Abstract
An episode of status epilepticus (SE), if left untreated, can lead to death, or brain damage with long-term neurological consequences, including the development of epilepsy. The most common first-line treatment of SE is administration of benzodiazepines (BZs). However, the efficacy of BZs in terminating seizures is reduced with time after the onset of SE; this is accompanied by a reduced efficacy in protecting the hippocampus against neuronal damage, and is associated with impaired function and internalization of hippocampal GABAA receptors. In the present study, using Fluoro-Jade C staining, we found that administration of diazepam to rats at 3 h after the onset of kainic acid-induced SE, at a dose sufficient to terminate SE, had no protective effect on the hippocampus, but produced a significant reduction in neuronal degeneration in the amygdala, piriform cortex, and endopiriform nucleus, examined on days 7−9 after SE. Thus, in contrast to the hippocampus, the amygdala and other limbic structures are responsive to neuroprotection by BZs after prolonged SE, suggesting that GABAA receptors are not significantly altered in these structures during SE.
Keywords: Amygdala, Hippocampus, Benzodiazepines, Status epilepticus, Neurodegeneration, Epileptogenesis
Introduction
Status epilepticus (SE) in humans is an acute medical emergency associated with significant morbidity and mortality (Krumholz et al. 1995; Cascino et al. 1998; Logroscino et al. 2002). Seizures during SE are generalized and enduring, and if not controlled timely, brain damage may occur (Treiman 2007) leading to neurological, cognitive, or other behavioral deficits (Krumholz et al. 1995). Furthermore, after an episode of SE, patients have a high risk of developing epilepsy (Hesdorffer et al. 1998). This is because SE can trigger epileptogenesis, a process whereby—in the absence of further seizures after the initial SE episode—structural and functional alterations take place in certain brain regions that play a key role in epilepsy, culminating in the appearance of spontaneous seizures and eventually the development of epilepsy. SE has been successfully used in animal models as a trigger for epileptogenesis and development of temporal lobe epilepsy (TLE). In the SE animal model, neuronal loss and reorganization of neuronal circuits during epileptogenesis are similar to neuropathological findings in TLE patients (for a review see Pitkänen et al. 2007), particularly in regard to the neurodegeneration that occurs in limbic structures (Tuunanen et al. 1996; Covolan and Mello 2000; Aroniadou-Anderjaska et al. 2008).
The first-line treatment for SE is administration of benzodiazepines (BZs), which increase the efficacy of GABAA receptor-mediated inhibition by binding to specific sites on GABAA receptors and allosterically modulating the GABAA receptor complex (Macdonald and Olsen 1994; McKernan and Whiting 1996). However, BZs lose their efficacy if not administered early (Treiman et al. 1998), and higher concentrations, or more aggressive second- and third-line antiepileptic drugs must be used (Bleck 1999; Jones et al. 2002; Riss et al. 2008). The weakening anti-convulsant effect of BZs with increasing duration of SE has been associated with impaired function and internalization of GABAA receptors in the hippocampus (Goodkin et al. 2005; Naylor et al. 2005; Feng et al. 2008). This is consistent with the lack of protection against neuronal loss in the hippocampus when the BZ diazepam is administered 3 h after the onset of SE (Pitkänen et al. 2005). It is unknown, however, if other limbic structures that play an important role in seizure generation also lose their responsiveness to BZs soon after the onset of SE. Despite the loss of hippocampal responsiveness to BZs as SE progresses, these drugs maintain some effectiveness in reducing seizures, particularly if the doses are increased (Treiman 1990; Jones et al. 2002). This suggests that other brain regions that are important in sustaining SE may remain responsive to benzodiazepines even at later stages of SE. As a first step in investigating this question, we compared the protective effect of diazepam against neuronal degeneration in the hippocampus and in the amygdala, as well as the piriform cortex and endopiriform nucleus, when the drug is administered at 3 h after SE. Neuronal degeneration was examined on days 7−9 after SE.
We studied the amygdala because of its important role in TLE (Quesney 1986; Pitkänen et al. 1998; Aroniadou-Anderjaska et al. 2008), as well as in the generation and spread of seizure activity in the SE and kindling animal models of TLE (White and Price 1993a, b; Mohapel et al. 1996). The volume of the amygdala is often reduced in TLE patients (Cendes et al. 1993; Cendes et al. 1994; Wolf et al. 1997; Van Paesschen et al. 2001), while in SE animal models the amygdala suffers extensive neuronal loss (Tuunanen et al. 1996; Hsieh 1999). Similarly, the piriform cortex and the adjacent endopiriform nucleus also undergo substantial neuronal loss after SE (Ben-Ari 1985; Covolan and Mello 2000; Druga et al. 2003; Chen and Buckmaster 2005) and may play an important role in epileptogenesis (for a review see Majak and Moryś 2007). We found that diazepam did not reduce neuronal degeneration in the hippocampus, while it had a significant protective effect in the amygdala, the piriform cortex and the endopiriform nucleus.
