Abstract
Sulfur mustard (SM), also known as mustard gas, is an alkylating compound used as a chemical weapon in World War I and by Iraqi forces against Iranians and indigenous Iraqi Kurds during the Iran-Iraq War of the 1980s. Although SM is a proven carcinogen there are conflicting views regarding the carcinogenicity of a single exposure. The present study characterizes lung cancers formed in mustard gas victims from the Iran-Iraq War.
Methods and Materials
Demographic information and tumor specimens were collected from 20 Iranian male lung cancer patients with single high-dose SM exposures during the Iran-Iraq war. Formalin fixed, paraffin-embedded lung cancers were analyzed by immunohistochemistry for p53 protein. In addition, DNA was extracted from the tissues, PCR amplified and sequenced to identify mutations in the p53 and KRAS genes associated with SM exposure.
Results
A relatively early age of lung cancer onset (ranging from 28 to 73 with a mean of 48) in mustard gas victims, particularly those in the non-smoking population (mean age of 40.7), may be an indication of a unique etiology for these cancers. Seven of the 20 patients developed lung cancer before the age of 40. Five of 16 cancers from which DNA sequence data was obtainable provided information on eight p53 mutations (within exons 5–8). These mutations were predominately G to A transitions; a mutation consistent with the DNA lesion caused by SM. Two of the lung cancers had multiple p53 point mutations, similar to results obtained from factory workers chronically exposed to mustard agent. No mutations were detected in the KRAS gene.
Discussion
The distinguishing characteristics of lung carcinogenesis in these mustard gas victims suggest that a single exposure may increase the risk of lung cancer development in some individuals.
Keywords: Mustard Gas, Lung, Cancer, Iran, P53
INTRODUCTION
The alkylating compound sulfur mustard (SM) has been employed as a chemical warfare agent since its first use in World War I (1). The most recent application of this weapon was during the Iran-Iraq conflict by the Iraqi Baathist government. During this war, which lasted from 1980–88, Iraqi dictator Saddam Hussein made frequent use of SM as a battlefield force multiplier and also extensively targeted unprotected civilians in Iran and within the Kurdish regions of Iraq (2). Over 50,000 survivors of SM attacks remain alive in Iran and as a group suffer from high rates of chronic illnesses, particularly inflammatory conditions of the respiratory system, skin and eye (3–5), which are the major target organs of SM. Latent respiratory syndromes include asthma, chronic bronchitis, bronchiectasis, bronchiolitis, bronchial stenosis, and sinusitis (3, 4). The low cost and ease with which SM may be manufactured, transported and deployed, along with negligible odor and ability to cause permanent injuries after a few seconds exposure have made it historically the most frequently used chemical weapon during military operations. A particularly insidious feature of SM has also increased its potential as a combat force multiplier: Tissue damage due to the agent typically does not appear for 16 hours or more after exposure. Hence civilians and Iranian troops with often heavy SM exposure, would assume that absence of symptoms after light, or no decontamination meant that the danger had passed. Such victims typically developed symptoms within a day, often full-body blistering, loss of most skin, deep tissue injury and organ failure (2). These characteristics also make future battlefield and terrorist use of this chemical a real threat. Nevertheless, there are few comprehensive studies on the long-term health consequences of SM exposure, a gap in medical knowledge with potential for significantly adverse impact on public health management of future mass casualties.
The enormous pool of Iranian SM victims, with carefully documented exposure and medical histories, therefore offer an unparalleled resource and opportunity for developing a mechanistic understanding of SM-associated chronic health problems. In the present report we examine selected clinical, pathological and genetic features of Iranian SM victims suffering from lung cancer as an element of a broader investigation to assess the potential carcinogenic risk associated with an acute SM exposure.
The long-term effects of SM exposure may develop as a result of two major processes. One pathway leading to chronic illness in SM victims occurs due to a failure of host immunoregulatory mechanisms to resolve inflammation triggered by the episulfonium ion. Here, high levels of inflammatory cytokines inhibit apoptosis of polymorphonuclear leukocytes, particularly neutrophils, thus prolonging their active state and amplifying damage done by these cells (6, 7). A second major pathway leading from SM exposure to disease can occur when the compound causes mutations in tumor suppressor and oncogenes, such as p53 or KRAS. SM is a known alkylating agent, and under conditions of chronic exposure, is a recognized carcinogen (8). The mechanism of SM-induced carcinogensis begins with cyclization of SM in the aqueous environment of a victim, to a highly reactive episulfonium ion which may alkylate DNA. If these are not repaired, these lesions can lead to nucleotide substitutions (9), most commonly the G to A transition (10). This mutation may inactivate tumor suppressor genes such as p53, and greatly increase susceptibility to lung cancer, as was observed in Japanese mustard gas factory workers chronically exposed to SM (11–13). Although chronic SM is a known carcinogen (14–17), there are conflicting views regarding the carcinogenicity of a single exposure (18–21). From a public health perspective, acute exposure is a much more relevant exposure scenario in either past and potential future military conflicts or terrorist attacks.
