Abstract
Background
Methadone is an effective and inexpensive opioid for cancer pain treatment. It has been reported as difficult to use in the outpatient setting due to its variable relative potency and long half-life. The purpose of this study was to determine the outcome of methadone initiation or rotation for cancer pain treatment in outpatient settings.
Methods
Chart review of 189 consecutive patients who underwent methadone initiation or rotation in our palliative care outpatient center. Data were collected regarding demographic and clinical characteristics, symptoms, and opioid side effects at baseline and for 2 follow up visits(F1,F2). Failure was defined as methadone discontinuation by the palliative care physician or patient's hospitalization for uncontrolled pain or methadone-related side effects at F1.
Results
100(53%) initiations and 89(47%) rotations were conducted. Success rates for methadone initiation and rotation were 82/89(92%) and 85/100(84%) respectively. Mean(standard deviation) age was 60(11) years. 100(53%) patients were female, 138(73%) white, 182(96%) had solid cancers. The main reason for rotation was pain (65/89 patients, 47%). Median(interquartile range, IR) pain scores (Edmonton Symptom Assessment System/0–10) were 6(5–8), 4(3–6), and 3(2–5) at baseline, F1, and F2, respectively(p<0.0001). Median(IR) daily methadone dose for initiation and rotation was 10(5–15)mg and 15(10–30)mg at F1(p<0.0001) and 10(8–15)mg and 18(10–30)mg at F2(p<0.0001), respectively. Constipation and nausea improved (p<0.005) after initiation/rotation to methadone. Frequency of sedation, hallucinations, myoclonus, and delirium did not increase after initiation/rotation to methadone.
Conclusions
Outpatient methadone initiation and rotation for cancer pain treatment were safe, with high success rate and low side effect profile.
Keywords: methadone, pain, neoplasms, outpatients, palliative care
INTRODUCTION
Pain is extremely common in patients with advanced cancer, with prevalence as high as 80%. The vast majority of patients with cancer pain require opioids for treatment 1, 2. As a result of a major effort by various international groups, the use of opioids has improved significantly and patients now have earlier exposure to opioids, generally receive higher dosages, and are better managed than in the past 3. This highly desirable increase in the use of opioids, combined with increased vigilance has resulted in increased detection of several side effects. Side effects can range from constipation and nausea to the more serious opioid induced neurotoxicity which includes delirium, seizures, and myoclonus. Opioid toxicity is due to both the effect of the parent drug as well as to accumulation of opioid metabolites (e.g., morphine-3-glucuronide and morphine-6-glucucoronide for morphine) 4. Opioid rotation is required when there are intolerable side effects despite good analgesia or inadequate pain control despite dose escalation.
Methadone is a synthetic drug that was developed after the Second World War as an alternative to morphine, having a broad-spectrum of suggested actions: μ receptor agonist, NMDA receptor antagonist, monoamine reuptake inhibitor, and α receptor agonist, and has been increasingly used as a second line opioid in recent years in the management of cancer-related pain 5. It has complex pharmacodynamic and pharmacokinetic properties that make it difficult to use. It is an ideal drug for opioid rotation because of its efficacy, higher oral bioavailability, no known active metabolites, incomplete cross tolerance with other mu receptor agonists and lower cost. Several prospective and retrospective trials have demonstrated successful rotation to methadone from other strong opioids in the setting of intolerable side effects and inadequate analgesia despite dose escalation 6–8. However, it has the disadvantage of having a slow elimination phase (15–60 hours) and high inter-individual variation in the equianalgesic ratio with other opioids, and that largely restricted methadone use to experienced physicians in pain medicine 9.
It is also a desirable alternative first line strong opioid particularly in developing countries where cost is an important consideration. There is growing preliminary evidence supporting the use of methadone as a first line strong opioid 10, 11. Largely, the use of methadone is limited by its complex pharmacokinetic and pharmacodynamic properties such as the reported elimination metabolism through cytochromes 3A4 and 2B6 12, the participation of P-glycoprotein on the defense against central methadone intoxication13, or concerns related to its potential cardiotoxic effects (with prologantion of the QT interval)14, 15 which could impede its concomitant use with specific drugs inhibiting the cited cytochromes or P-Glycoprotein, or cause QT interval prolongation.
Unfortunately, there are few reports 16, 17 on the initiation and/or rotation of methadone in the outpatient setting for cancer pain. The objective of this retrospective study was to determine the efficacy and safety of methadone initiation (in strong opioid naïve patients) or rotation from another strong opioid in treating cancer-related pain in an outpatient palliative care clinic at a comprehensive cancer center.
METHODS
This study was reviewed and approved by the Institutional Review Board and was granted a waiver of informed consent. We reviewed the electronic charts of 189 consecutive patients who were prescribed methadone for the first time at the Palliative Care Outpatient Clinic (PCOC) at the University of Texas M. D. Anderson Cancer Center between April 2003 and March 2007.
Patients were included in the study if (a) they were receiving methadone for the first time in the outpatient setting and (b) the previous opioid was completely stopped at the day of methadone initiation. Patients were excluded of the study if they were (a) receiving opioids via intrathecal pump, (b) receiving opioids for reasons other than pain (e.g. dyspnea), or (c) admitted to the hospital after the initiation of methadone for reasons other than pain or methadone-related toxicities.
Strong opioid-naïve patients were started on methadone as the first line opioid when concerns regarding the utilization of other opioids were present (ie. renal failure) or when logistic issues were identified, such as the cost of medications for patients with limited resources. Rotations for pain were made by the palliative care specialist after patients underwent multiple unsuccessful titrations with the previous opioid agonist. Rotation usually was decided when the patient presented early signs or was considered at risk for dose-limiting side effects. Rotations because of side effects took place when patients developed severe side opioid toxicity such as delirium, myoclonus, and/or sedation.
In our PCOC, patients were initiated on regular methadone 5mg twice a day with an additional prescription of rescue methadone, according to minor modifications to procedures previously described by our group10. In the context of opioid rotation we applied the usual strategy of variable MEDD:methadone conversion ratios according to the dose of previous opioid (5:1 when previous MEDD ≤ 90mg/day, 8:1 when it was between 91 and 300mg/day, and 12:1 when it was ≥ 301mg/day)18. These rotation ratios are modified by the attending palliative care physician based on specific clinical characteristics. The most common modifier is the reason for rotation: patients being rotated because of severe intractable pain were rotated to a lower MEDD:methadone ratio as compared to patients rotated due to opioid neurotoxicity.