Methods
Animals
Experiments were performed on male Sprague Dawley rats (Taconic Farms, Rockville, MD), 5−6 weeks old, weighing 170−200 g at the start of the experiments. Animals were individually housed in an environmentally controlled room (20−23°C, 12-h light/12-h dark cycle, lights on 06:00 a.m.), with food and water available ad libitum. All animal experiments were in accordance with our institutional guidelines after obtaining approval of the Institutional Animal Care and Use Committee (IACUC).
Induction of SE
Five rats implanted with four cortical screw electrodes plus four non-implanted rats were injected (i.p.) with kainic acid (KA) to induce SE, following a modified titration protocol (Hellier et al. 1998). The electrode-implanted rats were allowed 1 week of recovery before KA injections. Rats were initially treated with 7.5−8 mg/kg KA dissolved in 0.1 M phosphate buffered normal saline (PBS; 5 mg/ml), followed by subsequent doses of 4−5 mg/kg until the onset of SE. For implanted rats, SE onset was defined by 5 min of continuous generalized electrographic seizure activity in all four cortical electrodes involved with no breaks longer than 10 s; SE was allowed to continue for 180 min, until termination of both behavioral and electrographic seizures with 25 mg/kg of diazepam (i.p.). This group of rats will be referred to as KA +DZP group (n = 5). The four non-implanted rats were given KA to induce SE, using behavioral assessments to determine the necessity of additional injections and the onset of SE (defined by the first generalized behavioral seizure; Stage 4 from Racine 1972). In this group, diazepam was not used to terminate SE (KA group). Behavioral SE was observed for more than 4 h after the first Stage 4 behavioral seizure. SE was still ongoing when monitoring was stopped. There were no mortalities. Previous studies have shown that SE induced by KA results in behavioral seizures that can continue for more than 7 h (Tuunanen et al. 1999), and epileptiform spiking for up to 12−14 h (Pitkänen et al. 2005) after KA injections. The KA group received 16.6 ± 0.67 KA injections, and the KA +DZP group received 20.5 ± 2.55 KA injections to induce SE (no significant difference between the number of KA injections needed in the two groups; P < 0.31).
The KA +DZP group was electrode-implanted so that we can determine the efficacy of diazepam to terminate electrographic seizures in addition to the behavioral seizures. In previous experiments, we have examined neuronal degeneration (using Fluoro-Jade C) in “sham rats” that were electrode-implanted but received no other treatment, and control rats which did not receive any treatment. There were no degenerating cells in the amygdala or hippocampus in either group. Therefore, the electrode implantation in the group which received diazepam is unlikely to have affected the results.
Fixation and tissue processing
The four rats of the KA group and the five rats of the KA +DZP group were used for morphological analysis of the amygdala, hippocampus, piriform cortex, and endopiriform nucleus. Seven to nine days after KA-induced SE, rats were deeply anesthetized using ketamine (60 mg/kg i.p.) and medetomidine (0.5 mg/kg i.p.) and transcardially perfused with PBS (100 ml) followed by 4% paraformaldehyde (250 ml). The brains were removed and post-fixed overnight at 4°C, then transferred to a solution of 30% sucrose in PBS for 72 h, and frozen with dry ice before storage at −80°C until sectioning. A one-in-six series of sections containing the rostro-caudal extent of the amygdala was cut at 40 μm on a sliding microtome. One series of sections was mounted on slides (Superfrost Plus, Daigger, Vernon Hills, IL) in PBS for Nissl staining with cresyl violet. An adjacent series of sections was also mounted on slides for Fluoro-Jade C (FJ) staining.