Studies of cancer incidence in survivor populations with single, high-dose SM exposures have thus far failed to demonstrate strong correlations between exposure and disease occurrence. Kang and Bulman followed the outcome of US veterans exposed to SM in World War II (21). Although analysis of these patients showed no significant increase in lung cancer risk, exposure incidences were relatively low and smoking habits were not considered. There is no study in the medical literature addressing the affect of a single, high-dose SM exposure on the long-term risk of lung cancer. Now, after two decades, Iranians with well-documented exposures to mustard agent in the 1980s can be studied to help clarify the lung cancer risk associated with a battlefield exposure to SM.
Multiple lines of evidence will be required before a causative link between acute mustard agent exposure and lung cancer development can be established. Ongoing epidemiological studies are being pursued in Iran to determine if mustard-exposed individuals are at greater risk for lung cancer development, but these efforts will require an extended period of time before a sufficient number of lung cancer cases develop, and may take years to complete. For the present study, we have collected data from Iranian SM victims with unambiguous, documented exposure histories, who eventually developed lung cancer. Records from the Iran-Iraq war also provide information on the precise exposure timeframes during the eight year conflict. Here, specific personal attributes of victims and molecular/genetic features of their cancers were examined to evaluate carcinogenic effect of SM exposure on lung tissue. These studies include a mutational analysis of two tumor suppressor genes: p53 and KRAS, both of which are known indicators of gene-environment interactions impacting cancer risk. In summary, our data support the view that a single exposure to mustard agent may trigger cancer development in some individuals.
METHOD AND MATERIALS
Patient Population
The present study was conducted using medical record data provided by Janbazan Medical and Engineering Research Center (JMERC) and archived paraffin-embedded lung tumor samples from pathology departments of major Iranian medical centers treating persons exposed to chemical weapons during the Iran-Iraq war. Here, data and samples were drawn from a subject population of 20 Iranian males with single, battlefield SM exposures resulting in acute and chronic symptoms including skin and respiratory injuries. All exposures occurred in the years 1982–1988 and subjects were subsequently diagnosed between the ages of 28 and 73 with three major forms of lung carcinoma (Table 1). Subjects were randomly selected from among deceased individuals for whom complete records and samples existed. Time intervals between exposure to the weapon and onset of disease ranged from 5–20 years. Subjects included 5 current or former smokers, 9 non-smokers and 6 with indeterminate histories of tobacco use (Table 1). We were unable to assemble a significant number of matched tumor samples from patients without a history of SM exposure and smoking that were preserved in a manner that allowed DNA extraction. We therefore used International Agency for Research on Cancer (IARC) database as our baseline for p53 mutational frequencies in lung tumors (8).
Table 1.
Biodata, p53 gene expression and mutational spectrum for 20 Iranian males exposed to sulfur mustard during military operations 1982–88.
| Subject | Age of onseta |
Latency interval (years)b |
Pathology results: tumor classification |
Smoking | P53 (IHC)c |
DNA sequence changed |
P53 gene exon no. |
P53 gene codon changee |
Predicted phenotypic changef |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 35 | 16 | Adeno-carcinoma | - | - | - | - | - | - |
| 2 | 33 | 10 | Muco-epidermal carcinoma | - | - | GCC→GTC | 5 | 161 | Ala→Alasilent) |
| 3 | 39 | 9 | Small cell carcinoma | - | + | - | - | - | - |
| 4 | 38 | 6 | Adeno-carcinoma | - | + | GAG→GGG GAG→GAA TGT→TAT |
5 5 7 |
171 180 238 |
Glu→Gly Glu→Glu (silent) Cys→Tyr |
| 5 | 51 | 16 | Adeno-carcinoma | - | + | - | - | - | - |
| 6 | 43 | 13 | Adeno-carcinoma | + | + | - | - | - | - |
| 7 | 67 | 11 | Adeno-carcinoma | + | _ | - | - | - | - |
| 8 | 55 | 15 | Small cell carcinoma | + | - | - | - | - | - |
| 9 | 53 | 5 | Small cell carcinoma | Unknown status | + | - | |||
| 10 | 28 | 7 | Small Cell carcinoma | _ | + | - | - | - | - |
| 11 | 73 | 14 | Poorly differentiated. carcinoma | + | + | - | - | - | -- |
| 12 | 47 | 12 | Adeno-carcinoma | Unknown status | _ | - | - | - | - |
| 13 | 40 | 20 | Small cell carcinoma | Unknown status | + | CGC→CAC | 5 | 156 | Arg-→His |
| 14 | 66 | 17 | Small cell carcinoma | + | + | CCC→CTC GGC→GTC |
5 7 |
151 245 |
Pro→Leu Gly→Val |
| 15 | 51 | 14 | Adeno-carcinoma | _ | + | * | |||
| 16 | 53 | 18 | Poorly dif. Carcinoma | _ | + | - | - | - | - |
| 17 | 54 | 16 | Adenocarcinoma | Unknown status | _ | Exons 5&8* | - | ||
| 18 | 38 | 18 | Adenocarcinoma | _ | + | Exons 5&8* | - | ||
| 19 | 35 | 18 | Small cell carcinoma | Unknown status | + | GAG→GTG | 8 | 285 | Glu→Val |
| 20 | 49 | 7 | Small cell carcinoma | Unknown status | _ | * | - |
Age at which cancer diagnosis was made and samples for the present study were collected.
Time interval between mustard exposure and cancer diagnosis (latency).