Data were collected regarding patients’ demographics, diagnosis, current use of opioids (drug names and total daily dose), date of methadone initiation, and reason for rotation (if previously on opioids). Predictors of poor response to opioid therapy such as presence of delirium, psychological distress, and neuropathic and incidental pain 19 were also noted.
Symptoms (namely pain, nausea, and drowsiness) were recorded for the first visit to the PCOC and two consecutive follow ups using the Edmonton Symptom Assessment System (ESAS), a widely used and validated tool to assess nine symptoms (pain, nausea, drowsiness, dyspnea, anxiety, depression, anorexia, sleep, and fatigue) and general feeling of wellbeing in a 0–10 scale 20, 21. Other opioid side effects such as sedation, hallucinations, myoclonus, delirium and constipation are routinely assessed in our PCOC and were considered present when reported by the attending palliative care physician in the patient’s chart.
The total daily opioid dosage was calculated for the first visit to the PCOC and two consecutive follow ups by converting the total opioid dosage during 24 hours to an equivalent dose of oral morphine (Morphine Equivalent Daily Dose, MEDD), following standard equianalgesic conversion tables. 22 The ratio between MEDD and methadone was calculated by dividing the MEDD previous to rotation by the methadone daily dose at stable pain control (defined as ESAS pain change equal or lower than 1 point or equal or lower than 30%).
Successful initiation/rotation to methadone was evaluated at the first follow up visit. Complete success of methadone initiation or rotation was defined as (a) improvement greater or equal to 30% or greater or equal to 2 points in the ESAS-pain score for strong opioid-naïve patients who initiated methadone because of pain or for patients who were rotated to methadone from other strong opioids for uncontrolled pain in the absence of side effects; (b) reported evidence of disappearance of side effects at the first follow up in the cases in which the reason for methadone initiation was the development of side effects with the previous strong opioid; (c) improvement greater or equal to 30% or greater or equal to 2 points in the ESAS-pain score with reported disappearance of side effects for those patients rotated to methadone in the setting of uncontrolled pain and dose-limiting side effects with the previous opioid; and (d) reported evidence of resolution of the logistical issue with the previous opioid (ie. Resolution of non-adherence related to financial burden as detected and reported by the specialist palliative care physician as the result of routinely performed adherence assessments), with stable pain control. Methadone initiation or rotation in the outpatient setting was considered a failure if the drug was discontinued, if the patient was admitted to the hospital because of pain or methadone-related side effects on or before the first follow up, or if the patient was lost to follow up. All cases which did not fall into the complete success or failure categories were considered partial successes.
Data obtained from the second follow up were analyzed with regards to pain control and opioid side effects.
Descriptive statistics were used to summarize the data. χ2 tests were used to determine associations between categorical variables. Differences between continuous variables were analyzed using t tests for normally distributed data and Wilcoxon rank-sum tests for non-normally distributed data. Significance levels less than 0.05 were considered statistically significant. Spearman’s correlation coefficient was calculated to evaluate the association between MEDD of the previous opioid and the MEDD/methadone dose ratio. In order to further identify factors associated with the MEDD/Methadone ratios at stable pain control, we performed multivariate analyses of variance based on the ranks of the ratios, because these values were not normally distributed. We performed these analyses first only including the variables found to be univariately associated with the ratios at significance levels <0.20, then deleted variables one at a time that were not statistically significant (p<0.05) from the multivariate model.
RESULTS
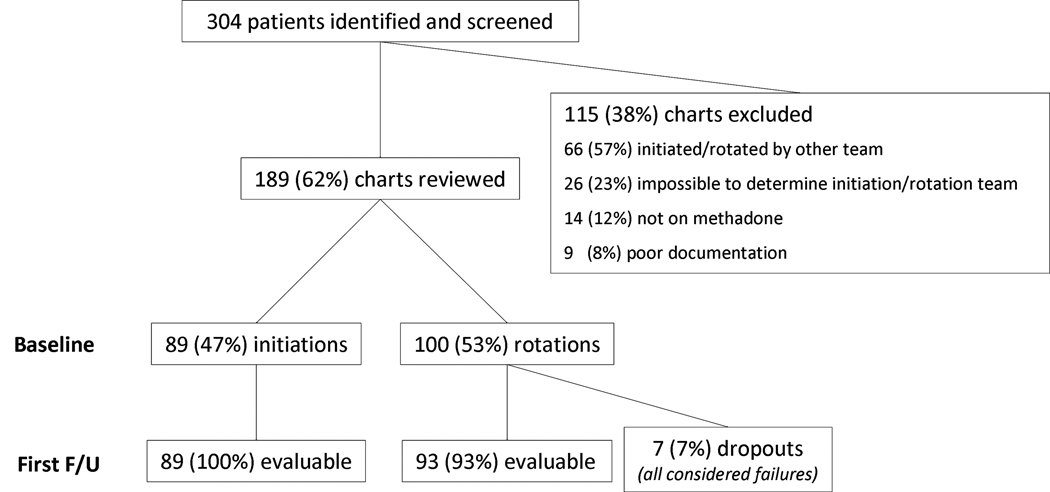
One hundred and eighty-nine patients underwent methadone initiation or rotation in the PCOC. Data collection is described in the flowchart in figure 1. Patient characteristics are summarized in table 1. There were no significant differences between groups.
Figure 1.
Table 1.
