of the tongue (see Results), taste bud cell counts and neurofilament immunohistochemistry were done only on the first millimeter of tongue.
Taste bud analysis
Immunohistochemistry
Taste buds were visualized using an antibody to cytokeratin 8 (Fig. 1), a protein that, in the mouse oral cavity, is found exclusively within taste buds (Knapp et al., 1995; Zhang et al., 1995). The tissue was postfixed in 4% paraformaldehyde /0.01 M PBS solution and rinsed with PBS. Slides were reacted with an antibody to cytokeratin 8 (a monoclonal antibody against intermediate filaments generated from rat spleen, TROMA-I, 1:60; in 0.3% Triton X-100/PBS solution, Sigma, St. Louis, MO), covered with parafilm strips, and stored overnight at 4°C. Troma I (i.e., cytokeratin 8) was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences (Iowa City, IA). The Troma I antiserum was characterized with Western blot analysis that stained a single band of ≈55 kD molecular weight (manufacturer’s technical information) from preimplanted embryos (Brulet et al., 1980). Slides were rinsed in PBS and placed in rhodamine (TRITC)-conjugated donkey antirat IgG secondary (1:333, Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 hour. After a final rinse in PBS, slides were air-dried and coverslipped with Krystalon mounting medium (Harleco, Gibbstown, NJ).
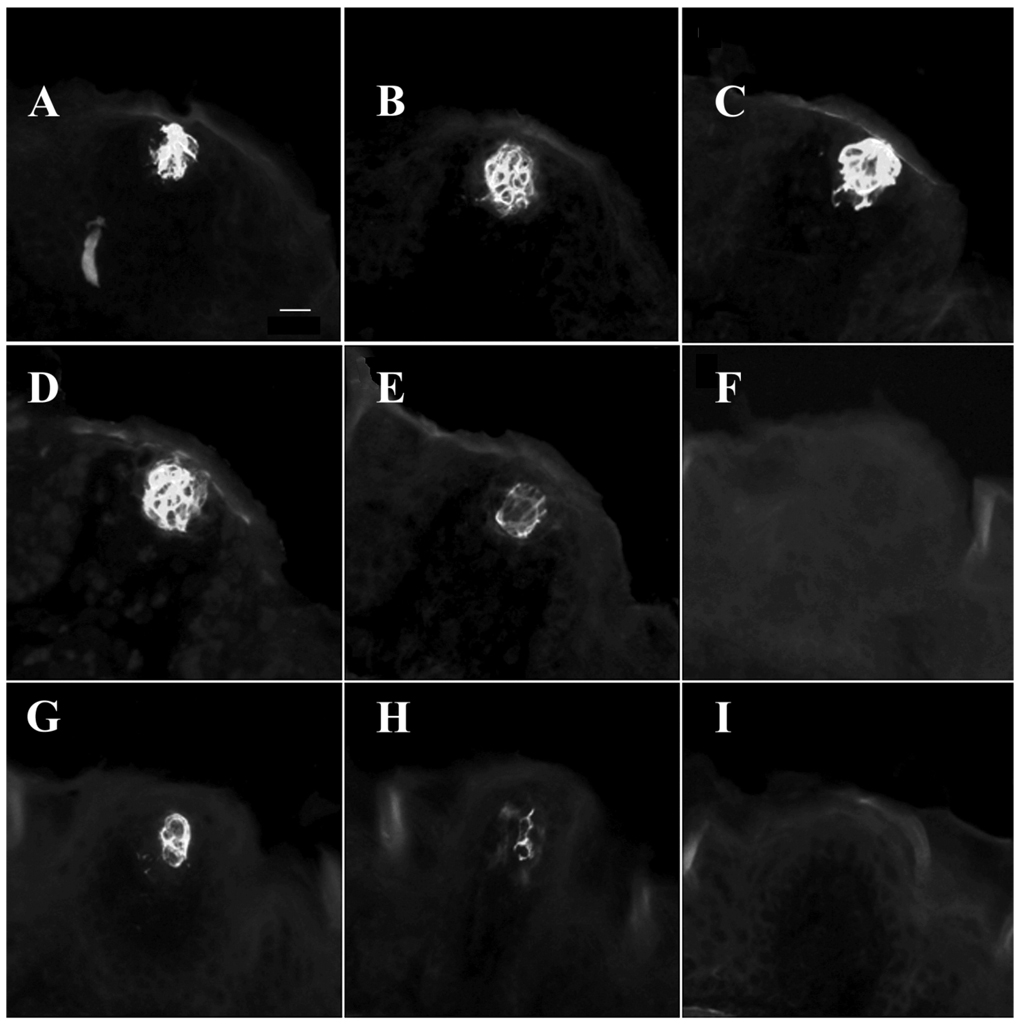
Fig. 1.
Cytokeratin 8-positive taste buds. Taste bud cells immunopositive for cytokeratin 8 appear as white. A–E: Serial sections of a taste bud from an intact (control) mouse. F–I: A taste bud from a mouse 10 days after an ipsilateral chorda-lingual transection. Scale bar = 20 µm.
Microscopic analysis
Fungiform taste buds were counted and taste bud volumes were measured using a compound epifluorescent microscope (Leica) and Neurolucida imaging software (MicroBrightField, Williston, VT). A single taste bud from an intact tongue could generally be found in 3–5 sections (i.e., 36–60 µm; Fig. 1). The area from each section was multiplied by section thickness and summed to obtain the total taste bud volume. The approximate distance from the tip of the tongue and the approximate distance from the midline of the tongue were also recorded for regional analysis.
Taste bud cell counts
To see if taste bud degeneration after nerve transection was due to a loss of taste bud cells or due to a change in taste bud morphology (e.g., cell shrinkage), control mice and a group of mice with unilateral chorda-lingual transection sacrificed 10 days postsurgery were stained with Sytox (Molecular Probes, Eugene, OR), a fluorescent nuclear stain. Staining with Sytox al-lowed counting of individual cells within the taste bud and estimating the mean size of the taste bud cell nucleus. After reacting the slides for cytokeratin 8 as described earlier, slides were rinsed and exposed to a 1-µM solution of Sytox in DMSO for 30–40 seconds. After a brief rinse with distilled water, slides were allowed to dry in the dark and coverslipped with Krystalon mounting medium.
Imaging
All of the profiles of a single taste bud were optically sectioned on an Olympus confocal laser microscope (IX70 inverted microscope, Melville, NY) with a 488 nm argon laser and a 543 nm HE/NE laser to visualize both the Sytox nuclear stain and the rhodamine cytokeratin 8 stains, respectively. Images were captured using FluoView software (Olympus). Taste buds were serially reconstructed using Neurolucida software, and the number of cells in each taste bud was counted. A cell was counted if the nucleus was completely encompassed by cytokeratin 8 stain. Sizes of cell nuclei were estimated by tracing the perimeter of all nuclei observed in the centermost section of each taste bud; the area of the nuclei were calculated using Neurolucida imaging software (MicroBrightField).
Analysis
Taste bud volumes among animals within each experimental group belonged to a homogenous population (using Levene’s test for homogeneity of variance, P > 0.05); therefore, taste buds were pooled and a single mean taste bud volume was calculated for each group. Mean taste bud numbers and volumes were compared using a one-way ANOVA. When overall significance was determined, a Tukey post-hoc test was used to identify which groups within the analysis were significant. When only two groups were compared, an independent T-test was used. Significance for all analyses was defined as P < 0.05 using two-tailed tests.