Detectable expression of p53 protein (immunoreactivity) in tumor samples.
Mutation detected in a particular tumor sample.
Codon number within cDNA p53 sequence used in the present study.
Predicted phenotypic alteration (amino acid change) are shown for formalin-fixed, paraffin-embedded tumor samples taken from each subject.
Samples in which DNA extraction was not possible.
Mustard exposure, inclusion and exclusion criteria
Mustard exposure in this study is defined as any contact with SM in liquid or vapor form, resulting in transient or permanent disability. This definition is based on standards developed in a comprehensive national survey accomplished during the timeframe 1997–2000 that established a uniform convention for designation of Iranian citizens with war-related chemical injuries. Under this convention, mustard exposure is defined as any contact with SM in liquid or vapor form, resulting in transient or permanent disability. Here the minimum threshold for SM-induced disability is defined according to known primary effects of the agent on its major target organs: eyes, skin and lungs. Threshold exposure definitions for each organ are as follows: Eye: edema and visible inflammation of ocular membranes; Skin: redness accompanied by obvious blistering; and Lung: edema accompanied by inflammation and either a productive cough, or hemoptysis in the form of bloody streaks or expectoration of clots. These criteria take into account the highly variable length of time and concentration ranges of SM that personnel are typically subjected to under battlefield conditions and make no attempt to correlate SM dosage with symptoms. Some estimation of SM dosage sustained by subjects of this study may nevertheless be estimated based on reference ranges of the agent known to produce particular outcomes. Acute exposure guideline levels (AEGLs) for SM have been developed by the U.S. National Advisory Committee (NAC). Exposure to SM at 0.60 milligrams/cubic meter (mg/m3) of air for 10 minutes; or 0.013 mg/m3 for 8 hours constitute the threshold level at which edema of the eyes, sensitivity to light, and eye irritation occur (22). These ocular symptoms also define the threshold level for SM exposure established by Janbazan organization. Therefore participants in this study were exposed to at least the level of SM identified by the NAC as needed to produce critical ocular symptoms. Patients participating in this study were selected on the basis of documented exposure to SM based on official certification from the Iranian Veteran’s Affairs organization (Janbazan). This documentation included records of medical treatment showing the type and extent of mustard-associated injury and/or disability. Patients with histories of serious major disease other than lung cancer were excluded from this study. This investigation was conducted under the approval of Janbazan organization’s ethics committee.
Tissue preparation and DNA Extraction
Lung cancer tissue was obtained from biopsies or surgical sample, fixed with formalin and embedded in paraffin. Neoplastic lesions from representative areas of 4–5 unstained slides containing 10 μm thick tissue slices were scraped into a microcentrifuge tube. After paraffin removal with xylene, tissues were rehydrated and the DNA was isolated using the PicoPure™ DNA extraction Kit (Arcturus Engineering Inc., Mountain View, CA), according to the manufacturer’s instructions.
DNA Amplification and Sequencing
The extracted DNA was amplified by the polymerase chain reaction (PCR), using a nested primer approach. The primer sets for P53 exons 5, 6, 7 and 8 are shown in Table 2. Initial amplification reactions yielded the target amplimer with a number of off-target products. The specific product was then selectively amplified using nested p53 primers positioned a few bases downstream of the initial primers. The cycling conditions were as following: denaturation at 95°C for 2 minutes, followed by 35 cycles with denaturation at 95°C for 30 seconds, annealing at 56.2°C (exons 5 and 8) or 64.4°C (exons 6 and 7) for 30 seconds, and then elongation at 74°C for 30 seconds. In the last cycle, the elongation step was extended to 10 minutes. Aliquots of the PCR products were examined by electrophoresis on 1.5% agarose gel containing ethidium bromide to determine whether a specific product was generated by the amplification. PCR products were prepared for sequencing by treatment of an 8 μl aliquot of the PCR reaction with 3 μL ExoSAP-IT (Amersham Biosciences) at 37°C for 15 min, followed by inactivation at 80°C for 15 min. Primer extension sequencing was performed by GENEWIZ, Inc (South Plainfield, NJ) using Applied Biosystems BigDye version 3.1 (Foster City, CA). The reactions were then run on Applied Biosystem’s 3730xl DNA Analyzer. All PCR products were sequenced in both directions. A double peak on the sequence chromatograph was considered as a potential point mutation, which was then confirmed by comparison to the opposite strand. Mutations were confirmed by re-amplification and single strand sequencing.
Table 2.
Primer sets for PCR amplification of lung tumor tissue p53 exons 5, 6, 7 and 8.