Demographic characteristics
| Initiation N=89 |
Rotation N=100 |
Total N=189 |
P value | |
|---|---|---|---|---|
| Mean (SD) Age, in years | 60 (11) | 58 (11) | 60 (11) | 0.2 |
| Female gender | 45 (51%) | 55 (55%) | 100 (53%) | 0.6 |
| Race | ||||
| African-American | 11 (12%) | 4 (4%) | 15 (8%) | 0.06 |
| Hispanic | 7 (8%) | 10 (10%) | 17 (9%) | 0.6 |
| Caucasian | 62 (70%) | 77 (77%) | 139 (73%) | 0.4 |
| Other | 9 (10%) | 9 (9%) | 18 (10%) | 0.8 |
| Cancer Site | ||||
| Head/Neck/Lung | 36 (40%) | 36 (36%) | 72 (38%) | 0.6 |
| Urologic | 15 (17%) | 16 (16%) | 31 (16%) | 1.0 |
| Breast | 7 (8%) | 15 (15%) | 22 (12%) | 0.2 |
| Gastrointestinal | 10 (11%) | 9 (9%) | 19 (10%) | 0.6 |
| Hematological | 2 (2%) | 5 (5%) | 7 (3%) | 0.5 |
| Other | 11 (12%) | 10 (10%) | 21 (11%) | 0.7 |
| Cancer Status | ||||
| Metastatic | 58 (65%) | 72 (72%) | 130 (69%) | 0.4 |
| Locally advanced/recurrent | 28 (32%) | 25 (25%) | 53 (28%) | 0.4 |
| No evidence of disease | 3 (3%) | 3 (3%) | 6 (3%) | 1.0 |
| Poor prognostic factors | ||||
| Neuropathic pain | 52 (58%) | 57 (57%) | 109 (58%) | 0.9 |
| Incidental pain | 23 (26%) | 23 (23%) | 46 (24%) | 0.7 |
| Psychological distress | 32 (36%) | 39 (39%) | 71 (38%) | 0.8 |
| CAGE positive | 11 (12%) | 13 (13%) | 24 (13%) | 1.0 |
Using a conservative intention-to-treat approach, all 7 patients who didn’t have any follow ups were considered failures. Reasons for rotation among these cases are described in table 2. Because of documentation limitations, it was not possible to determine the reason for rotation to methadone in 4/100 (4%) cases.
Table 2.
Methadone initiation/rotation outcomes – number of successes (complete and partial) and failures of methadone rotation and initiation.
| Complete success |
Partial success |
Overall Success |
Failure | |
|---|---|---|---|---|
| Reasons for Rotation | ||||
| Pain (n=65) | 28 (48%) | 30 (52%) | 58/65 (89%) | 7/65 (11%) |
| Pain and Side Effects (n=23) | 10 (59%) | 7 (41%) | 17/23 (74%) | 6/23 (26%) |
| Side effects only (n=5) | 5 (100%) | 0 (0) | 5/5 (100%) | 0/23 (0) |
| Logistic (n=3) | 2 (67%) | 1 (33%) | 3/3 (100%) | 0/23 (0) |
| Unclear (n=4) | 2 (100%) | 0 (0) | 2/4 (50%) | 2/4 (50%) |
| Rotation total (n=100) | 47 (55%) | 38 (45%) | 85/100 (85%)* | 15/100 (15%) |
| Initiation (n=89) | 53 (65%) | 29 (35%) | 82/89 (92%)* | 7/89 (8%) |
p=0.17 for rotation versus initiation
Median (interquartile range, IR) time between consult and first follow up was 13(6–21) days, and did not differ between initiation and rotation patients (p=0.22). Median (IR) time between first and second follow ups was 8(1–5) days for initiation patients and 15(10–25) days for rotation patients (p<0.0001). Median (IR) time between baseline and second follow up was 37(23–60) days (p=0.59).
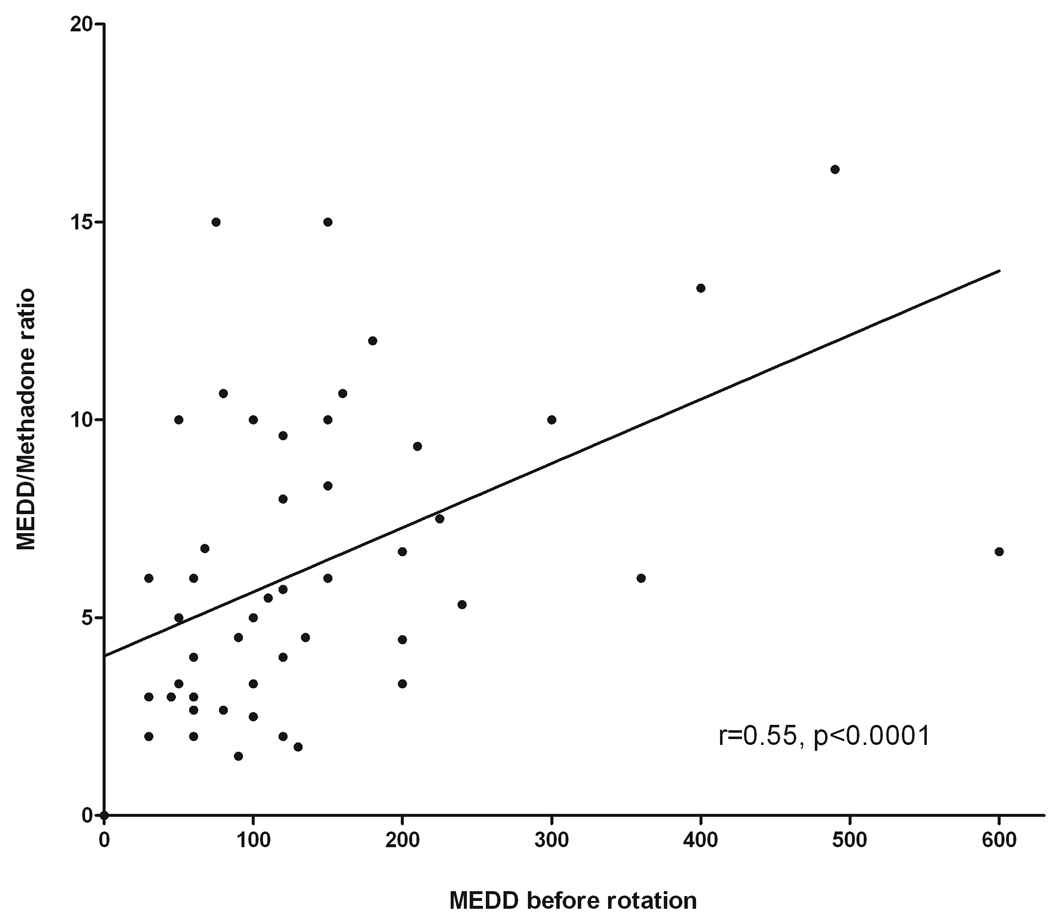
Median (IR) MEDD before methadone initiation was 100 (60–185) mg/day. The median (IR) methadone dose at first follow up was 10 (5–15)mg/day for initiation patients and 15 (10–30) for rotation patients (p<0.0001) and at second follow up it was 10 (8–15)mg/day for initiation patients and 18(10–30) for rotation patients (p<0.0001). Median dose increase between first and second followup was 1.75(0–5)mg (p<0.0001) for initiation and 2.5(0–10)mg for rotation patients (p=0.12). Considering previous reports of variable dose-dependent ratio for conversions from other opioids to methadone 23–26, the correlation between the MEDD before rotation and the MEDD/methadone dose ratio at stable pain control was studied and its results are shown in figure 2. The findings confirm the significant correlation between previous opioid dose and opioid/methadone ratio (r=0.55, p<0.0001). The MEDD/methadone ratio was univariately analyzed according to age, gender, reason for rotation, and MEDD of the previous opioid. Multivariate analyses were then performed with the three variables (age, reson for rotation, and MEDD before rotation) found to be significant in the univariate analyses. Age was not significant and was removed from the model. Results for both univariate and multivariate analysis are summarized in table 3.