Neurofilament visualization
Tongue sections from mice sacrificed 10 and 20 days after chorda-lingual transection were reacted for TROMA-I and neurofilament 150 kD. Sections stored at −80°C were allowed to thaw at room temperature before fixation in acetone at −20°C for 10 minutes. Slides were air-dried, then rinsed with 0.1 M PBS solution before incubating in anti-cytokeratin 8 (Troma I, 1:60) and anti-neurofilament 150 kD (1:2,000; Chemicon International, Temecula, CA, product number AB1981) with 0.3% Triton overnight at 4°C. Anti-neurofilament 150 kD is a polyclonal antibody raised in rabbit against highly purified bovine neurofilament polypeptide. Neurofilament 150 kD was purified and separated using batchwise hydroxylapatite chromatography (Liem and Hutchison, 1982) and high-performance liquid chromatography (Karlsson et al., 1987). Antibody specificity was shown using both inhibition enzyme-linked immunosorbent assay and immunoblotting techniques from bovine brainstem (Karlsson et al., 1989). The latter revealed a single band of ≈150 kD. Tissue was then rinsed in PBS and placed in a mixture of rhodamine-conjugated donkey antirat IgG (1:333) to visualize anti-cytokeratin 8 and Alexa Fluor 488 goat antirabbit IgG (1:250; Molecular Probes) for 45 minutes. Slides were rinsed briefly in distilled water and allowed to dry before they were coverslipped with Krystalon. In order to visualize finer neural processes, we also used an antibody to PGP 9.5 along with Troma I on another set of tissue. This polyclonal antibody (AbD Serotec, Raleigh, NC, Cat. No. 7863-0504) was raised in rabbit against PGP 9.5 from pathogen-free human brain, but works well in mice due to high species cross-specificity. When tested under reducing conditions, the antibody gave a single band of 27 kD (manufacturer’s product sheet) when tested against rat brain. Similar to cytokeratin 8 staining, slides were rinsed with 0.1 M PBS and placed in antibody (1:400, 0.3% Triton) overnight at 4°C. On the following day, tissue was placed in Alexa Fluor 488 goat antirabbit IgG (1:250) secondary for 1 hour, rinsed in PBS, and then placed in Troma I primary (1:60, no Triton) for 1 hour at room temperature. After a brief rinse, the slides were reacted with rhodamine-conjugated donkey antirat IgG (1:333) for an hour. Tissue was examined under the Olympus confocal laser microscope to see if fibers were present in the cut side of the tongue at 10 or 20 days after chorda-lingual transection. No staining was observed when either the neurofilament 150 antibody or the PGP 9.5 antibody were omitted from the primary incubation.
For figure plates, Fireworks (Macromedia, San Francisco, CA) and Photoshop (Adobe Systems, San Jose, CA) were used to compose images from digital files. Images were enhanced only for contrast and brightness.
RESULTS
Characterization of taste buds in intact mice
Taste bud numbers
In control mice, taste buds in the fungiform papilla on the anterior 5 mm of the tongue had a characteristic onion-like appearance (Fig. 1) with a mean (±SEM) of 54.6 ± 1.1 (n = 16) taste buds per side of the tongue. To identify regional differences in taste bud size and number the tongue was divided along both the anterior–posterior and medial–lateral axis (Fig. 2).
Fig. 2.
Diagram from the tongue of an intact mouse to illustrate the divisions used for analyses. The mouse tongue was divided into anterior (anteriormost 1 mm) and intermediate (next 4 mm) divisions for anterior–posterior analyses and medial (midline to 350 µm lateral from midline), middle (next 550 µm), and lateral divisions for medial–lateral analyses. CV, circumvallate papilla; FP, foliate papillae; IE, intermolar eminence. Scale bar = 1 mm.
There was a pronounced anterior-to-posterior gradient in taste bud densities in control mice. The anteriormost region of the tongue had the greatest density of taste buds; the first millimeter of tongue had a mean of 34.4 ± 1.1 taste buds on each side of the tongue, while the next 4 mm had a mean of 20.2 ± 0.9 taste buds per side (t = 10.5; P < 0.001; Table 1, intact data). Thus, ≈63% of mouse taste buds on the first 5 mm of tongue were on the anteriormost millimeter. The tongue was further divided along the medial–lateral axis. Taste buds located ≈350 µm to the midline were classified as “medial” taste buds. “Middle” taste buds were located between 350 and 900 µm lateral from the midline, and taste buds located 900 mm from the midline to the lateral margin of the tongue were termed “lateral” taste buds (Fig. 2). In control mice there was no significant difference in the number of taste buds among regions along the medial–lateral axis (data not shown).
TABLE 1.
Mean Number and Volume of Fungiform Taste Buds in Intact Mice and After Chorda-lingual Nerve Transection (CLX)
| Mean # of TBs | Mean TB Volume (× 104 µm3) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CLX | SE | Sig | CLX | SE | Sig | Intact | SE | Sig | |
| Intact | |||||||||
| Anterior | 34.4 | 1.1 | N/A | N/A | 2.2 | 0.1 | |||
| Intermediate | 20.1 | 0.9 | N/A | N/A | 2.8 | 0.1 | |||
| Total | 54.6 | 1.1 | N/A | N/A | 2.5 | 0.0 | |||
| 5 Day CLX | |||||||||
| Anterior | 32.3 | 3.4 | 1.4 | 0.1 | * | 2.5 | 0.1 | * | |
| Intermediate | 12.5 | 2.5 | * | 1.4 | 0.1 | * | 3.1 | 0.1 | * |
| Total | 44.8 | 4.3 | * | 1.4 | 0.1 | * | 2.8 | 0.1 | * |
| 10 Day CLX | |||||||||
| Anterior | 26.0 | 0.0 | 1.3 | 0.1 | * | 2.9 | 0.1 | * | |
| Intermediate | 8.0 | 4.0 | * | 1.4 | 0.2 | * | 3.3 | 0.2 | * |
| Total | 34.0 | 4.0 | * | 1.4 | 0.1 | * | 3.1 | 0.1 | * |
| 15 Day CLX | |||||||||
| Anterior | 17.3 | 4.1 | * | 1.0 | 0.2 | * | 2.4 | 0.1 | |
| Intermediate | 0.0 | 0.0 | * | N/A | N/A | 2.6 | 0.1 | ||
| Total | 17.3 | 4.1 | * | 1.0 | 0.2 | * | 2.5 | 0.1 | |
| 20 Day CLX | |||||||||
| Anterior | 17.0 | 1.2 | * | 1.0 | 0.2 | * | 2.8 | 0.1 | * |
| Intermediate | 0.7 | 0.7 | * | 0.9 | 0.5 | * | 2.6 | 0.1 | |
| Total | 17.7 | 1.8 | * | 1.0 | 0.2 | * | 2.7 | 0.1 | * |
The mean number of taste buds decreases significantly after nerve transection. The decrease occurs sooner and more robustly in the posterior field than the anterior. By 15 days few taste buds remain on the posterior field. In intact animals taste buds on the anterior 1 mm of the tongue are smaller than more posterior taste buds (P < 0.05). Remaining fungiform taste buds after transection are smaller on the ipsilateral, transected side of the tongue, but increase in size on the side of the tongue contralateral to nerve transection. The plus (+) denotes a significant difference from corresponding lingual area (anterior or posterior) in intact mice (P < 0.05).