| Exon | PCR cycle | Strand | Sequence | Length (bp) | Optimal Annealing Temp (°C) |
|---|---|---|---|---|---|
| 5 | outer | Up-stream | cacttgtgccctgacttt | 18 | 56.2 |
| Down-Stream | cctggggaccctgggcaa | 18 | “ | ||
| inner | Up-stream | ttgtgccctgactttcaa | 18 | “ | |
| Down-stream | ggggaccctgggcaacca | 18 | “ | ||
| 6 | outer | Up-stream | cgacagggctggttgcccaggg | 22 | 64.5 |
| Down-Stream | agggccactgacaaccacc | 19 | “ | ||
| inner | Up-stream | cagggctggttgcccagggtcc | 22 | “ | |
| Down-Stream | gccactgacaaccaccctta | 20 | “ | ||
| 7 | outer | Up-stream | cttgccacaggtctccccaa | 20 | 64.5 |
| Down-Stream | aagcagaggctggggcacagcagg | 18 | “ | ||
| inner | Up-stream | gccacaggtctccccaaggc | 18 | “ | |
| Down-Stream | cagaggctggggcacagcaggcca | 22 | “ | ||
| 8 | outer | Up-stream | taggacctgatttccttactgcct | 22 | 56.2 |
| Down-Stream | tgaatctgaggcataactgca | 19 | “ | ||
| inner | Up-stream | gacctgatttccttactgcctctt | 22 | “ | |
| Down-Stream | atctgaggcataactgcaccct | 20 | “ |
The present study also analyzed the KRAS gene for presence of activating point mutations. The oligonucleotide primers used for the amplification of KRAS at codons 12 and 13 were:
Up-stream: 5′-GACTGAATATAAACTTGTGG-3′;
Down-stream: 5′-CTATTGTTGGATCATATTCG-3′.
The cycling conditions were as performed for p53 gene, except the annealing temperature was 55 °C. As indicated in the Results, none of our samples harbored a KRAS mutation.
Immunohistochemistry
Four-micrometer sections of paraffin-embedded tissue were cut and immunostained using the avidin-biotin-peroxidase complex (ABC) method (23). Sections were deparafinnized by baking at 60°C, rinsed with xylene and re-hydrated. Epitope retrieval was performed by boiling in 10 mM citrate buffer (pH 6.0) plus 0.05% Tween 20. Slides were then incubated in blocking solution (25% goat serum, 1% BSA, 0.1% cold fish gelatin, 0.1% Triton X-100, 0.05% Tween 20, 0.05% sodium azide, 10 mM PBS, ph 7.2) for 30 min and incubated in p53 DO-1 antibody (Santa Cruz Biotechnology) at 1:200 in primary antibody dilution buffer (1% BSA, 0.1% cold fish gelatin, 0.05% sodium azide, 10 mM PBS, pH 7.2) at 4°C overnight. Slides were rinsed in washing buffer (10 mM PBS, pH 7.2, 0.05% Tween 20), followed by peroxidase blocking with 3% H2O2 for 10 min. Slides were incubated with biotinylated secondary antibody at a 1:500 dilution in secondary antibody dilution buffer (10 mM PBS, pH 7.2, 0.05% sodium azide) for 30 min. Slides were rinsed in washing buffer followed by incubation with HRP-streptavidin (1:500) in HRP-Streptavidin dilution buffer (10 mM PBS, pH 7.2, 0.05% Thimerosal) for 30 min. Detection was carried out using DAB solution as per the manufacturer’s instructions. Slides were counterstained with hematoxylin dehydrated and mounted.
RESULTS
Subjects for the present study were 20 males exposed to a single, high dose of SM during military service in the timeframe 1982–88. All subjects were observed to experience acute and chronic symptoms of mustard toxicity including skin injury and respiratory difficulties and were included in this study based on the criteria described in the Methods and Materials. The distribution of subjects according to age, gender, smoking history, time lapse between exposure and lung cancer diagnosis, tumor pathology, p53 IHC and p53 sequencing data is shown in Table 1.
Of the cancers that could be accurately assessed pathologically, the most frequently occurring type of lung cancer was Adenocarcinoma (9/20), followed by Squamous Cell Carcinoma (SCC) (4/20) and Small Cell Carcinoma (3/20). In addition, a single case of mucoepidermal lung cancer was observed in a 33 year old individual. This type of lung cancer is rare, accounting for less than a few percent of all lung cancers.
Examination of the individuals’ age at lung cancer diagnosis shows ages ranging from 28 to 73, with seven of the 20 patients developing lung cancer before the age of 40. This finding was of interest, since lung cancer typically develops after the age of 50 and peaks at approximately 60 years of age (24). Overall, the mean age at diagnosis was 47.4 years. On average, the time lapse between SM exposure and lung cancer development was 13.1 years.