Figure 2.
Table 3.
Median MEDD/Methadone ratio at stable pain control
| Variable | N | Median MEDD-Methadone ratio (interquartile range) |
Univariate analysis p value |
Multivariate analysis* p value |
|---|---|---|---|---|
| Age | ||||
| ≤ 65 years | 47 | 6:1 (3:1–8:1) | ||
| > 65 years | 16 | 9:1 (6:1–10:1) | 0.0302 | - |
| Gender | ||||
| Female | 34 | 6:1 (4:1–10:1) | ||
| Male | 29 | 6:1 (3:1–10:1) | 0.8302 | - |
| Reason for rotation | ||||
| Pain alone | 43 | 6:1 (3:1–8:1) | ||
| Side effects (± pain) | 16 | 9:1 (6:1–11:1) | 0.0266 | 0.0032 |
| Previous opioid MEDD | ||||
| ≤ 90mg/day | 29 | 5:1 (3:1–6:1) | ||
| > 90mg/day | 34 | 8:1 (5:1–10:1) | 0.0022 | 0.0005 |
Multivariate model R-square: 0.269, p=0.0002
Reasons for opioid rotation are described in Table 2. Success (complete and partial) of methadone initiation after one follow up occurred in 82/89 (92%) initiation patients and in 85/100 (85%) rotation patients (p=0.2). Reasons for failure of the rotation to methadone were non improvement/increase in pain in 12/15 cases (80%) and appearance/persistance of side effects in 3/15 cases (20%). For opioid naïve patients initiating methadone, reasons for failure were non improvement in pain in 3/7 cases (43%), non-adherence in 1/7 cases (14%), and appearance/persistency of side effects in 3/7 cases (43%).
There were 29/89 partial successes (35%) among initiation patients between baseline and first followup. Reasons for partial successes were pain in 21/29(72%) patients, non-adherence in 3/29(10%) patients, and side effects in 5/29(17%) patients. Among rotation patients, there were 38/85 partial successes (44%) between baseline and first followup. In this group, reasons for partial successes were pain in 28/38(74%), non-adherence in 3/38(9%), and side effects in 7/38(18%) patients.
Data was available for 63/89(70%) rotation and 63/93(68%) initiation patients at the time of second follow up. At this timepoint, 53/63(84%) rotation and 59/61(96%) initiation patients continued receiving methadone (p=0.03).
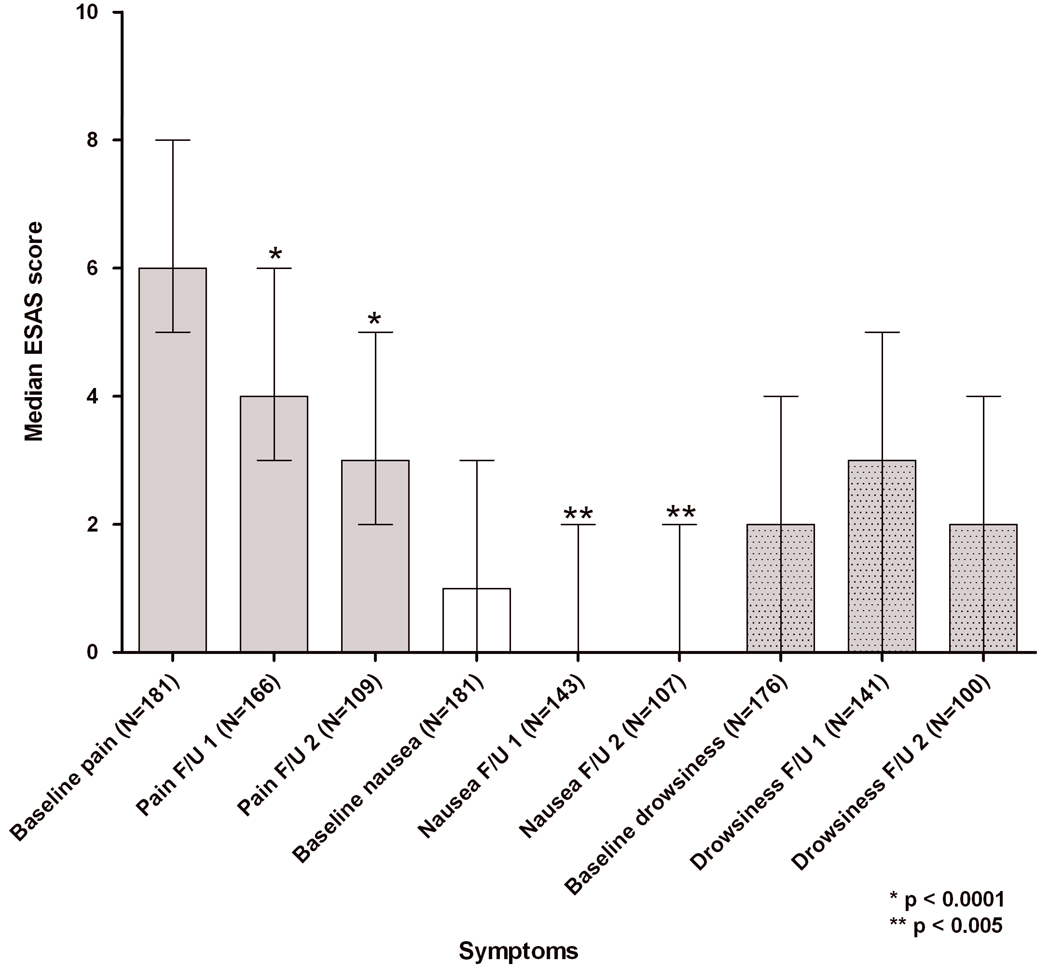
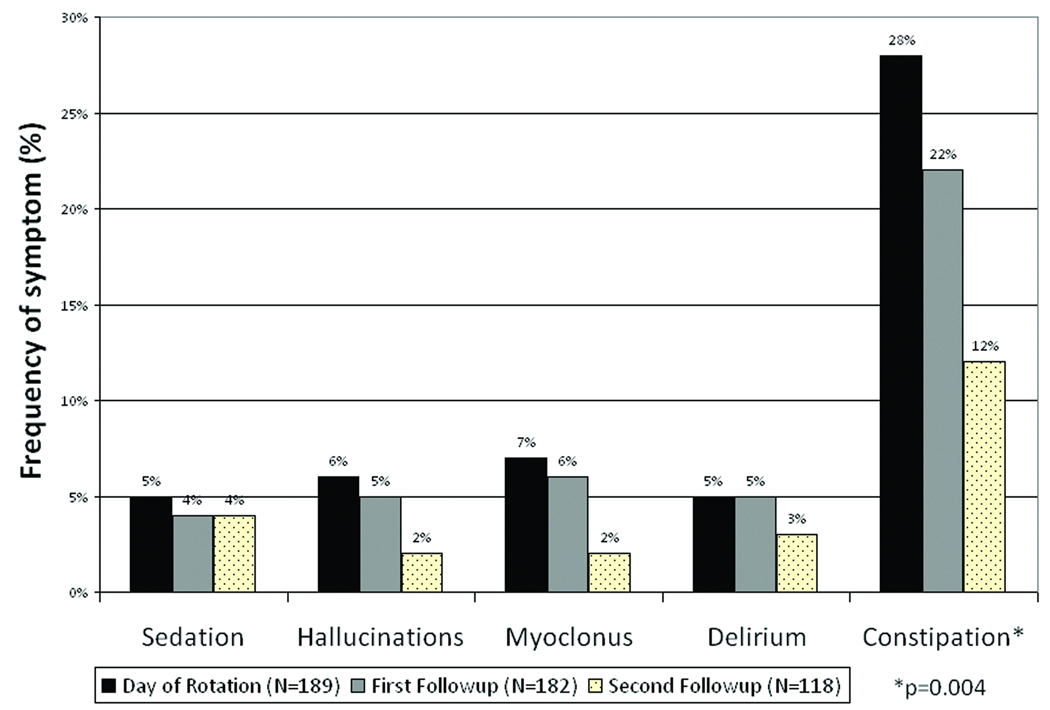
ESAS pain scores improved in all groups, regardless of their reason for initiation. Nausea and drowsiness also reduced in all groups, as summarized in Figure 3. Sedation, hallucinations, myoclonus, delirium, and constipation, symptoms frequently related to opioid toxicity, did not increase in frequency after methadone initiation/rotation, as described in Figure 4.
Figure 3.
Figure 4.
DISCUSSION
