Abstract
Background and Purpose
FTY720 is a known sphingosine-1-phosphate (S1P) receptor agonist. In the present study we investigated the neuroprotective effect of postischemic administration of FTY720 in rats with 2 hours transient middle cerebral artery occlusion (MCAO).
Methods
One hundred eleven male rats were randomly assigned to sham-operated and MCAO treated with vehicle, 0.25mg/kg and 1mg/kg of FTY720, another selective S1P receptor-1 (S1P1) agonist SEW2871 (5mg/kg), or 0.25mg/kg of FTY720+ a S1P antagonist VPC23019 (0.5mg/kg). Drugs were injected intraperitoneally immediately after reperfusion. Neurological score and infarct volume were assessed at 24 and 72 hours after MCAO. Western blotting, immunohistochemistry, and Terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-biotin nick end-labeling (TUNEL) were conducted at 24 hours after MCAO.
Results
FTY720 significantly reduced infarct volume and improved neurological score at 24 and 72 hours after MCAO compared with the vehicle group. SEW2871 showed similar neuroprotective effects to FTY720, while VPC 20319 abolished the neuroprotective effects of FTY720. FTY720 significantly retained Akt and extracellular-signal regulated kinase phosphorylation and Bcl-2 expression, and decreased cleaved caspase-3 expression and TUNEL-positive neurons at 24 hours after MCAO. VPC23019 blocked the antiapoptotic effects of FTY720.
Conclusions
These data suggest that activation of S1P1 by FTY720 reduces neuronal death after transient MCAO.
Keywords: cerebral ischemia, FTY720, Sphingosine 1-phosphate receptor-1, apoptosis
Introduction
Sphingosine 1-phosphate (S1P) is a bioactive metabolic product of sphingolipids, generated by sphingosine kinase (SPHK). Recent studies have shown that S1P binds to the S1P family of G protein-coupled receptors and regulates multiple cellular events.1 FTY720 is a known agonist of S1P receptor-1 (S1P1), -3 (S1P3), -4 (S1P4), and -5 receptors (S1P5).2 FTY720 exerts immunomodulatory actions by affecting lymphocyte production3, trafficking4, and apoptosis5 via S1P receptors. FTY720 has been demonstrated to reduce ischemia reperfusion injury in kidney6 and liver7 through the S1P1 modulation causing a transient lymphopenia by a reversible redistribution of lymphocytes. However, it is not clear whether FTY720 possess neuroprotective effect via S1P1 in ischemic stroke.
In this study, we hypothesized that FTY720 may exert neuroprotection via S1P1 mediated antiapoptotic mechanisms in an experimental transient middle cerebral artery occlusion (MCAO) model in rats. We administered FTY720 intraperitoneally 2 hours after MCAO (immediately after reperfusion) and evaluated the extent of ischemic injury and apoptosis.
Materials and Methods
Experimental animals
All experiments were approved by the institutional Animal Care and Use Committee of Loma Linda University. One hundred eleven male Sprague-Dawley rats (Harlan, Indianapolis, Ind) weighing 330–400g were randomly divided into the following groups: preoperation (n=5), sham-operated (n=10), MCAO treated with vehicle (dimethyl sulfoxide [DMSO]: n=30), MCAO with low (n=30) and high (n=8) doses of FTY720, MCAO with SEW2871 (n=9) and MCAO with low dose of FTY720+VPC23019 (n=19).
MCAO Model
Anesthesia was induced with 4% isoflurane and maintained with 2.5% isoflurane, 30% oxygen, and 70% medical air via a face mask. Arterial blood gas analysis, mean arterial blood pressure, heart rate, and blood glucose before, during, and after MCAO were analyzed via the left femoral artery. The rectal temperature was monitored and kept at 37.0±0.5°C by using a feedback-regulated heating system during surgery.
Transient focal ischemia was induced by occluding the MCA using the intraluminal technique.8 Briefly, the left common carotid artery (CCA) was exposed and 3-0 nylon suture coated with poly-L-lysine was introduced into the left internal carotid artery through the CCA. After 2 hours the suture was withdrawn to allow MCA reperfusion. Neurological score was measured 10 min before reperfusion using a modification of the neurologic score of Bederson et al.9 Accordingly, grade 0 was recorded in the absence of observable deficits, grade 1 in the presence of forelimb flexion, grades 2 and 3 when there was decreased resistance to lateral push in the absence or presence of circling, respectively, and grade 4 was assigned to comatose animals. Rats with grades 0 and 4 were excluded from further experiments.
Administration of Drugs
Five groups of rats with transient MCAO received DMSO, low dose (0.25mg/kg; FTY group) and high dose (1mg/kg; FTY-high group) FTY720 in DMSO, a selective S1P1 agonist SEW2871 (5mg/kg; SEW group) in DMSO, or low dose FTY720+S1P1, S1P3, S1P4 antagonist VPC23019 (0.5mg/kg; VPC+FTY group) in DMSO/1N HCL (95:5) intraperitoneally immediately after reperfusion. The dissolving manner and dosage were followed as previously described:10,11 DMSO (1.1g/mL/kg) was used at a concentration ≤0.4mL.