Although DNA extraction from lung tumor samples of SM victims was difficult due to variable tissue fixation procedures used in Iran, we were able to obtain information on the p53 mutational status in 18 of the subjects. Mutations identified and described in these studies were determined by comparison of experimental results with reference data of known normal human p53 sequences published in GenBank
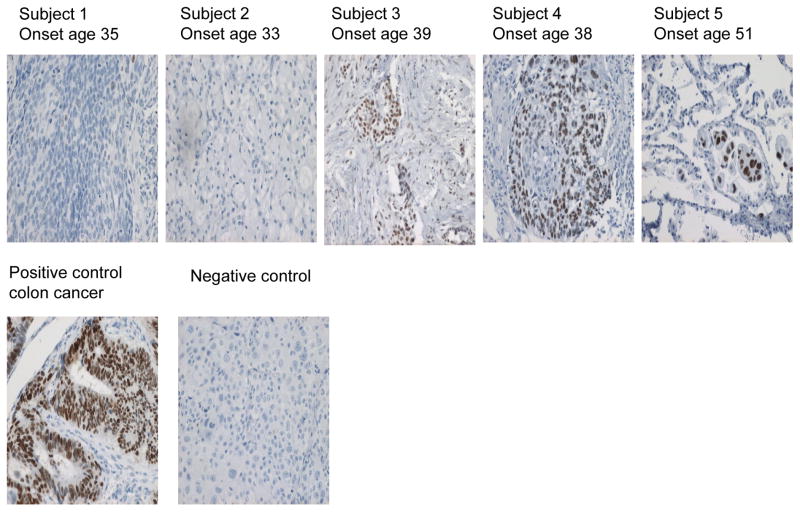
Table 1 shows the location and nature of the base change in these subjects, as well as the predicted consequence on the p53 protein. A total of eight mutations were identified. Five of these were G to A transitions, with single A to G, G to T and A to T changes also observed. The frequent G to A base change in these patients matched that most frequently observed mutation in chronically exposed factory workers (11). Interestingly, two of the cases had multiple point mutations (Table 1). Case 3 showed 3 point mutations: one silent mutation in codon 180 and 2 missense mutations in codons 171 and 238. In this case, all silent and missense mutations were G to A transitions. In Case 14, we observed two missense point mutations: G to A and G to T at codons 151 and 245, respectively. As discussed below, multiple p53 point mutations are relatively rare in lung cancer, although one such case was observed in an individual chronically exposed to SM (11). We also evaluated possible mutations in the KRAS gene of these tumors, but did not find any mutations in codons 12 or 13 of this gene. Finally, IHC for the p53 protein was performed on the lung tumor samples. Just over half (13/20) showed expression of p53, which is similar to the frequency described in other lung cancer studies. In a number of instances p53 reactivity was observed in the absence of a detected mutation. This inconsistency may be due to false-positive IHC staining (25). Alternatively, the p53 mutation may lay outside the region analyzed by sequencing. Representative IHC results from tumors taken from subjects 1–5 are shown in Figure 1, with corresponding demographic and genetic data provided in Table 1. Figure 1 also shows an isotype-matched negative control and a colon carcinoma used as a positive control.
Figure 1.
P53 expression in paraffin-embedded lung tumors. Lung tumor samples were obtained from 5 Iranian males exposed to SM between 1982 and 1988. Tissues were paraffin-embedded, cut into 4 μm sections and analyzed by immunostaining. Visualization of p53 protein expression was accomplished using the p53 DO-1 antibody, with HRP and DAB reagents for detection, and a hematoxylin counterstain. IHC results and age of cancer onset are shown for tumor samples taken from subjects 1–5 shown in Table 1. A section of p53+ colon cancer is included as a positive control; and tissue stained with an isotype-matched control antibody included as a negative control.
DISCUSSION
All subjects of the present study had sustained single, high-dose SM exposures during the Iran-Iraq war and subsequently developed lung cancer. Analysis of tissue samples in the context of each subject’s biodata yielded information relevant to characterization of cancer risk and pathogenesis in mustard-exposed populations. Major significant findings included an overall younger age of cancer onset in SM victims than that typically found for lung cancer (Table 1). In addition, p53 mutations in tumors taken from participants in this study included G to A transitions; a mutation consistent with the DNA lesion caused by SM (Table 1). Additionally the presence of double and triple point mutations in the p53 gene was noted in our subjects; an observation that was also made in factory workers chronically exposed to mustard agent (11). The significance of the sequence data must nevertheless be interpreted with caution. G to A transitions in p53 may indeed be triggered by direct SM-DNA reactivity, however there are other etiologic agents that may induce the same mutation that are also associated with lung cancer (26). For example, chronic pulmonary inflammation suffered by SM victims could contribute to a characteristic mutational spectrum independently of direct SM-DNA reactivity. Nonetheless, this study provides insight as to the spectrum of mutations that may be associated with tumors in persons with single, high-dose SM exposure; along with other features of illness in this population, notably early age of cancer onset.
The relatively early age of onset of lung cancer in SM victims may be an indication of a unique etiology for some of these cancers. The range of lung cancer diagnosis in our subjects was from 28 to 73, with seven of the 20 patients developing lung cancer before the age of 40. Lung cancer typically develops after the age of 50, peaks at approximately 60 years of age, and it is unusual among people under 40 (24). Few studies have been published on lung cancer in Iran, but available data indicates that the mean age of lung cancer is higher than 60, while the mean of age among SM victims was 48 (±12) (27). The causal inference was stronger for those who are in their 20s or 30s at the time of diagnosis.