Taste bud volumes
The mean taste bud volume on the anterior 5 mm of tongue in control mice was 2.5 ± 0.1 × 104 µm3 (n = 201). The first millimeter of tongue (anterior tongue) contained significantly smaller taste buds (2.2 ± 0.1 × 104 µm3; n = 112; T = 6.4, P < 0.001) than in the more posterior region (designated the intermediate tongue, 2.8 ±0.1 × 104 µm3; n = 89; Table 1). Taste buds decreased in size farther away from the midline (F = 4.7, P < 0.01). Medial taste buds did not differ significantly from middle taste buds in mean taste bud volume (2.6 ± 0.1 × 104 µm3, n = 63; and 2.5 ± 0.1 × 104 µm3, n = 68, respectively), but medial taste buds were significantly larger than lateral taste buds (2.3 × 104 µm3, P < 0.01, data not shown). There were no interaction effects between anterior–posterior taste bud position and medial–lateral taste bud position, although it did approach significance (F = 2.8, P = 0.07). Thus, in the mouse the volume of a fungiform taste bud was affected by its position along both the anterior–posterior axis and the medial–lateral axis of the tongue.
Unilateral chorda-lingual nerve transection: ipsilateral effects
After transection, both number of taste buds (F = 52.5, P < 0.001; see Table 1) and the size of remaining taste buds (F = 91.0, P < 0.001; see Table 1) on the ipsilateral tongue decreased dramatically in all groups. At 5 days after transection, mice had ≈82% of control taste buds (44.8 ± 4.3 and 54.6 ± 1.1, respectively; P = 0.03). This number dropped significantly to 32% at 15 (17.3 ± 4.1; P < 0.001) and 20 days (17.7 ± 1.8; P < 0.001) following nerve transection (Table 1). Mean taste bud volume also decreased significantly by 5 days (1.5 ± 0.1 × 104; P < 0.001) and significantly decreased further at 15 days posttransection (1.0 ± 0.1 × 104, P < 0.001). Chorda-lingual transection eliminated the effect of anterior–posterior position on mean taste bud volume (see Table 1).
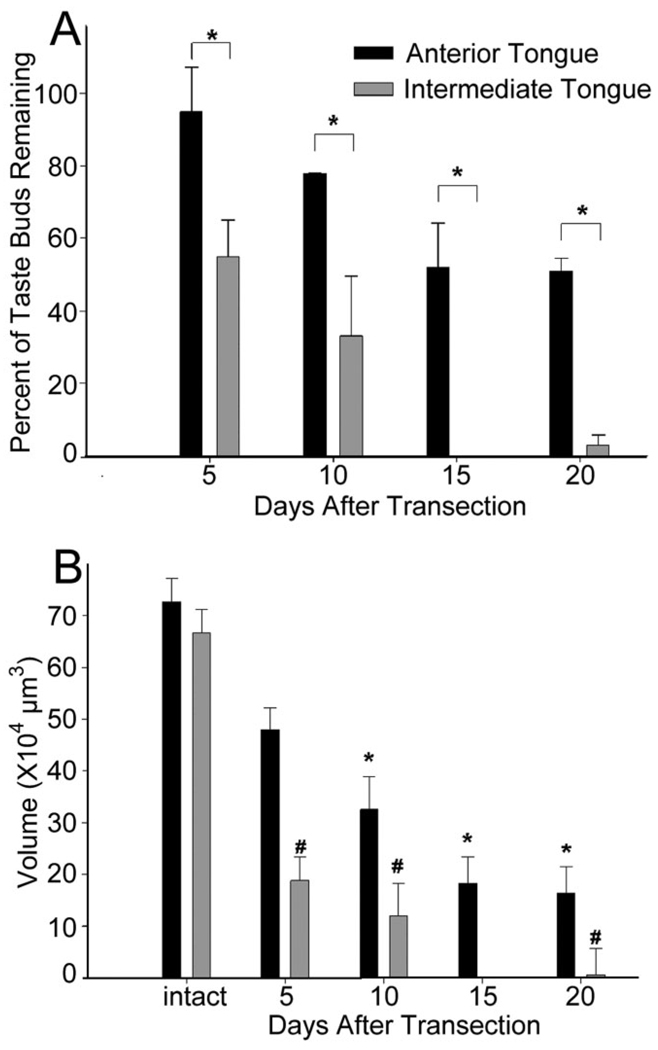
Interestingly, the loss of taste buds along the tongue was not uniform; a much higher percentage of taste buds were lost on the intermediate tongue compared to the anterior tongue, while no medial–lateral differences were observed (Fig. 3A). Although the anterior tongue lost more total taste buds, almost no taste buds remained on the intermediate portion of the tongue by 15 days posttransection. This difference was quantified by analyzing the difference in the total amount of taste tissue (total taste bud volume) between the anterior and intermediate tongue after chorda-lingual nerve transection. Total taste bud volume was defined as the total amount of lingual tissue immunopositive for cytokeratin 8 and was derived by multiplying the mean taste bud volume for the anterior and intermediate tongue by the total number of taste buds in each region of the tongue. These two numbers represent an estimate of the total taste bud volume for each animal (Fig. 3B).
Fig. 3.
A: Percent of taste buds remaining after nerve transection: anterior vs. intermediate tongue. The intermediate tongue was particularly susceptible to taste bud loss. The anterior tongue lost as much as half its taste bud population after chorda-lingual transection. The intermediate tongue lost 100% and 97% of taste buds at 15 and 20 days, respectively. Asterisks (*) denote significant differences between anterior and intermediate regions at each time period posttransection. B: Total volume of taste buds (±SEM) on the anterior and intermediate tongue after transection. The intermediate tongue had significantly fewer taste buds, and in turn much less total taste tissue than the anterior tongue (P’s < 0.01). Asterisks (*) indicate significant differences from control volumes on the anterior tongue. The number symbol (#) represents significant differences between taste bud volumes on the intermediate tongue from control volumes.
Although the anterior and intermediate tongue had similar decreases in taste bud volume 20 days after chorda-lingual transection, the intermediate tongue lost significantly more tissue than the anterior tongue (F = 47.7, P = 0.002). By 20 days, the mean total taste bud volume on the anterior tongue was 16.0 ± 2.2 × 104 µm3 (n = 3), and the intermediate tongue had a mean of 0.6 ± 0.6 × 104 µm3 (n = 3, P < 0.01; Fig. 3B). While there was no anterior–posterior difference for total taste volume in intact mice, there was 26.8 times more taste tissue on the anterior tongue than on the intermediate tongue at 20 days posttransection.
Unilateral chorda-lingual nerve transection: contralateral effects
The side of the tongue contralateral to chorda-lingual nerve transection was also analyzed. Although none of the groups differed in taste bud number on the intact side of the tongue, there was a change in mean taste bud volume (F = 8.5, P < 0.001; Table 1). At 5 days posttransection there was a significant increase in mean taste bud volume on the side of the tongue contralateral to nerve transection compared to intact controls (P = 0.004). Taste bud volumes on the contralateral tongue peaked at 10 days (P < 0.001) and declined but remained significantly greater than controls at 20 days following nerve transection (P = 0.001). The mean volume for the group surviving for 15 days posttransection did not differ significantly from controls.