Caution in the use of methadone in the outpatient setting was previously reccomended in several articles by our group and others 27–30. We present the results of a retrospective chart review of 189 advanced cancer patients who underwent initiation or rotation to methadone in an outpatient palliative care clinic. In the majority (88%) of the patients, this strategy was successful. The use of methadone as a first line opioid and especially in the outpatient setting is still scarce, probably due to concerns about the complex management of this drug and its side effects 9. In our study, 89 patients were started on methadone as first strong opioid, and the overall success rate was of 92%. In a randomized study by Mercadante et al with 108 patients receiving slow release morphine, fentanyl, or methadone as initial strong opioid, no difference was found between the three drugs regarding effectiveness, need for co-analgesia, and safety. Methadone had the advantage of lower cost; However, it required more dose titration prompting the authors to suggest that its use requires more expertise in pain management compared with other opioids 11. Our group has also reported in a randomized, double blind study of methadone versus morphine as first line opioid for the treatment of cancer pain that no difference was found regarding tolerability and analgesia between the drugs 10.
The use of methadone in the process of opioid rotation has been more extensively studied. In general, the switch to methadone has been considered safe and effective. However, the majority of the studies were performed in the inpatient setting 6, 8, 23, 31–34. Our study shows that in the context of opioid rotation, the use of methadone for cancer-related pain in the outpatient setting is successful (85% of the cases). Specifically, in the outpatient setting, Hagen et al. reviewed 29 charts of patients with cancer-related pain who underwent rotation to methadone in the outpatient setting and found that rotation to methadone was successful in 17 patients (62%) within a 32 day average. The authors concluded that patients can be safely and effectively rotated to methadone in the outpatient setting despite a long period to reach pain stability 16. Hernansanz et al reported success in 10/14 (71%) cases in which opioid rotation was performed in the home setting in advanced cancer patients. Our study builds up on this previous evidence, shedding light on the possibility of safely utilizing this drug in the outpatient setting.
After two follow up visits, methadone was continued in the majority of patients in our study, more frequently among opioid-naïve patients than in patients undergoing opioid rotation. This is consistent with previous data showing that the titration of methadone was easier in patients who were not previously receiving opioids 35.