Many studies have shown that mutation of the p53 gene can contribute to lung cancer development, with mutations in this gene found in over half of all lung cancers (28, 29). The protein product of the p53 gene is involved in DNA damage response. Consequently, this gene may be a preferred target for environmental carcinogens, which act as DNA damaging agents. Moreover, carcinogens leave molecular fingerprints on the p53 gene. Thus, the study of the p53 mutational spectrum has been a useful approach for implicating suspected carcinogens to different human cancers (30, 31). However, at the time of this writing, only one report has been published characterizing p53 mutations in SM-exposed individuals. This is an article describing p53 mutations in a small Japanese Japanese population chronically exposed to SM through work at a factory producing mustard agent. The p53 analysis performed on 12 tumors isolated from Japanese factory workers showed six out of twelve lung tumors had at least one mutation in p53 (within exons 5–8); and two of the twelve tumor samples had double G to A transitions. It was concluded that these unusual double mutations may be characteristic of lung tumors caused by interaction of SM with DNA and potentially reflects the high mutagenic capacity of mustard agent. Interestingly, we likewise observed multiple point mutations in two of our cases (Table 1). Case 3 showed 3 point mutations: one silent mutation in codon 180 and 2 missense mutations in codons 171 and 238. In this case, all silent and missense mutations were G to A transitions. In Case 14, we observed two missense point mutations: G to A and G to T on codons 151 and 245, respectively. Over 2000 p53 mutations have been reported in human lung cancers, and few double mutations have been documented thus far (11, 32, 33). In a study of 15 atomic-bomb exposed patients with lung adenocarcinoma or squamous cell carcinoma, two patients were found to have double mutations in p53. Development of lung cancer in these patients was reported in association with radiation exposure (34). Hayes et al. also reported that an excess risk of lung cancer is associated with chronic exposure to chromate (35), which were also found to carry multiple mutations in the p53 gene (36). In conclusion, multiple point mutations may be attributed to exposure to strong exogenous carcinogens, such as SM.
It is of interest to note that only one of the observed mutations was among the most common mutations reported for p53. Approximately 30% of p53 mutations in the world are in “hot spot” codons 175, 245, 248, 273, and 282. Among p53 mutations in lung cancers world-wide, GC base-pairs are predominantly attacked (78–86%) (32, 37, 38). Such mutations have been attributed to spontaneous deamination, or the selective carcinogen targeting, of 5-methycytosine (39, 40). However, among the 8 mutations detected in SM exposed lung cancer patients, only one transition at CpG site dinucleotides was found. The under-representation of CpG mutations further suggests a unique etiology of lung cancers formed in SM victims.
Notwithstanding the strengths of this and a previous study of individuals chronically exposed to SM, there are a number of concerns and limitations. For example, analysis of additional p53 mutations in SM victims could generate a mutational spectrum more similar to that found in other lung cancers. In addition, our present study cannot provide an estimate of the increased lung cancer risk associated with an acute exposure to SM in the general population, nor can it substitute for epidemiological studies. Since genetic variation is one of the most important factors contributing to the risk development of cancers in humans, an individual’s sensitivity to SM is likely depend in part on their innate ability to metabolize and detoxify carcinogens and detect and repair DNA lesions. The individuals studied here may represent a highly susceptible subpopulation that might not be present at a high frequency in the general population. The later concern has been a significant motivator for an on going epidemiological study among SM-exposed Iranian populations in a newly developed program called “Study of Surveillance Health Analysis of Mustard Chemical Exposure” (SHALAMCHE) (41). The program in its present form is configured as a prospective and historical cohort study designed to track the health status of Iranian war veterans and civilians registered in the National Warfare Victims Database in Tehran. Since June 2002, 13441 participants (8500 mustard-exposed men and 4941 controls) were enrolled in SHALAMCHE, with substantially more on a weekly basis. Eventually, this program will provide enormous insight into how SM exposure affects multiple health parameters including epidemiology of lung cancer development SM-exposed Iranian population, including the degree to which an acute SM exposure increases the risk of lung cancer development in a general population. The present report represents an initial contribution to the dynamic database that will result from implementation of the SHALAMCHE program.
Acknowledgments
The authors would like to acknowledge Colleen Spurling for her assistance in analyzing the sequence data; and Stephanie C. Fox, J.D., President of QueenBeeEdit Inc. for her hard work preparing the present manuscript for publication. This work was supported by a grant from the NIEHS to CG (5R21ES013775–02).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Alireza Hosseini-khalili, Email: alireza_hosseini50@yahoo.com, University of Connecticut, USA; Janbazan Medical and Engineering Research Center, Iran Storrs, CT UNITED STATES.
David D Haines, University of Connecticut, USA.
Ehsan Modirian, Janbazan Medical and Engineering Research Center, Iran.
Mohammadreza Soroush, Janbazan Medical and Engineering Research Center, Iran.
Shahriar Khateri, Janbazan Medical and Engineering Research Center, Iran.
Rashmi Joshi, University of Connecticut, USA.
Kazem Zendehdel, Cancer Research Center, the Cancer Institute, Tehran University of Medical Sciences, Iran.
Mostafa Ghanei, Research center for chemical injuries, Baqiyatallah University of medical sciences, Tehran, Iran.
Charles Giardina, Department of Molecular and Cell Biology, University of Connecticut, USA.