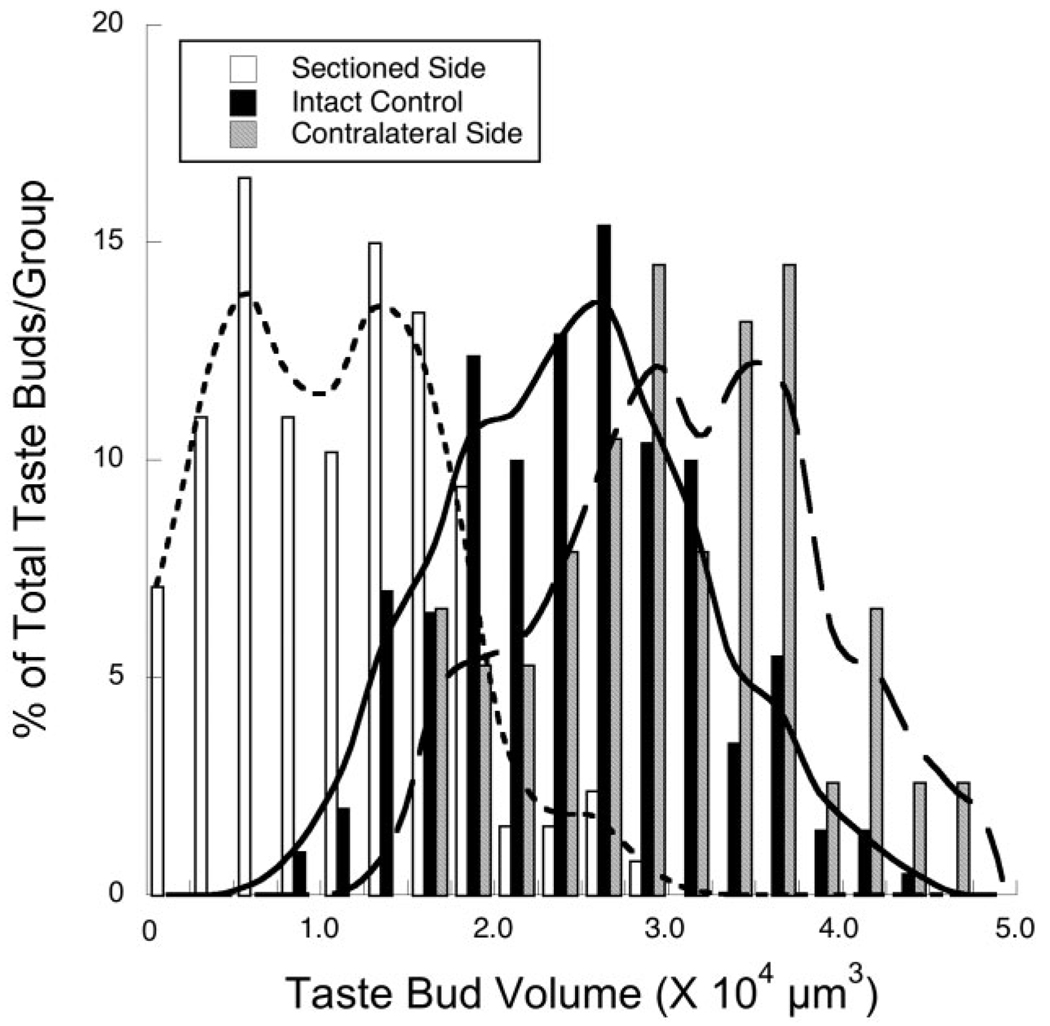
Distribution of taste bud volume changes
The experimentally induced changes in taste bud volumes may have been due to a selective loss of large taste buds on the side of the tongue unilateral to the nerve section and/or a selective loss of small taste buds on the side of the tongue contralateral to the nerve section. Alternatively, entire distributions may have shifted to the left (ipsilateral effects) or to the right (contralateral effects) compared to intact controls. Figure 4 shows that similar shapes of the frequency distributions are seen for taste bud volumes on the sectioned side of the tongue, intact controls, and on the contralateral side of the tongue (Fig. 4). The experimentally induced alterations are due to a significant shift in the frequency distribution of taste bud volumes to smaller taste buds on the ipsilateral side and to larger taste buds on the contralateral side (Fig. 4). Therefore, there is not a selective loss of large or small taste buds in the distributions from the experimental groups.
Fig. 4.
Frequency distributions (percent of the total number/group) of taste bud volumes from the side of the tongue ipsilateral to the nerve section (open bars; curve fit by dotted line), the intact control tongue (solid bars; curve fit by solid line), and the side contralateral to the nerve transection (gray bars; curve fit by dashed line).
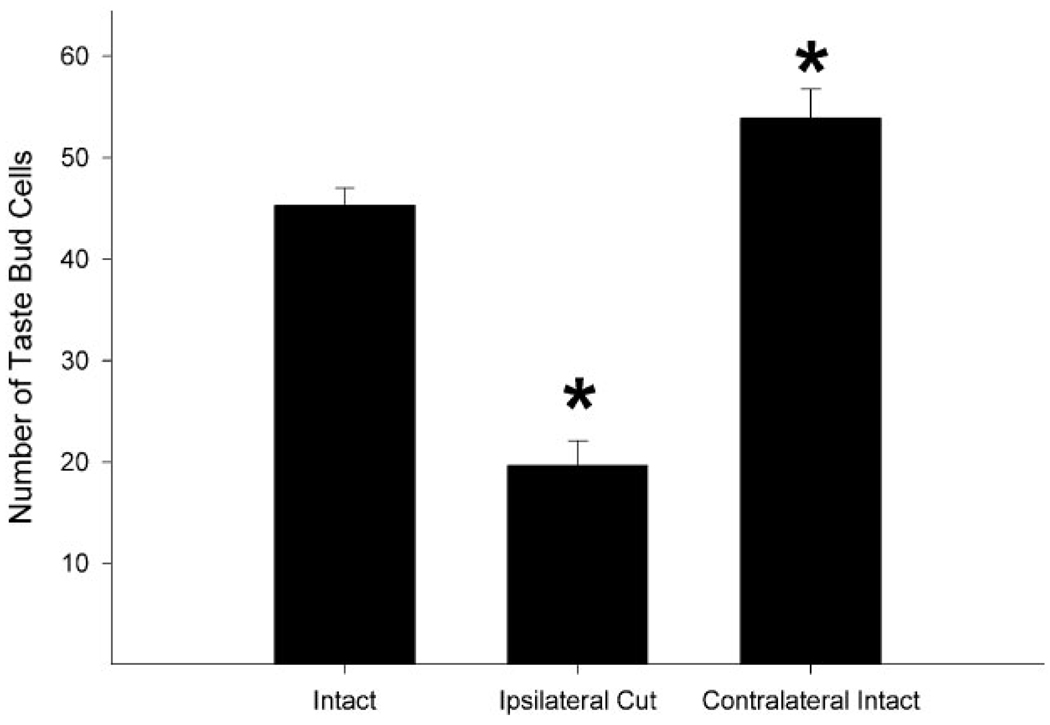
Taste bud cell number
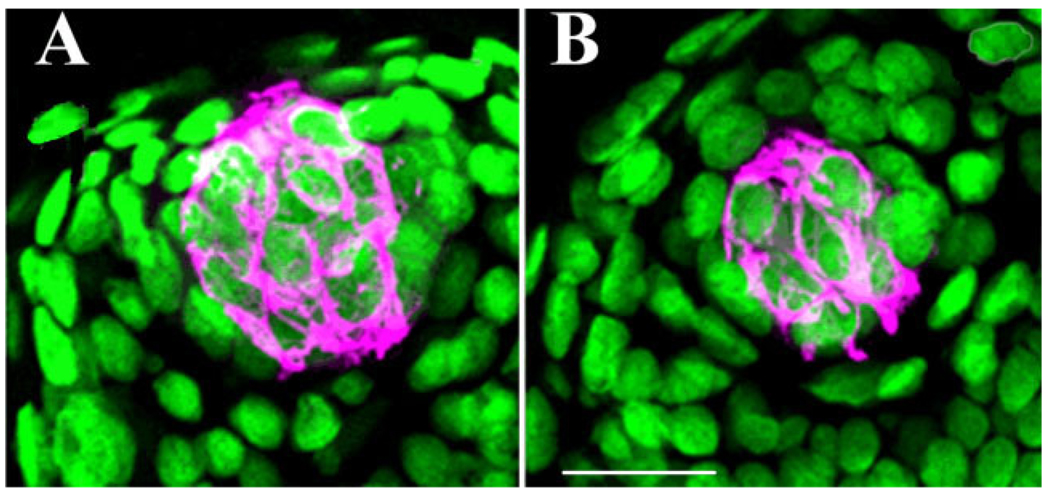
Sytox staining revealed significant differences in the number of taste bud cells per taste bud (F = 54.5, P < 0.001; Fig. 5, Fig. 6). At 10 days after transection there were significantly fewer taste bud cells/taste bud on the side of the tongue ipsilateral to transection (19.7 ± 2.4, n = 20, P < 0.001) compared to controls (45.3 ± 1.7, n = 19). Concomitantly, taste buds on the contralateral tongue had more cells (53.9 ± 2.9, n = 20, P = 0.04) compared to control mice. Further, taste bud cell nuclei did not differ in size between intact and chorda-lingual transected sides of the tongue (21.7 ± 1.2 µm3 and 23.2 ± 1.2µm3, respectively, n = 152, t = 0.8, P = 0.4). These data show taste buds ipsilateral to nerve transection are getting smaller due to loss of taste bud cells rather than a decrease in the size of individual taste bud cells.
Fig. 5.
Two taste buds immunopositive for cytokeratin 8 (magenta) and the nuclear stain Sytox (green). A taste bud from a control mouse (A) and from the transected side of the tongue in a mouse sacrificed 10 days after nerve transection (B). Scale bar = 20 µm.
Fig. 6.
Mean number of Sytox/cytokeratin 8-positive cells per taste bud in unoperated control mice (intact) and in the transected (ipsilateral cut) and intact (contralateral intact) sides of the tongue of mice 10 days after receiving chorda-lingual nerve transection. Taste buds on the transected side of the tongue had significantly fewer cells than those in control mice and on the intact side of the tongue at 10 days post-nerve transection (*P’s < 0.001). Also, the side of the tongue contralateral to the nerve transection had more mean taste buds compared to intact control mice (*P = 0.04).
Innervation
It was important to confirm the efficacy of the chorda-lingual nerve transection and determine if fungiform papilla ipsilateral to the nerve cut contained nerve fibers. In addition, Hård af Segerstad et al. (1989) demonstrated that nongustatory nerves maintain taste buds in the absence of chorda tympani nerve innervation, and Kinnman and Aldskogius (1988) found some lingual nerve fibers contralateral to chorda-lingual nerve transection crossed the midline and innervated taste buds on the denervated side of the tongue. To determine if remaining fungiform taste buds were maintained by nerve innervation, we sampled mice at 10 and 20 days post-chorda-lingual transection and visualized nerve fibers using a neurofilament antibody and an antibody to PGP 9.5.