Measurement of the area of early ischemic brain injury
Animals were decapitated 24 (n=8) and 72 (n=9) hours after MCAO. Their brains were cut into 2 mm-thick coronal slices using a rodent brain matrix. Six selected sections (±5 mm, ±3 mm, and ±1 mm from the bregma) were stained for 10 min in a 2% solution of 2,3,5-triphenyltetrazolium chloride at 37°C. The area of ischemic brain injury was measured by a blinded observer with Image J software (version 1.40; National Institutes of Health, Bethesda, MD). Infarct areas were corrected to compensate for edema formation by subtracting the area of the intact ipsilateral hemisphere from the area of the intact contralateral hemisphere. Then the infarct areas on each slice were added together and multiplied by slice thickness to give the infarct volume.
Neurological Scoring
A 25-point scoring system was used to evaluate the neurological deficit at 24 and 72 hours after MCAO in a blind fashion using a modification of the method described by Garcia et al. (1995).12
Western blot analysis
The left ischemic cortex in MCA region (MCA cortex) was obtained from healthy controls and 24hours after MCAO (n=5, respectively). Protein concentration was determined using DC protein assay (Bio-Rad, Hercules, CA). Individual samples (50μg each) were subjected to SDS-polyacrylamide gel electrophoresis, and then transferred to a nitrocellulose membrane for 80 min at 70V (Bio-Rad, Hercules, CA). Blotting membranes were incubated for 2 hours with 5% nonfat milk in Tris buffered saline containing 0.1% Tween 20 and then incubated overnight at 4°C with the following primary antibody: anti-phospho-extracellular-signal regulated kinase (ERK) 1/2, anti-caspase-3 (1:200, Santa Cruz Biotechnology, Santa Cruz, CA), anti-phospho-Akt (Ser473) and anti-Bcl-2 (1:1000, Cell Signaling Technology, Danvers, MA) antibodies. The membranes were incubated for 1 hour with secondary antibodies (1:2000, Santa Cruz Biotechnology, Santa Cruz, CA) and processed with enhanced chemiluminescence reagent kit (ECL plus kit; Amersham Bioscience, Arlington Heights, IL). The images were scanned and analyzed semiquantitatively in a blind fashion using the Image J software. Changes in phosphorylation of Akt-Ser-473 and ERK-1, and expression of Bcl-2 and 17kDa (cleaved) caspase-3 were expressed as a percentage of the preoperative level. β-actin (Santa Cruz Biotechnology, Santa Cruz, CA) was used as an internal control for every experiment.
Histology
Samples from sham-operated, vehicle, FTY, and VPC+FTY groups (n=5, respectively) were used for experiments. At 24 hours after MCAO, the brains were fixed by cardiovascular perfusion with phosphate-buffered saline and 10% paraformaldehyde. The brains were quickly removed and postfixed in 10% paraformaldehyde followed by 30% sucrose (weight/volume) for 3 days. Ten-micron-thick coronal sections at the level of bregma+1 mm (rostrally) were cut on cryostat (Leica Microsystems LM3050S) and mounted on poly-L-lysine-coated slides.
Immunofluorescence staining
Double–fluorescence labeling was performed at 24 hours after MCAO as described previously.13 The following primary antibodies were used: 1) anti-S1P1 antibody (1:100, Cayman Chemical, Ann Arbor, MI), 2) anti-phospho-Akt (Ser473) antibody IHC-specific (1:100, Cell Signaling Technology, Danvers, MA), and 3) anti-NeuN antibody (1:200; Chemicon International, Temecula, CA). For negative controls the primary antibodies were omitted and the same staining procedures were followed.
Terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-biotin nick end-labeling (TUNEL) staining
We immunostained brain sections with anti-NeuN antibodies and then subjected the sections to TUNEL staining with an in situ cell death detection kit (Roche Inc., Mannheim, Germany). A mixture of FITC-labeled nucleotides and terminal deoxynucleotidyl transferase was applied onto brain sections for 60 min at 37°C in a dark humidified chamber as previously described.14 Incubation with labeling solution without the enzyme served as a negative labeling control. The apoptotic neurons were counted in the periinfarct cortex (three fields were counted in each case at ×400 magnification) in a blinded manner. The number of cells was expressed as cells per mm2.
Statistical Analysis
All values are expressed as the mean±SD. Statistical differences among the various groups were assessed with one-way analysis of variance followed by a Tukey-Kramer post hoc analysis. The differences between the two groups were compared using unpaired t test. Differences of P<0.05 were considered significant.
Results
Mortality
The mortality rate was as follows: 10% (3 of 30 rats) in the vehicle group and 10% (3 of 30 rats) in the FTY group within 72 hours, and 11% (1 of 9 rats) in the SEW group and 5% (1 of 19 rats) in the VPC+FTY group within 24 hours. No rats in the sham-operated and FTY-high groups died.
Physiological parameters
No statistical differences were observed among the groups with regard to physiological parameters (Table 1).
Table 1.