Fluoro-Jade C staining
Fluoro-Jade C (Histo-Chem, Jefferson, AK) was used to identify dying neurons in the amygdala, hippocampus, piriform cortex, and endopiriform nucleus, at 7−9 days after SE. Mounted sections were air-dried overnight, and then immersed in a solution of 1% sodium hydroxide in 80% ethanol for 5 min. The slides were then rinsed for 2 min in 70% ethanol, 2 min in dH20, and incubated in 0.06% potassium permanganate solution for 10 min. After a 2 min rinse in dH20, the slides were transferred to a 0.0001% solution of Fluoro-Jade C dissolved in 0.1% acetic acid for 10 min. Following three 1-min rinses in dH20, the slides were dried on a slide warmer, cleared in xylene for at least 1 min and cover slipped with DPX (Sigma).
Evaluation of Fluoro-Jade C
Tracings of the amygdala, dorsal hippocampus, piriform cortex, and endopiriform nucleus from an adjacent series of Nissl stained sections were superimposed on the FJ-stained sections. For qualitative analysis of FJ-stained sections, the following rating system was used to determine the score for the extent of degeneration in each structure: 0 = no damage; 1 = minimal damage (1−10%); 2 = mild damage (10−25%); 3 = moderate damage (25−45%); and 4 = severe damage (>45%). Qualitative assessments were made from 6 sections per animal, and the average for each animal was recorded. For quantitative analysis, FJ positive cells were counted in each outlined structure at 20×, and recorded as a density (number of cells per mm2) from, on average, six sections.
Statistical analysis
All statistical values are presented as mean ± SEM. The KA group and the KA +DZP group were compared using the unpaired Student's t test. Differences between the two groups were considered statistically significant when P < 0.05. Sample sizes (n) refer to the number of rats.
Results
Neuropathology of limbic structures
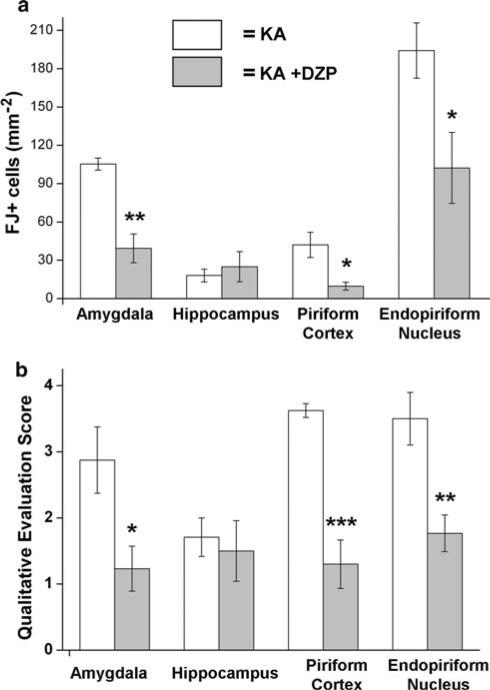
Neuronal damage of limbic structures occurs both acutely (Tuunanen et al. 1999; Covolan and Mello 2000) and as a delayed process in the weeks, or even months, after SE (Tuunanen et al. 1996; Chen and Buckmaster 2005). In the present study, degenerating neurons were identified using FJ, a fluorescent marker that binds to irreversibly damaged neurons (Schmued et al. 1997). We characterized the extent of neurodegeneration in different limbic structures during epileptogenesis triggered by KA-induced SE in two groups: rats in which SE was not terminated with diazepam (KA rats; n = 4) and rats in which SE was terminated after 3 h with diazepam (KA ?DZP rats; n = 5). In both groups, the region with the most extensive ongoing degeneration, having the highest density of FJ+ cells, was the endopiri-form nucleus, followed by the amygdala (Table 1; Fig. 3). To a lesser extent, neurodegeneration was also observed in the piriform cortex and hippocampus (Table 1; Fig. 3).
Table 1.
Data from quantitative and qualitative analysis of Fluoro-Jade C staining in different limbic structures from rats 7−9 days after KA-induced SE (KA group) or after KA-induced SE that was terminated 3 h after its onset, by administration of diazepam (KA +DZP group)
| KA group | KA +DZP group | p | % reduction | |
|---|---|---|---|---|
| Quantitative evaluation (number of FJ+ cells, mm−2) | ||||
| Amygdala | 105.27 ± 4.71 | 39.29 ± 11.26 | 0.0024 | 62.7 |
| Hippocampus | 18.09 ± 5.00 | 24.96 ± 11.79 | 0.640 | - |
| Piriform cortex | 42.06 ± 9.91 | 9.73 ± 3.04 | 0.0107 | 76.9 |
| Endopiriform nucleus | 194.19 ± 21.67 | 102.29 ± 27.85 | 0.0414 | 47.33 |
| Qualitative evaluation score of FJ staining | ||||
| Amygdala | 2.88 ± 0.50 | 1.23 ± 0.34 | 0.0264 | 57.2 |
| Hippocampus | 1.71 ± 0.29 | 1.50 ± 0.46 | 0.7303 | 12.2 |
| Piriform cortex | 3.63 ± 0.10 | 1.30 ± 0.37 | 0.0009 | 64.2 |
| Endopiriform nucleus | 3.50 ± 0.40 | 1.77 ± 0.28 | 0.0077 | 49.4 |
Values are presented as mean ± standard error of the mean
Qualitative scale: 0 = no damage, 1 = minimal damage (0−10%), 2 = mild damage (10−25%), 3 = moderate damage (25−45%), and 4 = severe damage (>45%)
Fig. 3.