Ten days after chorda-lingual transection the intact side of the tongue showed robust taste bud innervation; fibers could be seen within taste buds immediately adjacent to taste buds and in papillae surrounding the taste bud (Fig. 7A). Presumably, fibers contacting the taste bud belonged to the chorda tympani nerve, while surrounding fibers were part of the lingual nerve (Cheal and Oakley, 1977). On the cut side of the tongue, most papillae had no nerve fibers (Fig. 7B,C). However, there was a single case in which fibers entered a papilla at 10 days posttransection, but no taste bud was observed. In 20-day posttransected mice, a different picture emerged. Almost all papillae, regardless of whether they contained taste buds, had nerve fibers in the taste bud region of the papilla (Fig. 7E,F). Because Neurofilament 150 kD tends to stain larger fibers, we used an antibody to PGP 9.5, a neural marker that labels finer processes, to confirm both empty papillae and remnant fungiform taste buds lack innervation (Fig. 7G–I). The results indicate nerve fibers appear to invade fungiform papilla between 10 and 20 days posttransection, similar to the results obtained using the Neurofilament 150 kD antibody.
Fig. 7.
Innervation after chorda-lingual nerve transection. A–C: Profiles of taste buds from a mouse 10 days after unilateral chorda-lingual nerve transection. Fibers positive for neurofilament and PGP 9.5 are in green, cytokeratin 8-positive cells are in magenta. Taste buds on the contralateral, intact side of the tongue were richly innervated with neurofilament 150-positive fibers (A). On the transected side of the tongue, no fibers were observed in empty papilla (B) or papilla with remnant fungiform taste buds (C). The arrow indicates hypoglossal fibers innervating underlying muscle tissue. D–F: Profiles of taste buds 20 days after chorda-lingual nerve transection. These include an intact taste bud contralateral to nerve transection (D), neural fibers in an empty fungiform papilla (E), and fibers approaching a small, remnant fungiform taste bud (F). To confirm the lack of chorda-lingual fibers 10 days after nerve transection, G–I show PGP 9.5 immuno-like staining. Taste buds on the intact side of the tongue show prolific staining for PGP 9.5 (G), while the cut side lacks PGP 9.5 staining in empty fungiform papilla (H) and in the presence of a fungiform taste bud (I). Scale bars = 20 µm.
DISCUSSION
The results of this study are the first to show the morphological changes in mouse fungiform taste buds following axotomy of the chorda-lingual nerve. As such, they form the basis for similar studies using genetically engineered mice. Initially, we characterized fungiform taste bud structure and size in intact mice in order to provide the standard by which axotomy-induced changes could be compared.
Several patterns emerged from this analysis. The anterior tip of the tongue had many more taste buds than the intermediate tongue, while the intermediate tongue had larger taste buds. When both mean taste bud volume and number of taste buds were taken into account, the region-specific differences were eliminated, resulting in approximately the same amount of total taste tissue on the anterior tongue and the intermediate tongue. In addition, taste buds on the medial portion of the tongue were larger than taste buds on the lateral portion of the tongue.
Effects of chorda tympani-lingual nerve axotomy
Two unexpected results related to axotomy of the chorda-lingual nerve surfaced in the course of these studies. First, there was a large difference in the amount of taste bud degeneration between the tip of the tongue and the intermediate region of the tongue. Second, changes in taste bud volume on the side of the tongue contralateral to nerve transection occurred.