Arterial blood gas analysis, mean arterial blood pressure (BP), heart rate (HR), and blood glucose (BG) before, during, and after MCAO.
| Group | pH | PO2 (mmHg) | PCO2 (mmHg) | BP (mmHg) | HR (/min) | BG (mg/dl) |
|---|---|---|---|---|---|---|
| Before MCAO | ||||||
| DMSO | 7.41±0.04 | 209±8 | 41±4 | 136±10 | 317±16 | 266±32 |
| FTY | 7.41±0.03 | 213±7 | 41±4 | 139±9 | 311±11 | 260±21 |
| FTY-high | 7.40±0.04 | 200±26 | 41±3 | 139±7 | 322±16 | 261±20 |
| SEW | 7.42±0.03 | 212±9 | 42±3 | 138±9 | 315±16 | 274±34 |
| VPC | 7.44±0.01 | 200±22 | 40±3 | 139±9 | 324±20 | 269±30 |
| During MCAO | ||||||
| DMSO | 7.37±0.06 | 207±18 | 36±6 | 135±13 | 316±26 | 188±33 |
| FTY | 7.33±0.06 | 226±20 | 41±6 | 130±11 | 317±21 | 198±33 |
| FTY-high | 7.35±0.07 | 230±21 | 42±4 | 133±16 | 313±22 | 205±46 |
| SEW | 7.34±0.05 | 210±17 | 37±6 | 130±11 | 323±19 | 193±28 |
| VPC | 7.36±0.06 | 218±29 | 37±7 | 125±11 | 323±24 | 220±41 |
| After MCAO | ||||||
| DMSO | 7.38±0.07 | 204±5 | 33±3 | 134±15 | 302±37 | 179±33 |
| FTY | 7.39±0.07 | 206±7 | 34±2 | 127±13 | 315±29 | 207±45 |
| FTY-high | 7.40±0.06 | 203±11 | 34±2 | 131±15 | 306±34 | 213±45 |
| SEW | 7.39±0.07 | 206±6 | 33±3 | 128±13 | 315±29 | 196±43 |
| VPC | 7.41±0.05 | 199±6 | 32±2 | 135±11 | 326±24 | 208±37 |
Values are the mean±SD. There are no significant differences among the groups.
Infarct volume and neurological score
The neurological score before reperfusion was not significantly different among the groups (data not shown). The total infarct volume in the FTY group was significantly smaller than in the vehicle group at both 24 (105±61 mm3 vs. 225±94 mm3; Fig. 1A) and 72 (119±84 mm3 vs. 198±68 mm3; Fig. 2A) hours after MCAO. This reduction of infarct size was derived from reduced infarct volume in the cortex (63±62 mm3 vs. 141±60 mm3; Fig. 2B), but not in subcortex (57±27 mm3 vs. 57±18 mm3; Fig. 2C) at 72 hours after MCAO. A significant improvement in neurological score was observed between the FTY and vehicle groups at both 24 (14±4 vs. 10±2; Fig. 1B) and 72 (17±4 vs.11±4; Fig. 2D) hours after MCAO. FTY-high group had a similar neuroprotective effect compared with FTY group at 24 hours after MCAO (108±62 mm3 and 14±4, respectively; Fig. 1A and B).
Figure 1.
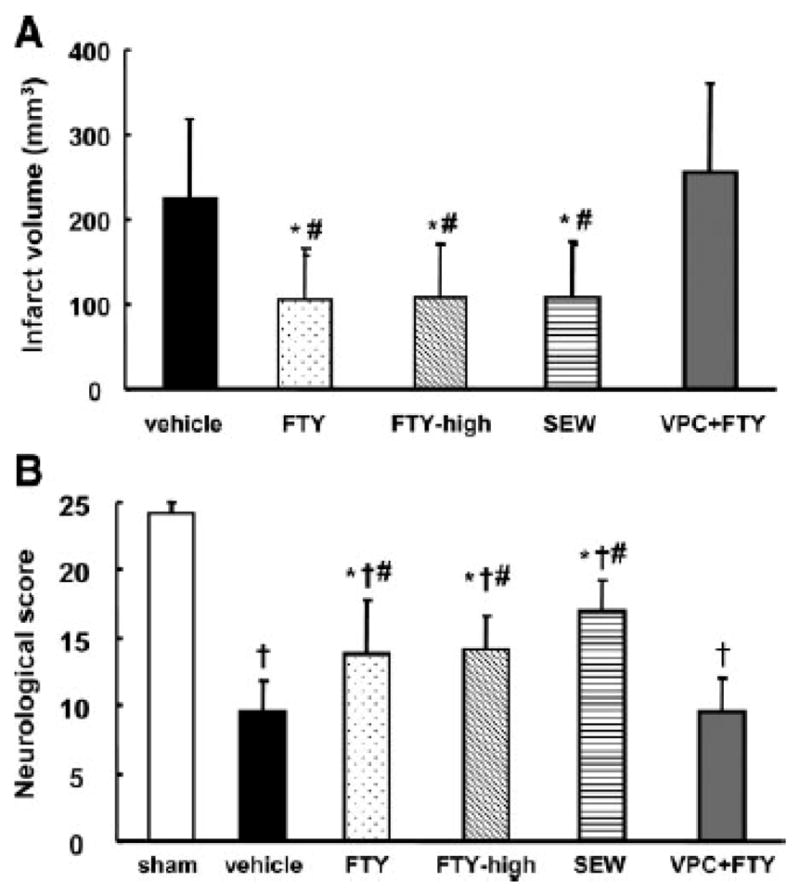
Quantitative analysis of total infarct volume (A) and neurological score (B) in groups treated with vehicle (n=8), 0.25mg/kg of FTY720 (FTY; n=8), 1mg/kg of FTY720 (FTY-high; n=8), 5mg/kg of SEW2871 (SEW; n=8), 0.5mg/kg of VPC23019+0.25mg/kg of FTY720 (VPC+FTY; n=8), and/or sham-operation (sham; n=5) at 24 hours after MCAO. Values are the mean±SD; †P<0.05 vs. sham, *P<0.05 vs. vehicle, #P<0.05 vs. VPC+FTY, ANOVA.