References
- 1.Constanc M, Pechura DPR. Veterans at Risk: Health Effects of Mustard Gas and Lewisite. Washington DC: National Academic Press; 1993. pp. 218–221. [PubMed] [Google Scholar]
- 2.United Nations Security Council. Report; of Specialists Appointed by the Secretary General to investigate Allegations by the Islamic Republic of Iran Concerning the Use of Chemical Weapons. New York, NY: United Nations Security Council Publication; 1986. S/16433/1986. [Google Scholar]
- 3.Emad A, Rezaeian GR. The diversity of the effects of sulfur mustard gas inhalation on respiratory system 10 years after a single exposure: analysis of 197 cases. Chest. 1997;112:734–738. doi: 10.1378/chest.112.3.734. [DOI] [PubMed] [Google Scholar]
- 4.Ghanei M, Adibi I, Farhat F, Aslani J. Late respiratory effects of sulfur mustard: how is the early symptoms severity involved? Chron Respir Dis. 2008;5(2):95–100. doi: 10.1177/1479972307087191. [DOI] [PubMed] [Google Scholar]
- 5.Khateri S, Ghanei M, Keshavarz S, Soroush MR, Hains D. Incidence of lung, eye and skin lesions on late complications in 34,000 Iranian with wartime exposure to mustard agent. J Occu Environ Med. 2003;45:1136–1143. doi: 10.1097/01.jom.0000094993.20914.d1. [DOI] [PubMed] [Google Scholar]
- 6.Asensi V, et al. In vivo interleukin-6 protects neutrophils from apoptosis in osteomyelitis. Infect Immun. 2004;72(7):3823–8. 98. doi: 10.1128/IAI.72.7.3823-3828.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savill J, Haslett C. Granulocyte clearance by apoptosis in the resolution of inflammation. Semin Cell Biol. 1995;6:385–93. doi: 10.1016/s1043-4682(05)80009-1. [DOI] [PubMed] [Google Scholar]
- 8.International Agency for Research on Cancer (IARC) [Accessed January 30, 2006.];Monograph: Overall Evaluations of Carcinogenicity to Humans. 2004 Available at: http://www-cie.iarc.fr/monoeval/crthall.html.
- 9.Hemminki K, et al. Dna adducts, mutations, and cancer 2000. Regul Toxicol Pharmacol. 2000;32(3):264–75. doi: 10.1006/rtph.2000.1431. [DOI] [PubMed] [Google Scholar]
- 10.Shibata MA, et al. DNA methylation adduct formation and H-ras gene mutations in progression of N-butyl-N-(4-hydroxybutyl)nitrosamine-induced bladder tumors caused by a single exposure to N-methyl-N-nitrosourea. Carcinogenesis. 1994;15(12):2965–8. doi: 10.1093/carcin/15.12.2965. [DOI] [PubMed] [Google Scholar]
- 11.Takeshima Y, Inai K, Bennett WP, et al. P53 mutations in lung cancers from Japanese mustard gas workers. Carcinogenesis. 1994;15(10):2075–9. doi: 10.1093/carcin/15.10.2075. [DOI] [PubMed] [Google Scholar]
- 12.Adachi S, Takemoto K. Occupational lung cancer. A comparison between humans and experimental animals. Sangyo Igaku. 1987;29(5):345–57. doi: 10.1539/joh1959.29.345. [DOI] [PubMed] [Google Scholar]
- 13.Yamakido M, et al. Former poison gas workers and cancer: incidence and inhibition of tumor formation by treatment with biological response modifier N-CWS. Environ Health Perspect. 1996;104(Suppl 3):485–8. doi: 10.1289/ehp.96104s3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wada S, Nishimoto Y, Miyanishi M, Katsuta S, Nishiki M. Malignant respiratory tract neoplasms related to poison gas exposure. Hiroshima J Med Sci. 1962;11:81–91. [Google Scholar]
- 15.Weiss A, Weiss B. Carcinogenesis due to mustard gas exposure in man, important sign for therapy with alkylating agents. Dtsch Med Wochenschr. 1975;100(17):919–23. doi: 10.1055/s-0028-1106315. [DOI] [PubMed] [Google Scholar]
- 16.Yamakido M, Ishioka S, Hiyama K, Maeda A. Former poison gas workers and cancer: incidence and inhibition of tumor formation by treatment with biological response modifier N-CWS. Environ Health Perspect. 1996 May;104:485–8. doi: 10.1289/ehp.96104s3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Easton DF, Peto J, Doll R. Cancers of the respiratory tract in mustard gas workers. Br J Ind Med. 1988;45(10):652–9. doi: 10.1136/oem.45.10.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Case RAM, Lea AJ. Mustard gas poisoning, chronic bronchitis and lung cancer. An investigation into the possibility that poisoning by mustard gas in the 1914–1918 war might be a factor in the production of neoplasia. Br J Prev Soc Med. 1955;9:62–72. doi: 10.1136/jech.9.2.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beebe GW. Lung cancer in world war I veterans. Possible relation to mustard gas injury and 1918 influenza epidemic. J Nati Cancer Ins. 1960;25:1231–1252. [PubMed] [Google Scholar]
- 20.Norman JE., Jr Lung Cancer mortality in World War I veterans with mustard gas exposure (1919–1965) J Natl Cancer Inset. 1975;54(2):311–7. doi: 10.1093/jnci/54.2.311. [DOI] [PubMed] [Google Scholar]