Anterior versus intermediate tongue
Taste buds on the intermediate tongue are more susceptible to chorda-lingual transection than taste buds near the tip of the tongue. By 15 days posttransection, nearly half the taste buds on the anteriormost millimeter of tongue survived. In contrast, almost all posterior taste buds disappeared. This could be due to regional differences in cellular/molecular factors related to taste bud cell death. Sun and Oakley (2002) found that the anteriormost millimeter of the mouse tongue was dependent on epidermal growth factor receptor (EGFr) for normal development, while the mid-region of the tongue developed normally in the absence of epidermal growth factor receptors. Conversely, brain-derived neurotrophic factor (BDNF) null mutant mice had more taste buds remaining on the tip of the tongue than on more posterior regions (Mistretta et al., 1999). The regional difference in the susceptibility of adult mouse taste buds to survive chorda-lingual nerve transection seen here may relate to the regional differences in the molecular expression of these and/or other factors. For example, if the chorda tympani nerve provides trophic support in the form of anterograde transport of BDNF, then transection may differentially affect the posterior, BDNF-sensitive portion of the chorda tympani receptive field. Curiously, the reappearance of nerves into the anterior portion of the tongue by 20 days posttransection failed to restore taste buds, which is consistent with the hypothesis that the neural dependence of anterior tongue taste buds are distinct from more posteriorly located taste buds.
Neuroimmunological data from the rat taste system lends further support for a fundamental difference between the anterior and the mid-region of tongue. Nearly 50% of activated macrophages are located in the anterior-most 1.2 mm of tongue after chorda tympani transection (McCluskey, 2004; Cavallin and McCluskey, 2005). This macrophage infiltration may increase cell survival after nerve injury, as demonstrated in retinal ganglion cells after nerve injury (Fischer et al., 2000, 2001; Leon et al., 2000).
Contralateral changes
Mice with unilateral chorda-lingual transection showed an increase in taste bud size, due to an increase in taste bud cell number, on the contralateral, intact side. These findings were also confirmed in rats following unilateral chorda tympani transection (Shuler et al., 2004). Although the reasons for this are unclear, there is a precedence for such contralateral changes occurring after nerve injury (reviewed in Koltzenburg et al., 1999). For example, Rotshenker and Tal (1985) observed a 3-fold increase in sprouting at the neuromuscular junction contralateral to sciatic nerve lesion as soon as 5 days posttransection in the mouse. Similar results have been found in autonomic and sensory neurons (reviewed in Koltzenburg et al., 1999). Further, immunological changes in the lingual epithelium have been observed in the rat after unilateral chorda tympani nerve transection (McCluskey, 2004). The intact side of the tongue had ≈3 times the number of activated macrophages on the anterior tongue as control, sham-operated rats. Taken together, these changes may promote taste bud cell survival or the addition of cells to the taste bud resulting in larger taste buds in fungiform papilla contralateral to nerve injury.
Comparison with axotomy-induced changes in posterior tongue
The research in adult mouse taste bud degeneration following nerve damage has been limited to the circumvallate papillae (Takeda et al., 1996, 2000; Uchida et al., 2003). When innervation was removed from circumvallate taste buds, almost all taste buds completely degenerated by 11 days. The results from the current study extended the findings of Takeda et al. (1996, 2000) by showing that the fungiform taste buds also decrease in size due, at least in part, to a loss of taste receptor cells. This is consistent with the finding of increased apoptosis that was derived from mouse circumvallate papillae (Takeda et al., 1996). However, unlike circumvallate taste buds, a significant number of taste buds remained on the anteriormost tongue even 20 days after nerve transection, suggesting less of a neurotropic influence on taste buds on the anterior tongue. These remaining taste buds could not be explained by neural innervation; fibers were absent in both “empty” papillae and papillae containing taste bud remnants by 10 days posttransection. Therefore, while there are similarities in the ultimate effects of axotomy on circumvallate and fungiform taste buds, the time course and the extent of degeneration appears to be different.
Comparative analysis
Oakley et al. (1993) compared taste bud loss after chorda-lingual transection in the gerbil, rat, and hamster. They reported that gerbils had the most drastic loss of taste buds after 3 weeks, with the loss of 71% of taste buds, while hamsters and rats only lost 26% and 28% of taste buds, respectively. McCluskey and Hill (2002) reported a much greater loss of taste buds in the rat; almost 80% of fungiform taste buds were lost after a week posttransection. The difference between the two findings in rats may be due to the method used to identify and measure taste buds. Oakley et al. (1993) used a hematoxylin stain that reveals general morphological characteristics of taste buds and surrounding epithelial cells. McCluskey and Hill (2002) used a marker for taste bud cells, cytokeratin 19. Regardless, in all species (now including mice), some taste buds remained after chorda-lingual transection. In the mouse we found that 68% of taste buds were lost 20 days after nerve transection. Thus, mice appear to be more like gerbils in their nerve dependence than hamsters and rats. Unlike the circumvallate papilla, where complete taste bud loss occurred after denervation, neural dependence in mice fungiform taste buds was far from complete. Although it has been suggested this is due, at least in rats, to unidentified innervation (Hård af Segerstad et al., 1989; Oakley et al., 1993), the current study found almost no innervation in empty papilla or papilla with small, abnormal taste buds at 10 days posttransection. While this highlights one of the most interesting findings related to an apparent lack of neural dependence of taste buds, especially in the anteriormost portion of the mouse tongue, we cannot rule out the possibility that undetected neurons were present, supporting remnant taste buds.
Possible methodological differences in taste bud markers
Another potential reason that could account for differences between the current findings in mice and results seen in other species is the way taste buds are categorized and measured. As noted above, early studies that examined taste bud changes following taste nerve transections used hematoxylin and eosin stains. It is relatively straightforward to determine the boundaries of the central portion of the taste bud in hematoxylin-stained tissue because of the characteristic location of cell nuclei at the base of the taste bud and the translucent appearance of the apical portion (see Fig. 8A). However, the borders of taste buds become more ambiguous at the lateral margins of the taste bud (similar in appearance to Fig. 8C). In contrast, this ambiguity does not occur with the use of the cytokeratin antibody label as done here, but there is no assurance that both methods can be used to obtain similar measures of taste bud counts and taste bud volumes.
Fig. 8.
A fungiform papilla containing a taste bud (A,B) and another papilla containing a remnant or atrophic taste bud (C,D). Panels A and C were stained with hematoxylin and eosin and panels B and D were stained with the cytokeratin 8 antibody. The solid outline in A denotes the position of the taste bud as estimated from hematoxylin and eosin stained tissue. A similar structure of the same taste bud is also shown in B. Note that the papilla in C does not contain slender cells and nuclei in a position characteristic of taste buds, and that there are no immunopositive cells labeled in D. The label shown in D was the only immunopositive label in the entire papilla (magenta) and may represent remnant processes of the original taste bud; this papillae would be scored as not containing a taste bud. Scale bar = 20 µm.