Figure 2.
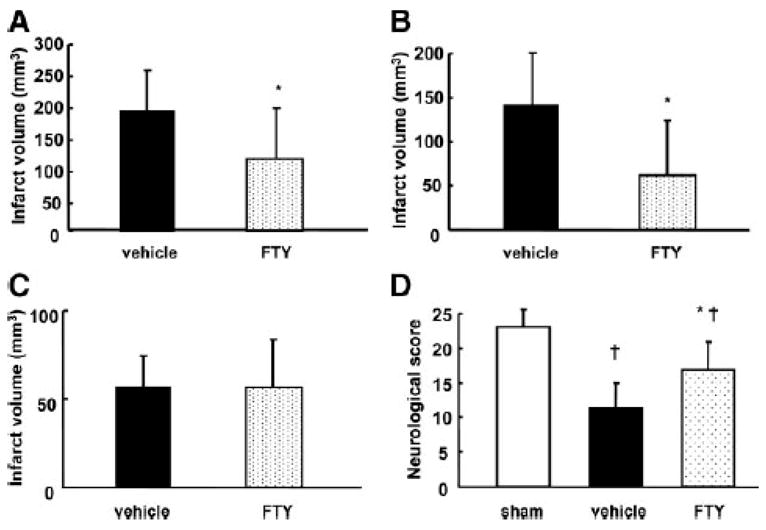
Quantitative analysis of infarct volume (A, total; B, cortex; C, subcortex) and neurological score (D) in groups treated with vehicle (n=9), 0.25mg/kg of FTY720 (FTY; n=9), and/or sham-operation (sham; n=5) at 72 hours after MCAO. Values are the mean±SD; †P<0.05 vs. sham, *P<0.05 vs. vehicle, unpaired t test or ANOVA.
To examine whether FTY has a neuroprotective effect via S1P1, we evaluated the effects of S1P1 related drugs. A selective S1P1 agonist SEW2871 showed similar neuroprotective effects to FTY720 in terms of infarct volume and neurological score (109±65 mm3, 17±2, respectively; Fig. 1A and B). On the other hand, a S1P antagonist VPC23019 abolished these protective effects by FTY720 (254±107 mm3, 10±3, respectively; Fig. 1A and B).
Phosphorylation of Akt and ERK and expression of Bcl-2 and caspase-3 after transient MCAO
To examine whether Akt and ERK underlies neuroprotection via S1P1 in the MCAO model, we measured phosphorylated Akt and ERK in the MCAcortex (Fig. 3A). Although phosphorylation of Akt (Fig. 3C) significantly decreased in the vehicle and VPC+FTY groups (24.5±18.7% and 31.5±13.8%, respectively), it was retained in the FTY group (130±63.2%).
Figure 3.
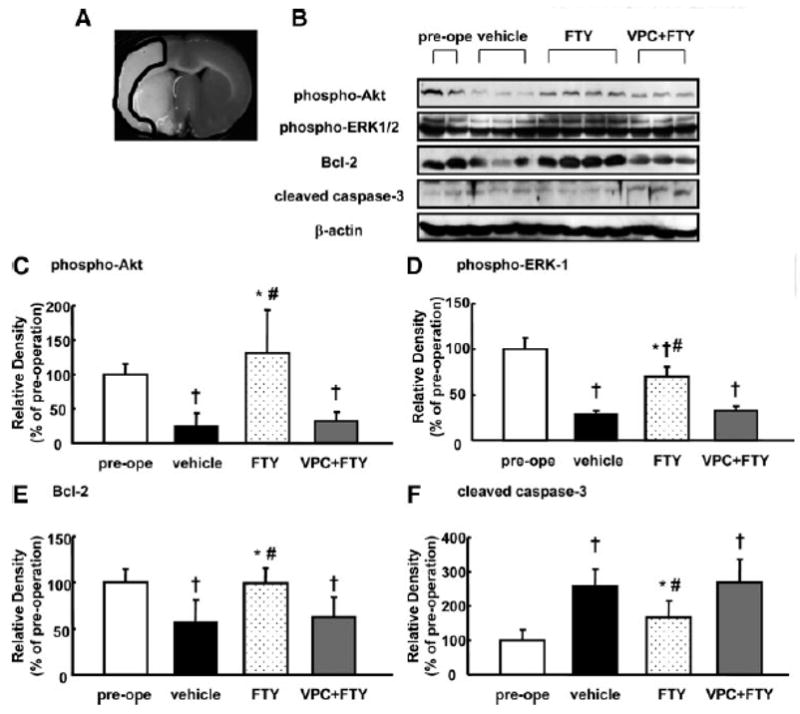
Changes in Akt and ERK phosphorylation, and Bcl-2 and cleaved caspase-3 expression in the infarct MCA region at 24 hours after MCAO (n=5). Representative ischemic brain (A) and Western blots (B), and quantitative analysis of Akt-Ser-473 (phosphor-Akt; C) and ERK-1 (phospho-ERK-1; D) phosphorylation and Bcl-2 (E) and 17kDa caspase-3 (cleaved caspase-3; F) expression in groups treated with vehicle, 0.25mg/kg FTY720 (FTY), 0.5mg/kg VPC23019+0.25mg/kg FTY720 (VPC+FTY) and preoperation (pre-ope). The band density values were calculated as a ratio of that of β-actin, and the values from the preoperation were used as 100%. Values are the mean±SD; †P<0.05 vs. sham, *P<0.05 vs. vehicle, #P<0.05 vs. VPC+FTY, ANOVA
Since the phosphorylation-related alterations were similar in ERK-1 (44kDa) and ERK-2 (42kDa) following ischemia,8 we focused on phosphorylation of ERK-1 in this study. Phosphorylation of ERK-1 (Fig. 3D) significantly decreased among all groups (vehicle; 28.6±4%, FTY; 70.2±10.4%, VPC+FTY; 32.7±4.6%), but the level in the FTY group was significantly preserved.