- 21.Bullman MA, Kang PH. A Fifty-year mortality follow-up study of veterans exposed to low-level chemical warfare agent, mustard gas. Ann Epidemiol. 2000;10:333–338. doi: 10.1016/s1047-2797(00)00060-0. [DOI] [PubMed] [Google Scholar]
- 22.Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for Sulfur Mustard (Update) Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service.); 2003. [PubMed] [Google Scholar]
- 23.Hsu SM, Raine L, Fanger H. A comparative study of the peroxidase–antiperoxidase method and an avidin– biotin complex method for studying polypeptide hormones with radioimmunoassay antibodies. Am J Clin Pathol. 1981;75:734 –738. doi: 10.1093/ajcp/75.5.734. [DOI] [PubMed] [Google Scholar]
- 24.O’Rourke MA, Feussner JR, Feigl P, Laszlo J. Age trends of lung cancer stage at diagnosis. Implications for lung cancer screening in the elderly. JAMA. 1987;258(7):921–6. [PubMed] [Google Scholar]
- 25.Baas IO, van den Berg FM, Mulder JW, Clement MJ, et al. Potential false-positive results with antigen enhancement for immunohistochemistry of the p53 gene product in colorectal neoplasms. J Pathol. 1996;178(3):264–7. doi: 10.1002/(SICI)1096-9896(199603)178:3<264::AID-PATH485>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 26.Yamada Y, Oghiso Y. Mutations in Tp53 gene sequences from lung tumors in rats that inhaled plutonium dioxide. Radiat Res. 1999 Dec;152(6 Suppl):S107–9. [PubMed] [Google Scholar]
- 27.Sadjadi A, Malekzadeh R, Derakhshan MH, et al. Cancer occurrence in Ardabil: results of a population-based cancer registry from Iran. Int J Cancer. 2003;107(1):113–8. doi: 10.1002/ijc.11359. [DOI] [PubMed] [Google Scholar]
- 28.Hofseth LJ, Hussain SP, Harris CC. P53: 25 years after its discovery. Trends Pharmacol Sci. 2004;25(4):177–81. doi: 10.1016/j.tips.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Wang W, El-Deiry WS. Restoration of p53 to limit tumor growth. Curr Opin Oncol. 2008;20(1):90–6. doi: 10.1097/CCO.0b013e3282f31d6f. [DOI] [PubMed] [Google Scholar]
- 30.Bennett WP, Hussain SP, Vahakangas KH, Khan MA, Shields PG, Harris CC. Molecular epidemiology of human cancer risk: gene-environment interactions and p53 mutation spectrum in human lung cancer. J Pathol. 1999;187(1):8–18. doi: 10.1002/(SICI)1096-9896(199901)187:1<8::AID-PATH232>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 31.Rengul CA, Mehmet O. P53 mutations as fingerprints of environmental carcinogens. Pure Appl Chem. 2000;72(6):995–999. [Google Scholar]
- 32.Kishimoto Y, Murakami Y, Shiraishi M, Hayashi K, Sekiya T. Aberrations of the p53 tumor suppressor gene in human non-small cell carcinomas of the lung. Cancer Res. 1992;52(17):4799–804. [PubMed] [Google Scholar]
- 33.Miller CW, Simon K, Aslo A, Kok K, Yokota J, Buys CH, Terada M, Koeffler HP. P53 mutations in human lung tumors. Cancer Res. 1992;52(7):1695–8. [PubMed] [Google Scholar]
- 34.Nakachi K, Harris CC, Tahara E. Japan-US Cooperative Cancer Research Seminar on molecular epidemiological characteristics of lung and colon cancer development among atomic-bomb survivors, Bethesda, USA, February 23–24, 2006. Cancer Sci. 2006;97(11):1279–82. doi: 10.1111/j.1349-7006.2006.00312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayes RB, Sheffet A, Spirtas R. Cancer mortality among a cohort of chromium pigment workers. Am J Ind Med. 1989;16:127–133. doi: 10.1002/ajim.4700160204. [DOI] [PubMed] [Google Scholar]
- 36.Kondo K, Hino N, Sasa M, Kamamura Y, Sakiyama S, Tsuyuguchi M, Hashimoto M, Uyama T, Monden Y. Mutations of the p53 gene in human lung cancer from chromate-exposed workers. Biochem Biophys Res Commun. 1997 Oct 9;239(1):95–100. doi: 10.1006/bbrc.1997.7425. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki H, Takahashi T, Kuroishi T, et al. P53 mutations in non-small cell lung cancer in Japan: association between mutations and smoking. Cancer Res. 1992;52(3):734–6. [PubMed] [Google Scholar]
- 38.Takahashi T, Takahashi T, Suzuki H, et al. The p53 gene is very frequently mutated in small-cell lung cancer with a distinct nucleotide substitution pattern. Oncogene. 1991;6(10):1775–8. [PubMed] [Google Scholar]
- 39.Ehrlich M, Zhang XY, Inamdar NM. Spontaneous deamination of cytosine and 5-methylcytosine residues in DNA and replacement of 5-methylcytosine residues with cytosine residues. Mutat Res. 1990;238(3):277–86. doi: 10.1016/0165-1110(90)90019-8. [DOI] [PubMed] [Google Scholar]
- 40.Pfeifer GP. Mutagenesis at methylated CpG sequences. Curr Top Microbiol Immunol. 2006;301:259–81. doi: 10.1007/3-540-31390-7_10. [DOI] [PubMed] [Google Scholar]
- 41.Zafarghandi MR, Soroush MR, Mahmoodi M, Holakoui K, Ardalan A, Dolatyari A, Abbasi A. Surveillance Health Analysis of Mustard Chemical Exposure (SHALAMCHE) Study. Design and Methods. Unpublished report. [Google Scholar]