To address this potential problem, we took tissue stained with cytokeratin 8 from a mouse 10 days after nerve transection and photographed fungiform taste buds ipsilateral and contralateral to the transection. We then stained the tissue for hematoxylin and eosin, as done by others (e.g., Oakley et al., 1993), and compared the appearance with the cytokeratin 8-stained image (Fig. 8). Of the 22 fungiform papillae examined, all cytokeratin 8-stained taste buds were similar in appearance and size to hematoxylin and eosin-stained taste buds. Specifically, the borders of taste buds stained with hematoxylin and eosin, especially in the center of the taste bud, were very similar to those identified with the cytokeratin 8 antibody (Fig. 8A,B). Conversely, a lack of the characteristic taste bud appearance in fungiform papillae (i.e., elongated cells with nuclei in the base) on the sectioned side of the tongue, as identified with hematoxylin and eosin staining, was matched with an absence of cellular staining with the cytokeratin 8 antibody (Fig. 8C,D). As an example, Figure 8C shows a section through the center of a taste bud that would be classified as a remnant or atrophic taste bud (Oakley et al., 1993), primarily because of the location of nuclei and lack of slender cells. Cytokeratin staining (Fig. 8D) also was not apparent for this papillae, with the exception of a small amount of staining in the apical portion of the papilla. For intact taste buds the primary difference between methods was that the fine characteristics of the taste buds and the margins were much easier to distinguish with the use of the cytokeratin 8 antibody (Fig. 8A,B); volumes were similar between taste buds stained with the two methods. Therefore, we believe that our measurements accurately identify taste buds at least as well as past methods, but have the added benefit of unambiguously identifying taste bud cells for additional measurements. Finally, to emphasize the confidence in using the antibody staining method, cytokeratin staining was used by Oakley et al. (1993), to complement the characterization of taste bud morphology with hematoxylin staining following nerve transection in rat and used more recently to exclusively examine taste bud volumes and morphology in mice with the bax gene deleted (Zeng et al., 2000).
SUMMARY
The gustatory system remains an excellent model system to examine nerve/target relationships. The findings presented here demonstrate that the mouse is an appropriate animal model to study these relationships and are similar to other rodent models in that there is a clear degeneration of fungiform taste buds following loss of innervation and with generally the same time course as seen in other rodents. However, there are clear distinctions that make the mouse an especially attractive model: a clear difference in susceptibility to axotomy between tip and mid-region of the anterior tongue and the ability to manipulate relevant genes. With the advent of recombinant DNA technology, the mouse model offers a genetic means to study the cellular and molecular mechanisms involved in regulation of cell turnover and nerve-target dependence in the gustatory system.
ACKNOWLEDGMENT
We thank Mr. William Goodwin for his tireless effort and patience sectioning mouse tongues.
Grant sponsor: National Institutes of Health (NIH); Grant number: DC006938.
LITERATURE CITED
- Agerman K, Hjerling-Leffler J, Blanchard MP, Scarfone E, Canlon B, Nosrat C, Ernfors P. BDNF gene replacement reveals multiple mechanisms for establishing neurotrophin specificity during sensory nervous system development. Development. 2003;130:1479–1491. doi: 10.1242/dev.00378. [DOI] [PubMed] [Google Scholar]
- Brulet P, Babinet C, Kemler R, Jacob F. Monoclonal antibodies against trophectroderm-specific markers during mouse blastocyst formation. Proc Natl Acad Sci U S A. 1980;77:4113–4117. doi: 10.1073/pnas.77.7.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain P, Frank ME, Barry MA. Recovery of chorda tympani nerve function following injury. Exp Neurol. 1996;141:337–346. doi: 10.1006/exnr.1996.0169. [DOI] [PubMed] [Google Scholar]
- Cavallin MA, McCluskey LP. Lipopolysaccharide-induced upregulation of activated macrophages in the degenerating taste system. J Neurosci Res. 2005;80:75–84. doi: 10.1002/jnr.20438. [DOI] [PubMed] [Google Scholar]
- Cheal M, Oakley B. Regeneration of fungiform taste buds: temporal and spatial characteristics. J Comp Neurol. 1977;172:609–626. doi: 10.1002/cne.901720405. [DOI] [PubMed] [Google Scholar]
- Cheal M, Dickey WP, Jones LB, Oakley B. Taste fiber responses during reinnervation of fungiform papillae. J Comp Neurol. 1977;172:627–646. doi: 10.1002/cne.901720406. [DOI] [PubMed] [Google Scholar]
- Farbman AI. Fine structure of degenerating taste buds after denervation. J Embryol Exp Morphol. 1969;22:55–68. [PubMed] [Google Scholar]
- Farbman AI. Renewal of taste bud cells in rat circumvallate papillae. Cell Tissue Kinet. 1980;13:349–357. doi: 10.1111/j.1365-2184.1980.tb00474.x. [DOI] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- Fischer D, Pavlidis M, Thanos S. Cataractogenic lens injury prevents traumatic ganglion cell death and promotes axonal regeneration both in vivo and in culture. Invest Ophthalmol Vis Sci. 2000;41:3943–3954. [PubMed] [Google Scholar]
- Fischer D, Heiduschka P, Thanos S. Lens-injury-stimulated axonal regeneration throughout the optic pathway of adult rats. Exp Neurol. 2001;172:257–272. doi: 10.1006/exnr.2001.7822. [DOI] [PubMed] [Google Scholar]
- Ganchrow JR, Ganchrow D. Long-term effects of gustatory neurectomy on fungiform papillae in the young rat. Anat Rec. 1989;225:224–231. doi: 10.1002/ar.1092250308. [DOI] [PubMed] [Google Scholar]
- Hård af Segerstad CH, Farbman AI, Hellekant G. Changes in number and morphology of fungiform taste buds after transection of the chorda tympani or chorda-lingual nerve. Chem Senses. 1989;14:335–348. [Google Scholar]
- Hendricks SJ, Brunjes PC, Hill DL. Taste bud cell dynamics during normal and sodium-restricted development. J Comp Neurol. 2004;472:173–182. doi: 10.1002/cne.20064. [DOI] [PubMed] [Google Scholar]
- Karlsson JE, Rosengren LE, Haglid KG. A rapid HPLC method to separate the triplet proteins of neurofilament. J Neurochem. 1987;49:1375–1378. doi: 10.1111/j.1471-4159.1987.tb01002.x. [DOI] [PubMed] [Google Scholar]
- Karlsson JE, Rosengren LE, Haglid KG. Polyclonal antisera to the individual neurofilament triplet proteins: a characterization using ELISA and immunoblotting. J Neurochem. 1989;53:759–765. doi: 10.1111/j.1471-4159.1989.tb11770.x. [DOI] [PubMed] [Google Scholar]
- Kinnman E, Aldskogius H. Collateral reinnervation of taste buds after chronic sensory denervation: a morphological study. J Comp Neurol. 1988;270:569–574. doi: 10.1002/cne.902700410. [DOI] [PubMed] [Google Scholar]