The Bcl-2 expression (Fig. 3E) significantly decreased in the vehicle and VPC+FTY groups (56.3±24.8% and 62.4±21.9%, respectively), while the administration of FTY retained the Bcl-2 level (99.7±16.3%).
On the other hand, the cleaved caspase-3 expression (Fig. 3F) significantly increased in the vehicle and VPC+FTY groups (260.2±47.4% and 269±66.4%, respectively), but FTY decreased the cleaved caspase-3 level (168±21.5%).
Immunohistochemical evaluations of S1P1, phosphorylated Akt and TUNEL in neurons
Immunofluorescence showed that S1P1 was expressed in neurons in periinfarct cortex at 24 hours after MCAO (Fig. 4A and B). In the periinfarct cortex, phosphorylated Akt in the FTY group was preserved better than in other groups (Fig. 4C). TUNEL-positive neurons were observed in the periinfarct cortex at 24 hours after MCAO (Fig. 5A) and a significant decrease in TUNEL-positive neurons was observed in the FTY group (425±194/mm2) compared with the vehicle (932±180 mm2) and VPC+FTY (936±228 mm2) groups (Fig. 5B). In the sham group, there were many phosphorylated Akt- and no TUNEL-positive neurons (data not shown).
Figure 4.
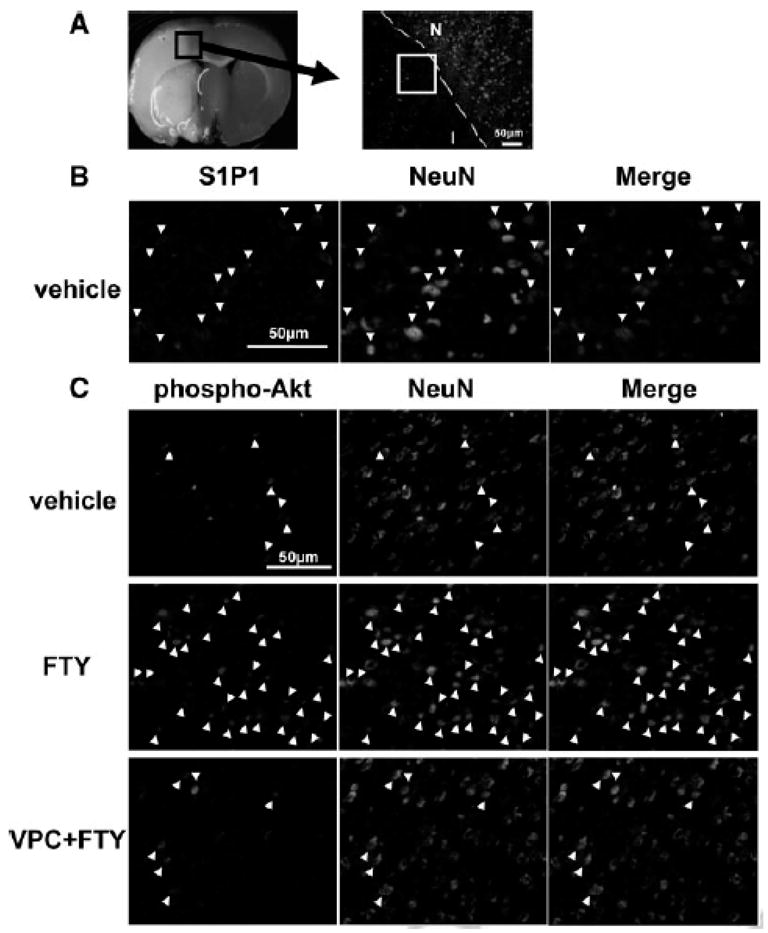
Representative ischemic brain and the area which is defined as periinfarct cortex (A) (stained by NeuN, I, infarct area; N, non-infarct area). Immunohistochemical colocalization of sphingosine-1-phosphate receptor-1 (S1P1; red, B) or phosphorylated Akt (phospho-Akt; red, C) with NeuN (green) positive cells (arrow head) in the periinfarct cortex at 24 hours after MCAO (n=5). Scale bar: 50μm
Figure 5.