Methadone used as first line strong opioid in general resulted in improvement in pain and nausea, with stable drowsiness. The improvement of pain is consistent throughout studies of methadone rotation/initiation. In a retrospective study of 196 patients who received methadone either as a first line opioid (54%) or for opioid rotation from morphine (18%), only pain and insomnia improved and other symptoms such as drowsiness, constipation and confusion worsened 36. The improvement in nausea shown in the current study might be related to advances in the interdisciplinary management of this symptom. Delayed onset sedation was previously described by our group in a study with 103 patients randomized to either morphine or methadone as first line opioid, in which those who received methadone reported more delayed onset sedation, with no difference in nausea, constipation, or confusion 10.
As observed in previous studies, patients who started or rotated to methadone showed considerable reduction in the frequency of constipation as reported by the attending palliative care physician, which has been previously reported and is postulated as being secondary to differences in methadone’s affinity for intestinal opioid receptors and also to bowel “withdrawal syndrome”, because of the significantly smaller doses of methadone needed to reach analgesia, binding to fewer intestinal opioid receptors as compared to other opioids 8, 37. Frequency of other symptoms related to opioid toxicity such as myoclonus, delirium, hallucinations and sedation did not increase with methadone rotation, however it is not possible to positively infer trends of reduction due to the number of drop offs between follow ups. Response to methadone when used for opioid rotation has been attributed to an absence of circulating metabolites as compared with other mu agonists 18.
It has been reported that methadone elimination mostly occurs through cytochrome 3A4 12. Even though very recently published evidence began to question this metabolic route in animal studies38, 39, it is still advisable to take in consideration that the use of potent cytochrome 3A4 inhibitors (such as fluconazole, ciprofloxacin, venlafaxine, fluoxetine, and macrolides)40, 41 has the potential to interfere in the elimination of methadone, increasing the risk of elevation of the plasma levels of the drug towards toxic levels. The same effect could be expected when inhibitors of cytochrome 2B6 (such as haloperidol, levopromazine, paroxetine, sertraline, fluoxetine, and chlorimipramine)40 are used concomitantly with methadone, since it is also related with methadone metabolism. Doxorubicin, vimblastin, and actinomycin, frequently used in cancer patients, are inhibitors of P-glycoprotein, and by impairing the ability of the blood-brain barrier to block excessive transference of methadone to the central nervous system, might predispose patients to have increasing plasmatic levels of methadone despite stable dosing.
Prolongation of the QT interval is associated with increased risk of severe arrhythmias42 and has been reported as associated to the use of methadone14, 15, 43. We did not find any clinical evidence of cardiac rhythm alterations after the introduction of methadone. However, since neither cardiac effects nor the concomitant use of other potentially arrhythmogenic drugs were formally assessed in this retrospective study, we are unable to draw definitive conclusions regarding the cardiac safety of methadone. Therefore, it is still advisable to obtain information regarding the presence of electrolyte imbalances or the concomitant use of other cardiotoxic drugs such as tricyclic antidepressants, diuretics, and antipsychotics 44.
The significant association between MEDD of the previous opioid (Figure 2) and the variable MEDD/Methadone ratio confirms previous reports from other authors 23–26, 45. We found in univariate analysis that greater MEDD/Methadone ratio are associated with older age, rotation because of side effects, and higher previous opioid doses. In the multivariate model, reason for rotation and previous opioid doses (grouped according to previouly published evidence45) remained independently associated with greater MEDD/Methadone ratio. Our findings in the outpatient setting are similar to those recently reported in a study with 54 patients who underwent rotation in the inpatient setting which showed that the median morphine-methadone ratio ranged from 2:1 to 15:1, being independently associated with previous opioid doses and reason for rotation 45. These findings suggest that methadone behaves in a different manner as compared to other opioids with regards to opioid sensitivity and caution should be exercised in patients who had escalation of the previous opioid.
We were not able to find a significant number of patients who were rotated to methadone because of financial issues in our limited sample. This might be due to the fact that when financial cost is identified by the palliative care team as a concern, patients are already started on methadone as a first line strong opioid. However it was impossible to obtain this detailed information for methadone initiations in this retrospective study. Our group and others 45–48 have already reported that methadone is a very inexpensive drug, being an ideal alternative to other opioids, particularly in developing countries where cost of treatment is often a barrier in providing excellent care to patients with cancer pain.
CONCLUSION
Methadone was effective both for use as first and second line opioid. Patients exhibited tolerable side effects to the drug. Our study adds to the growing evidence in the literature to recommend such use. Further prospective studies on the use of methadone as a first line strong opioid with longer followup periods are required to be able to confirm our clinical findings.
ACKNOWLEDGEMENT
We would like to thank Laura Navarrete, Timotheos Paraskevopoulos, Elva Ramirez, and Lynn Roberts for their invaluable help with data collection.
Sources of support: Eduardo Bruera is supported in part by NCI R01 grants CA122292-01 and CA124481-01, and NINR grant NR010162-01A1.
Footnotes
This study was partially presented at the 2008 Annual Meeting of the American Society of Clinical Oncology.
The authors report no conflicts of interest.