- Knapp L, Lawton A, Oakley B, Wong L, Zhang C. Keratins as markers of differentiated taste cells of the rat. Differentiation. 1995;58:341–349. doi: 10.1046/j.1432-0436.1995.5850341.x. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Wall PD, McMahon SB. Does the right side know what the left is doing? Trends Neurosci. 1999;22:122–127. doi: 10.1016/s0166-2236(98)01302-2. [DOI] [PubMed] [Google Scholar]
- Krimm RF, Hill DL. Innervation of single fungiform taste buds during development in rat. J Comp Neurol. 1998;398:13–24. [PubMed] [Google Scholar]
- Krimm RF, Hill DL. Neuron/target matching between chorda tympani neurons and taste buds during postnatal rat development. J Neurobiol. 2000;43:98–106. [PubMed] [Google Scholar]
- Krimm RF, Miller KK, Kitzman PH, Davis BM, Albers KM. Epithelial overexpression of BDNF or NT4 disrupts targeting of taste neurons that innervate the anterior tongue. Dev Biol. 2001;232:508–521. doi: 10.1006/dbio.2001.0190. [DOI] [PubMed] [Google Scholar]
- LeMaster AM, Krimm RF, Davis BM, Noel T, Forbes ME, Johnson JE, Albers KM. Overexpression of brain-derived neurotrophic factor enhances sensory innervation and selectively increases neuron number. J Neurosci. 1999;19:5919–5931. doi: 10.1523/JNEUROSCI.19-14-05919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI. Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci. 2000;20:4615–4626. doi: 10.1523/JNEUROSCI.20-12-04615.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebl DJ, Mbiene JP, Parada LF. NT4/5 mutant mice have deficiency in gustatory papillae and taste bud formation. Dev Biol. 1999;213:378–389. doi: 10.1006/dbio.1999.9385. [DOI] [PubMed] [Google Scholar]
- Liem RK, Hutchison SB. Purification of individual components of the neurofilament triplet: filament assembly from the 70 000-dalton subunit. Biochemistry. 1982;21:3221–3226. doi: 10.1021/bi00256a029. [DOI] [PubMed] [Google Scholar]
- McCluskey LP. Up-regulation of activated macrophages in response to degeneration in the taste system: effects of dietary sodium restriction. J Comp Neurol. 2004;479:43–55. doi: 10.1002/cne.20307. [DOI] [PubMed] [Google Scholar]
- McCluskey LP, Hill DL. Sensitive periods for the effect of dietary sodium restriction on intact and denervated taste receptor cells. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1275–R1284. doi: 10.1152/ajpregu.00282.2002. [DOI] [PubMed] [Google Scholar]
- Mistretta CM, Goosens KA, Farinas I, Reichardt LF. Alterations in size, number, and morphology of gustatory papillae and taste buds in BDNF null mutant mice demonstrate neural dependence of developing taste organs. J Comp Neurol. 1999;409:13–24. [PMC free article] [PubMed] [Google Scholar]
- Nosrat CA, Blomlof J, ElShamy WM, Ernfors P, Olson L. Lingual deficits in BDNF and NT3 mutant mice leading to gustatory and somatosensory disturbances, respectively. Development. 1997;124:1333–1342. doi: 10.1242/dev.124.7.1333. [DOI] [PubMed] [Google Scholar]
- Nosrat IV, Agerman K, Marinescu A, Ernfors P, Nosrat CA. Lingual deficits in neurotrophin double knockout mice. J Neurocytol. 2004;33:607–615. doi: 10.1007/s11068-005-3330-2. [DOI] [PubMed] [Google Scholar]
- Oakley B, Wu LH, Lawton A, deSibour C. Neural control of ectopic filiform spines in adult tongue. Neuroscience. 1990;36:831–838. doi: 10.1016/0306-4522(90)90026-z. [DOI] [PubMed] [Google Scholar]
- Oakley B, Lawton A, Riddle DR, Wu LH. Morphometric and immunocytochemical assessment of fungiform taste buds after interruption of the chorda-lingual nerve. Microsc Res Tech. 1993;26:187–195. doi: 10.1002/jemt.1070260302. [DOI] [PubMed] [Google Scholar]
- Ringstedt T, Ibanez CF, Nosrat CA. Role of brain-derived neurotrophic factor in target invasion in the gustatory system. J Neurosci. 1999;19:3507–3518. doi: 10.1523/JNEUROSCI.19-09-03507.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotshenker S, Tal M. The transneuronal induction of sprouting and synapse formation in intact mouse muscles. J Physiol. 1985;360:387–396. doi: 10.1113/jphysiol.1985.sp015623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuler MG, Krimm RF, Hill DL. Neuron/target plasticity in the peripheral gustatory system. J Comp Neurol. 2004;472:183–192. doi: 10.1002/cne.11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Oakley B. Development of anterior gustatory epithelia in the palate and tongue requires epidermal growth factor receptor. Dev Biol. 2002;242:31–43. doi: 10.1006/dbio.2001.0526. [DOI] [PubMed] [Google Scholar]
- Sun FJ, Wright DE, Pinson DM. Comparison of ketamine versus combination of ketamine and medetomidine in injectable anesthetic protocols: chemical immobilation in macaques and tissue reaction in rats. Contemp Top Lab Anim Med. 2003;42:32–37. [PubMed] [Google Scholar]
- Takeda M, Suzuki Y, Obara N, Nagai Y. Apoptosis in mouse taste buds after denervation. Cell Tissue Res. 1996;286:55–62. doi: 10.1007/s004410050674. [DOI] [PubMed] [Google Scholar]
- Takeda M, Suzuki Y, Obara N, Nagai Y. Induction of apoptosis by colchicine in taste bud and epithelial cells of the mouse circumvallate papillae. Cell Tissue Res. 2000;302:391–395. doi: 10.1007/s004410000294. [DOI] [PubMed] [Google Scholar]
- Uchida N, Kanazawa M, Suzuki Y, Takeda M. Expression of BDNF and TrkB in mouse taste buds after denervation and in circumvallate papillae during development. Arch Histol Cytol. 2003;66:17–25. doi: 10.1679/aohc.66.17. [DOI] [PubMed] [Google Scholar]
- Whitehead MC, Frank ME, Hettinger TP, Hou LT, Nah HD. Persistence of taste buds in denervated fungiform papillae. Brain Res. 1987;405:192–195. doi: 10.1016/0006-8993(87)91008-0. [DOI] [PubMed] [Google Scholar]
- Whitehead MC, Ganchrow JR, Ganchrow D, Yao B. Organization of geniculate and trigeminal ganglion cells innervating single fungiform taste papillae: a study with tetramethylrhodamine dextran amine labeling. Neuroscience. 1999;93:931–941. doi: 10.1016/s0306-4522(99)00115-3. [DOI] [PubMed] [Google Scholar]
- Yee CL, Jones KR, Finger TE. Brain-derived neurotrophic factor is present in adult mouse taste cells with synapses. J Comp Neurol. 2003;459:15–24. doi: 10.1002/cne.10589. [DOI] [PubMed] [Google Scholar]
- Yee C, Bartel DL, Finger TE. Effects of glossopharyngeal nerve section on the expression of neurotrophins and their receptors in lingual taste buds of adult mice. J Comp Neurol. 2005;490:371–390. doi: 10.1002/cne.20670. [DOI] [PubMed] [Google Scholar]
- Zeng Q, Kwan A, Oakley B. Gustatory innervation and bax-dependent caspase-2: participants in the life and death pathways of mouse taste receptor cells. J Comp Neurol. 2000;424:640–650. doi: 10.1002/1096-9861(20000904)424:4<640::aid-cne6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Zhang C, Cotter M, Lawton A, Oakley B, Wong L, Zeng Q. Keratin 18 is associated with a subset of older taste cells in the rat. Differentiation. 1995;59:155–162. doi: 10.1046/j.1432-0436.1995.5930155.x. [DOI] [PubMed] [Google Scholar]
- Zhang C, Brandemihl A, Lau D, Lawton A, Oakley B. BDNF is required for the normal development of taste neurons in vivo. Neuroreport. 1997;8:1013–1017. doi: 10.1097/00001756-199703030-00039. [DOI] [PubMed] [Google Scholar]