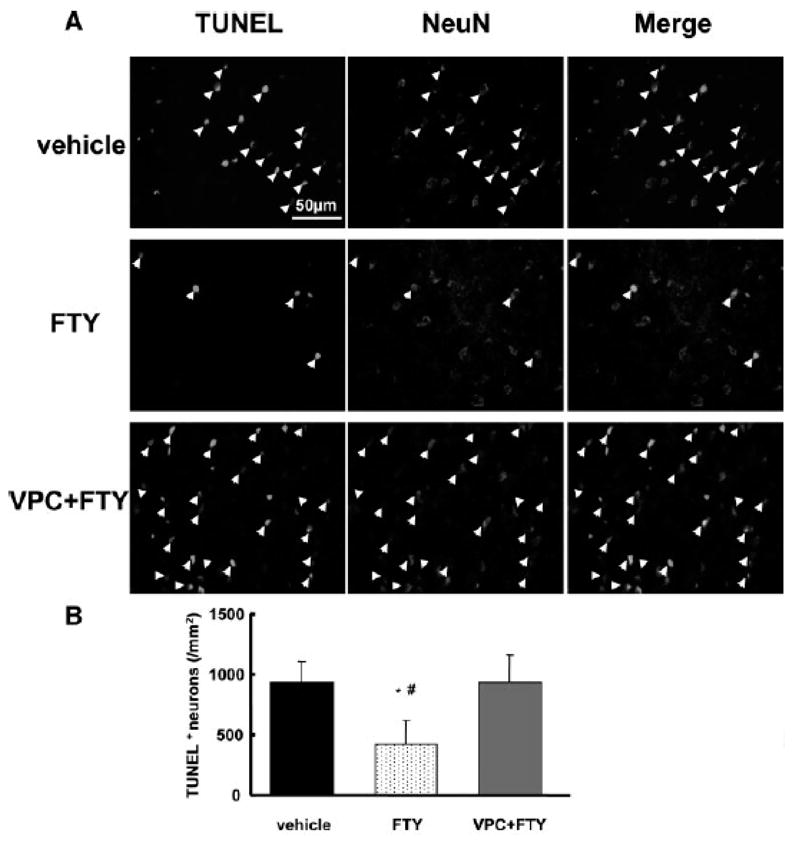
Evaluation of neuronal cell death in the periinfarct cortex at 24 hours after MCAO (n=5). A: Colocalization of TUNEL (green) and NeuN (red) positive cells (arrow head). B: Quantitative analysis of TUNEL positive neurons in the periinfarct cortex. Scale bar: 50μm, *P<0.05 vs. vehicle, #P<0.05 vs. VPC+FTY, ANOVA
Discussion
The present study demonstrated that activation of S1P1 is neuroprotective against cerebral ischemia. This conclusion is drawn from studies using FTY720 and SEW2871, two S1P1 agonists, and VPC23019, a S1P1 receptor antagonist. Moreover, this study showed that the neuroprotective effect of FTY720 is associated with deactivation of caspase-3, and activation of Akt and ERK.
FTY720 is rapidly phosphorylated in vivo by SPHK type-2 to form FTY720 phosphate, which behaves as a full agonist on S1P1, S1P4, and S1P5 at low nanomolar concentration.2 Neurons express mainly S1P1 and S1P3, with lower levels of S1P receptor-2 and no or negligible amounts of S1P5.15 Therefore, it can be speculated that FTY720 mainly acts as a S1P1 agonist in neurons. S1P1 is expressed on neuronal cell bodies and shows a widespread expression in the brain, with higher expression in the gray matter compared to the white matter.16 These findings correspond with our results that FTY720 significantly reduced infarct volume in the cortex but not in the subcortex.
Signal through S1P1 has been associated with activation of Akt and ERK.17 Akt activation is a principal factor in the prevention of apoptosis in animal models of cerebral ischemia and neuronal injury was correlated with Akt activity.8 In this study FTY720 decreased cleaved caspase-3 expression and TUNEL-positive cells, and phosphorylated Akt in neurons. So it is likely that Akt activation (phosphorylation) with FTY720 is a main factor to induce anti-apoptotic effect. On the other hand, whether activation of ERK is protective or detrimental to neurons is controversial. It is believed that elevated ERK phosphorylation plays a role in cell survival in the penumbra and ERK activity may block apoptosis by enhancing the level of the antiapoptotic protein Bcl-2 via CREB activation.18 In this study, ERK was dephosphorylated after MCAO but FTY720 maintained ERK phosphorylation and Bcl-2 expression compared with the vehicle and VPC+FTY groups. Therefore, we speculate that FTY720 activates Akt and ERK via S1P1 activation, rescuing neurons from cell death in periinfarct cortex affected by apoptotic mechanisms.
In contrast to its protective effects, FTY720 was shown to cause apoptosis in T-cell lines at micromolar concentrations.19 However, it is reported that the blood concentration range of FTY720 is lower than 100ng/mL when given to rats at 0.1 to 1 mg/kg.20 In fact, it is impossible for FTY720 to induce apoptotic cell death in lymphocytes at a dose range of 0.1 to 1 mg/kg in vivo.21,22 In our study, 0.25mg/kg and 1mg/kg of FTY720 showed similar neuroprotective effects. Therefore, it is safe to administer FTY720 less than 1mg/kg intraperitoneally.