REFERENCES
- 1.Goudas LC, Bloch R, Gialeli-Goudas M, Lau J, Carr DB. The epidemiology of cancer pain. Cancer Invest. 2005;23(2):182–190. [PubMed] [Google Scholar]
- 2.WHO. Geneve: WHO; Cancer Pain Relief. 1986
- 3.Bruera E, MacMillan K, Hanson J, MacDonald RN. Palliative care in a cancer center: results in 1984 versus 1987. J Pain Symptom Manage. 1990;5(1):1–5. doi: 10.1016/s0885-3924(05)80002-8. [DOI] [PubMed] [Google Scholar]
- 4.Lotsch J. Opioid metabolites. J Pain Symptom Manage. 2005;29(5 Suppl):S10–S24. doi: 10.1016/j.jpainsymman.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Davis MP, Walsh D. Methadone for relief of cancer pain: a review of pharmacokinetics, pharmacodynamics, drug interactions and protocols of administration. Support Care Cancer. 2001;9(2):73–83. doi: 10.1007/s005200000180. [DOI] [PubMed] [Google Scholar]
- 6.Mercadante S, Casuccio A, Calderone L. Rapid switching from morphine to methadone in cancer patients with poor response to morphine. J Clin Oncol. 1999;17(10):3307–3312. doi: 10.1200/JCO.1999.17.10.3307. [DOI] [PubMed] [Google Scholar]
- 7.Vigano A, Fan D, Bruera E. Individualized use of methadone and opioid rotation in the comprehensive management of cancer pain associated with poor prognostic indicators. Pain. 1996;67(1):115–119. doi: 10.1016/0304-3959(96)03112-0. [DOI] [PubMed] [Google Scholar]
- 8.Mercadante S, Casuccio A, Fulfaro F, Groff L, Boffi R, Villari P, et al. Switching from morphine to methadone to improve analgesia and tolerability in cancer patients: a prospective study. J Clin Oncol. 2001;19(11):2898–2904. doi: 10.1200/JCO.2001.19.11.2898. [DOI] [PubMed] [Google Scholar]
- 9.Shaiova L, Berger A, Blinderman CD, Bruera E, Davis MP, Derby S, et al. Consensus guideline on parenteral methadone use in pain and palliative care. Palliat Support Care. 2008;6(2):165–176. doi: 10.1017/S1478951508000254. [DOI] [PubMed] [Google Scholar]
- 10.Bruera E, Palmer JL, Bosnjak S, Rico MA, Moyano J, Sweeney C, et al. Methadone versus morphine as a first-line strong opioid for cancer pain: a randomized, double-blind study. J Clin Oncol. 2004;22(1):185–192. doi: 10.1200/JCO.2004.03.172. [DOI] [PubMed] [Google Scholar]
- 11.Mercadante S, Porzio G, Ferrera P, Fulfaro F, Aielli F, Verna L, et al. Sustained-release oral morphine versus transdermal fentanyl and oral methadone in cancer pain management. Eur J Pain. 2008;12(8):1040–1046. doi: 10.1016/j.ejpain.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Kharasch ED, Hoffer C, Whittington D, Sheffels P. Role of hepatic and intestinal cytochrome P450 3A and 2B6 in the metabolism, disposition, and miotic effects of methadone. Clin Pharmacol Ther. 2004;76(3):250–269. doi: 10.1016/j.clpt.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Thompson SJ, Koszdin K, Bernards CM. Opiate-induced analgesia is increased and prolonged in mice lacking P-glycoprotein. Anesthesiology. 2000;92(5):1392–1399. doi: 10.1097/00000542-200005000-00030. [DOI] [PubMed] [Google Scholar]
- 14.Krantz MJ, Lowery CM, Martell BA, Gourevitch MN, Arnsten JH. Effects of methadone on QT-interval dispersion. Pharmacotherapy. 2005;25(11):1523–1529. doi: 10.1592/phco.2005.25.11.1523. [DOI] [PubMed] [Google Scholar]
- 15.Martell BA, Arnsten JH, Krantz MJ, Gourevitch MN. Impact of methadone treatment on cardiac repolarization and conduction in opioid users. Am J Cardiol. 2005;95(7):915–918. doi: 10.1016/j.amjcard.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 16.Hagen NA, Wasylenko E. Methadone: outpatient titration and monitoring strategies in cancer patients. J Pain Symptom Manage. 1999;18(5):369–375. doi: 10.1016/s0885-3924(99)00083-4. [DOI] [PubMed] [Google Scholar]
- 17.Hernansanz S, Gutierrez C, Rubiales AS, Flores LA, del Valle ML. Opioid rotation to methadone at home. J Pain Symptom Manage. 2006;31(1):2–4. doi: 10.1016/j.jpainsymman.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Bruera E, Sweeney C. Methadone use in cancer patients with pain: a review. J Palliat Med. 2002;5(1):127–138. doi: 10.1089/10966210252785097. [DOI] [PubMed] [Google Scholar]
- 19.Fainsinger RL, Nekolaichuk CL. A "TNM" classification system for cancer pain: the Edmonton Classification System for Cancer Pain (ECS-CP) Support Care Cancer. 2008;16(6):547–555. doi: 10.1007/s00520-008-0423-3. [DOI] [PubMed] [Google Scholar]
- 20.Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7(2):6–9. [PubMed] [Google Scholar]
- 21.Chang VT, Hwang SS, Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer. 2000;88(9):2164–2171. doi: 10.1002/(sici)1097-0142(20000501)88:9<2164::aid-cncr24>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 22.Reddy SK. Pain Management. In: Elsayem A, Driver L, Bruera E, editors. The M.D. Anderson Symptom Control and Palliative Care Handbook. ed. 2nd. Houston: M.D. Anderson Cancer Center; 2004. p. 38. [Google Scholar]
- 23.Ripamonti C, De Conno F, Groff L, Belzile M, Pereira J, Hanson J, et al. Equianalgesic dose/ratio between methadone and other opioid agonists in cancer pain: comparison of two clinical experiences. Ann Oncol. 1998;9(1):79–83. doi: 10.1023/a:1008263910494. [DOI] [PubMed] [Google Scholar]
- 24.Walker PW, Palla S, Pei BL, Kaur G, Zhang K, Hanohano J, et al. Switching from methadone to a different opioid: what is the equianalgesic dose ratio? J Palliat Med. 2008;11(8):1103–1108. doi: 10.1089/jpm.2007.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pereira J, Lawlor P, Vigano A, Dorgan M, Bruera E. Equianalgesic dose ratios for opioids. a critical review and proposals for long-term dosing. J Pain Symptom Manage. 2001;22(2):672–687. doi: 10.1016/s0885-3924(01)00294-9. [DOI] [PubMed] [Google Scholar]
- 26.Lawlor PG, Turner KS, Hanson J, Bruera ED. Dose ratio between morphine and methadone in patients with cancer pain: a retrospective study. Cancer. 1998;82(6):1167–1173. [PubMed] [Google Scholar]