S1P is considered to be a prosurvival lipid because of its involvement in cellular differentiation, proliferation, migration, cytoskeletal reorganization, cellular proliferation, and survival.1 S1P has been reported to decrease 3 days after cerebral ischemia,23 and therefore deceased S1P may contribute to apoptosis after cerebral ischemia. FTY720 crosses blood-brain barrier, and remains a long time in brain,24 and activates S1P1. Thus, FTY720 may compensate for the decreased endogenous S1P and induce antiapoptotic mechanisms against cerebral ischemia.
In this study, we demonstrated that FTY720 has an antiapoptotic effect against cerebral ischemia. On the other hand, FTY720 was reported to inhibit harmful lymphocytes infiltration and act protectively against ischemia-reperfusion injury in kidney6 and liver.7 In addition, the effects of FTY720 on cerebral blood flow during reperfusion has not been studied. In this regard, further studies in cerebral ischemia are needed.
It has been suggested that FTY720 downregulated S1P1 and inhibited lymphocyte egress from secondary lymphoid tissues, being considered as the basis for the therapeutic effect of FTY720.20 However, in some other cell types, for example endothelial cells, sustained treatment is suggested not to induce receptor downregulation, and the consequent continued signaling persists.25 We showed that S1P1 were sufficiently expressed in neurons in periinfarct cortex after MCAO in this study.
In conclusion, FTY720 reduced neuronal injury and improved neurobehavior after cerebral ischemia by activation of Akt and ERK via S1P1, which prevented apoptosis. Clinical trials for treatment of multiple sclerosis26 and renal transplantation27 have demonstrated the efficacy of FTY720, which could be a novel compound available for treatment of stroke patients.
Acknowledgments
Acknowledgements and Funding
This study was partially supported by grants (NS053407) from the National Institutes of Health to J.H.Z.
Footnotes
The authors report no conflicts of interest.
References
- 1.Singh IN, Hall ED. Multifaceted roles of sphingosine-1-phosphate: how does this bioactive sphingolipid fit with acute neurological injury? J Neurosci Res. 2008;86:1419–33. doi: 10.1002/jnr.21586. [DOI] [PubMed] [Google Scholar]
- 2.Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Lynch KR. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–7. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 3.Yagi H, Kamba R, Chiba K, Soga H, Yaguchi K, Nakamura M, Itoh T. Immunosuppressant FTY720 inhibits thymocyte emigration. Eur J Immunol. 2000;30:1435–44. doi: 10.1002/(SICI)1521-4141(200005)30:5<1435::AID-IMMU1435>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 4.Brinkmann V, Pinschewer DD, Feng L, Chen S. FTY720: altered lymphocyte traffic results in allograft protection. Transplantation. 2001;72:764–9. doi: 10.1097/00007890-200109150-00002. [DOI] [PubMed] [Google Scholar]
- 5.Jung CG, Kim HJ, Miron VE, Cook S, Kennedy TE, Foster CA, Antel JP, Soliven B. Functional consequences of S1P receptor modulation in rat oligodendroglial lineage cells. Glia. 2007;55:1656–67. doi: 10.1002/glia.20576. [DOI] [PubMed] [Google Scholar]
- 6.Troncoso P, Ortíz M, Martínez L, Kahan BD. FTY 720 prevents ischemic reperfusion damage in rat kidneys. Transplant Proc. 2001;33:857–9. doi: 10.1016/s0041-1345(00)02349-6. [DOI] [PubMed] [Google Scholar]
- 7.Anselmo DM, Amersi FF, Shen XD, Gao F, Katori M, Lassman C, Ke B, Coito AJ, Ma J, Brinkmann V, Busuttil RW, Kupiec-Weglinski JW, Farmer DG. FTY720 pretreatment reduces warm hepatic ischemia reperfusion injury through inhibition of T-lymphocyte infiltration. Am J Transplant. 2002;2:843–9. doi: 10.1034/j.1600-6143.2002.20906.x. [DOI] [PubMed] [Google Scholar]
- 8.Hasegawa Y, Hamada J, Morioka M, Yano S, Kawano T, Kai Y, Fukunaga K, Ushio Y. Neuroprotective effect of postischemic administration of sodium orthovanadate in rats with transient middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2003;23:1040–51. doi: 10.1097/01.WCB.0000085160.71791.3F. [DOI] [PubMed] [Google Scholar]
- 9.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–6. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 10.Awad AS, Ye H, Huang L, Li L, Foss FW, Jr, Macdonald TL, Lynch KR, Okusa MD. Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol. 2006;290:F1516–24. doi: 10.1152/ajprenal.00311.2005. [DOI] [PubMed] [Google Scholar]