- 27.Oneschuk D, Bruera E. Respiratory depression during methadone rotation in a patient with advanced cancer. J Palliat Care. 2000;16(2):50–54. [PubMed] [Google Scholar]
- 28.Watanabe S, Tarumi Y, Oneschuk D, Lawlor P. Opioid rotation to methadone: proceed with caution. J Clin Oncol. 2002;20(9):2409–2410. doi: 10.1200/JCO.2002.20.9.2409. [DOI] [PubMed] [Google Scholar]
- 29.Quigley C. Opioid switching to improve pain relief and drug tolerability. Cochrane Database Syst Rev. 2004;(3):CD004847. doi: 10.1002/14651858.CD004847. [DOI] [PubMed] [Google Scholar]
- 30.Mancini I, Lossignol DA, Body JJ. Opioid switch to oral methadone in cancer pain. Curr Opin Oncol. 2000;12(4):308–313. doi: 10.1097/00001622-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Bruera E, Watanabe S, Fainsinger RL, Spachynski K, Suarez-Almazor M, Inturrisi C. Custom-made capsules and suppositories of methadone for patients on high-dose opioids for cancer pain. Pain. 1995;62(2):141–146. doi: 10.1016/0304-3959(94)00257-F. [DOI] [PubMed] [Google Scholar]
- 32.Morley JS, Watt JW, Wells JC, Miles JB, Finnegan MJ, Leng G. Methadone in pain uncontrolled by morphine. Lancet. 1993;342(8881):1243. doi: 10.1016/0140-6736(93)92228-l. [DOI] [PubMed] [Google Scholar]
- 33.Ripamonti C, Groff L, Brunelli C, Polastri D, Stavrakis A, De Conno F. Switching from morphine to oral methadone in treating cancer pain: what is the equianalgesic dose ratio? J Clin Oncol. 1998;16(10):3216–3221. doi: 10.1200/JCO.1998.16.10.3216. [DOI] [PubMed] [Google Scholar]
- 34.Tse DM, Sham MM, Ng DK, Ma HM. An ad libitum schedule for conversion of morphine to methadone in advanced cancer patients: an open uncontrolled prospective study in a Chinese population. Palliat Med. 2003;17(2):206–211. doi: 10.1191/0269216303pm696oa. [DOI] [PubMed] [Google Scholar]
- 35.Mercadante S, Casuccio A, Agnello A, Serretta R, Calderone L, Barresi L. Morphine versus methadone in the pain treatment of advanced-cancer patients followed up at home. J Clin Oncol. 1998;16(11):3656–3661. doi: 10.1200/JCO.1998.16.11.3656. [DOI] [PubMed] [Google Scholar]
- 36.De Conno F, Groff L, Brunelli C, Zecca E, Ventafridda V, Ripamonti C. Clinical experience with oral methadone administration in the treatment of pain in 196 advanced cancer patients. J Clin Oncol. 1996;14(10):2836–2842. doi: 10.1200/JCO.1996.14.10.2836. [DOI] [PubMed] [Google Scholar]
- 37.Daeninck PJ, Bruera E. Reduction in constipation and laxative requirements following opioid rotation to methadone: a report of four cases. J Pain Symptom Manage. 1999;18(4):303–309. doi: 10.1016/s0885-3924(99)00086-x. [DOI] [PubMed] [Google Scholar]
- 38.Kharasch ED, Bedynek PS, Park S, Whittington D, Walker A, Hoffer C. Mechanism of ritonavir changes in methadone pharmacokinetics and pharmacodynamics: I. Evidence against CYP3A mediation of methadone clearance. Clin Pharmacol Ther. 2008;84(4):497–505. doi: 10.1038/clpt.2008.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kharasch ED, Hoffer C, Whittington D, Walker A, Bedynek PS. Methadone pharmacokinetics are independent of cytochrome P4503A (CYP3A) activity and gastrointestinal drug transport: insights from methadone interactions with ritonavir/indinavir. Anesthesiology. 2009;110(3):660–672. doi: 10.1097/ALN.0b013e3181986a9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferrari A, Coccia CP, Bertolini A, Sternieri E. Methadone--metabolism, pharmacokinetics and interactions. Pharmacol Res. 2004;50(6):551–559. doi: 10.1016/j.phrs.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Haddad A, Davis M, Lagman R. The pharmacological importance of cytochrome CYP3A4 in the palliation of symptoms: review and recommendations for avoiding adverse drug interactions. Support Care Cancer. 2007;15(3):251–257. doi: 10.1007/s00520-006-0127-5. [DOI] [PubMed] [Google Scholar]
- 42.Viskin S. Long QT syndromes and torsade de pointes. Lancet. 1999;354(9190):1625–1633. doi: 10.1016/S0140-6736(99)02107-8. [DOI] [PubMed] [Google Scholar]
- 43.Fredheim OM, Borchgrevink PC, Hegrenaes L, Kaasa S, Dale O, Klepstad P. Opioid switching from morphine to methadone causes a minor but not clinically significant increase in QTc time: A prospective 9-month follow-up study. J Pain Symptom Manage. 2006;32(2):180–185. doi: 10.1016/j.jpainsymman.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 44.Al-Khatib SM, LaPointe NM, Kramer JM, Califf RM. What clinicians should know about the QT interval. JAMA. 2003;289(16):2120–2127. doi: 10.1001/jama.289.16.2120. [DOI] [PubMed] [Google Scholar]
- 45.Benitez-Rosario MA, Salinas-Martin A, Aguirre-Jaime A, Perez-Mendez L, Feria M. Morphine-Methadone Opioid Rotation in Cancer Patients: Analysis of Dose Ratio-Predicting Factors. J Pain Symptom Manage. 2009 doi: 10.1016/j.jpainsymman.2008.05.016. Epub ahead of print 2009/01/28. Available from http://download.journals.elsevierhealth.com/pdfs/journals/0885-3924/PIIS0885392408006532.pdf. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe S, Belzile M, Kuehn N, Hanson J, Bruera E. Capsules and suppositories of methadone for patients on high-dose opioids for cancer pain: clinical and economic considerations. Cancer Treat Rev. 1996;22 Suppl A:131–136. doi: 10.1016/s0305-7372(96)90075-4. [DOI] [PubMed] [Google Scholar]
- 47.Dickerson ED. Methadone: the question or the answer for US opioid therapy and pharmacoeconomics? Support Care Cancer. 2001;9(8):646–648. doi: 10.1007/s005200100266. [DOI] [PubMed] [Google Scholar]
- 48.Parsons HA, Shukkoor A, Quan H, Delgado-Guay MO, Palmer JL, Fainsinger R, et al. Intermittent subcutaneous opioids for the management of cancer pain. J Palliat Med. 2008;11(10):1319–1324. doi: 10.1089/jpm.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]