- 11.Landeen LK, Dederko DA, Kondo CS, Hu BS, Aroonsakool N, Haga JH, Giles WR. Mechanisms of the negative inotropic effects of sphingosine-1-phosphate on adult mouse ventricular myocytes. Am J Physiol Heart Circ Physiol. 2008;294:H736–49. doi: 10.1152/ajpheart.00316.2007. [DOI] [PubMed] [Google Scholar]
- 12.Garcia JH, Liu KF, Ho KL. Neuronal necrosis after middle cerebral artery occlusion in Wistar rats progresses at different time intervals in the caudoputamen and the cortex. Stroke. 1995;26:636–42. doi: 10.1161/01.str.26.4.636. [DOI] [PubMed] [Google Scholar]
- 13.Tsubokawa T, Solaroglu I, Yatsushige H, Cahill J, Yata K, Zhang JH. Cathepsin and calpain inhibitor E64d attenuates matrix metalloproteinase-9 activity after focal cerebral ischemia in rats. Stroke. 2006;37:1888–94. doi: 10.1161/01.STR.0000227259.15506.24. [DOI] [PubMed] [Google Scholar]
- 14.Sun Y, Zhou C, Polk P, Nanda A, Zhang JH. Mechanisms of erythropoietin-induced brain protection in neonatal hypoxia-ischemia rat model. J Cereb Blood Flow Metab. 2004;24:259–70. doi: 10.1097/01.WCB.0000110049.43905.AC. [DOI] [PubMed] [Google Scholar]
- 15.Dev KK, Mullershausen F, Mattes H, Kuhn RR, Bilbe G, Hoyer D, Mir A. Brain sphingosine-1-phosphate receptors: implication for FTY720 in the treatment of multiple sclerosis. Pharmacol Ther. 2008;117:77–93. doi: 10.1016/j.pharmthera.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Chae SS, Proia RL, Hla T. Constitutive expression of the S1P1 receptor in adult tissues. Prostaglandins Other Lipid Mediat. 2004;73:141–50. doi: 10.1016/j.prostaglandins.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Kluk MJ, Hla T. Signaling of sphingosine-1-phosphate via the S1P/EDG-family of G-protein-coupled receptors. Biochim Biophys Acta. 2002;1582:72–80. doi: 10.1016/s1388-1981(02)00139-7. [DOI] [PubMed] [Google Scholar]
- 18.Sawe N, Steinberg G, Zhao H. Dual roles of the MAPK/ERK1/2 cell signaling pathway after stroke. J Neurosci Res. 2008;86:1659–69. doi: 10.1002/jnr.21604. [DOI] [PubMed] [Google Scholar]
- 19.Matsuda S, Minowa A, Suzuki S, Koyasu S. Differential activation of c-Jun NH2-terminal kinase and p38 pathways during FTY720-induced apoptosis of T lymphocytes that is suppressed by the extracellular signal-regulated kinase pathway. J Immunol. 1999;162:3321–6. [PubMed] [Google Scholar]
- 20.Chiba K. FTY720, a new class of immunomodulator, inhibits lymphocyte egress from secondary lymphoid tissues and thymus by agonistic activity at sphingosine 1-phosphate receptors. Pharmacol Ther. 2005;108:308–19. doi: 10.1016/j.pharmthera.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki S, Enosawa S, Kakefuda T, Shinomiya T, Amari M, Naoe S, Hoshino Y, Chiba K. A novel immunosuppressant, FTY720, with a unique mechanism of action, induces long-term graft acceptance in rat and dog allotransplantation. Transplantation. 1996;61:200–5. doi: 10.1097/00007890-199601270-00006. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki S, Li XK, Enosawa S, Shinomiya T. A new immunosuppressant, FTY720, induces bcl-2-associated apoptotic cell death in human lymphocytes. Immunology. 1996;89:518–23. doi: 10.1046/j.1365-2567.1996.d01-777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura A, Ohmori T, Kashiwakura Y, Ohkawa R, Madoiwa S, Mimuro J, Shimazaki K, Hoshino Y, Yatomi Y, Sakata Y. Antagonism of sphingosine 1-phosphate receptor-2 enhances migration of neural progenitor cells toward an area of brain. Stroke. 2008;39:3411–7. doi: 10.1161/STROKEAHA.108.514612. [DOI] [PubMed] [Google Scholar]
- 24.Meno-Tetang GM, Li H, Mis S, Pyszczynski N, Heining P, Lowe P, Jusko WJ. Physiologically based pharmacokinetic modeling of FTY720 (2-amino-2[2-(-4-octylphenyl)ethyl]propane-1,3-diol hydrochloride) in rats after oral and intravenous doses. Drug Metab Dispos. 2006;34:1480–7. doi: 10.1124/dmd.105.009001. [DOI] [PubMed] [Google Scholar]
- 25.Brinkmann V, Cyster JG, Hla T. FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am J Transplant. 2004;4:1019–25. doi: 10.1111/j.1600-6143.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 26.Kappos L, Antel J, Comi G, Montalban X, O’Connor P, Polman CH, Haas T, Korn AA, Karlsson G, Radue EW FTY720 D2201 Study Group. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355:1124–40. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- 27.Tedesco-Silva H, Mourad G, Kahan BD, Boira JG, Weimar W, Mulgaonkar S, Nashan B, Madsen S, Charpentier B, Pellet P, Vanrenterghem Y. FTY720, a novel immunomodulator: efficacy and safety results from the first phase 2A study in de novo renal transplantation. Transplantation. 2005;79:1553–60. [PubMed] [Google Scholar]