Quantitative and qualitative analysis of Fluoro-Jade C staining reveal that termination of KA-induced SE by diazepam, 3 h after its onset, reduces neuronal degeneration during epileptogenesis in the amygdala, piriform cortex, and endopiriform nucleus, but not in the hippocampus. a Comparison of the number of Fluoro-Jade C positive stained cells per mm2 in the amygdala, hippocampus, piriform cortex and endopiriform nucleus of rats in which SE induced by kainic acid was not terminated (KA group; n = 4 rats) and rats in which SE was terminated after 3 h with diazepam (KA +DZP group; n = 5 rats). b Qualitative assessment of the extent of neurodegeneration produced similar relationships between the KA group and the KA +DZP group, as the quantitative evaluation; scale: 0 no damage, 1 minimal damage (0−10%), 2 mild damage (10−25%), 3 moderate damage (25−45%), 4 severe damage ([45%). Values are mean ± SEM, *P < 0.05, **P < 0.005; ***P < 0.001
Hippocampus
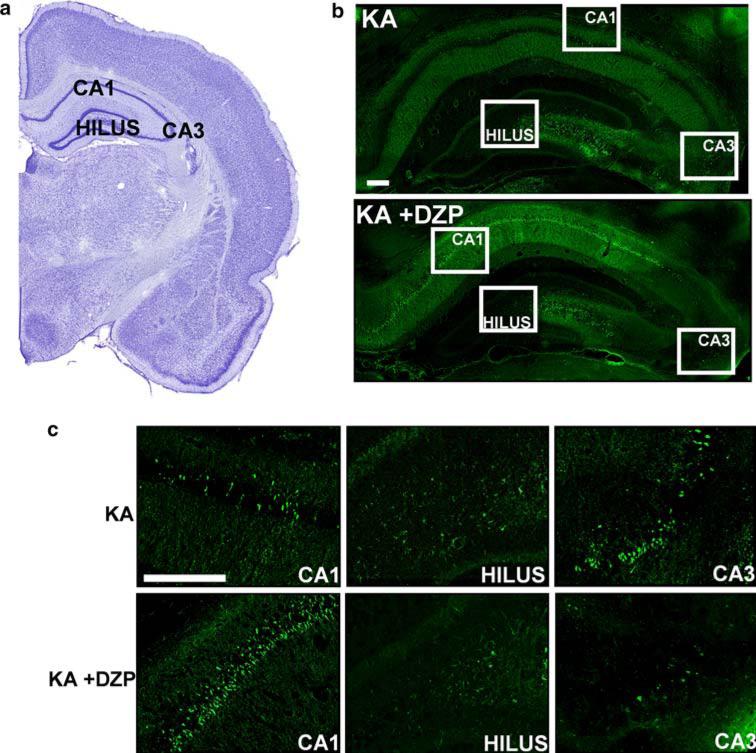
In the hippocampus of the KA rats, 18.09 ± 5.00 FJ+ cells were counted (see methods), on days 7−9 after KA-induced SE. The extent of neurodegeneration was not significantly different from that in the KA +DZP rats, in which 24.96 ± 11.79 FJ+ cells were counted in the hippocampus (Figs. 1, 3; Table 1). The qualitative scoring system also showed no significant difference in these groups, with a score of 1.71 ± 0.29 for the KA group and 1.50 ± 0.46 for the KA +DZP group. The most extensively damaged subfields were the CA3, CA1 and hilar region. Thus, administration of diazepam at 3 h after the onset of SE has no effect on the extent of neurodegeneration in the hippocampus, 7−9 days after SE.
Fig. 1.
Termination of KA-induced SE by diazepam, 3 h after its onset, does not reduce neuronal degeneration in the hippocampus during epileptogenesis. Nissl-stained (a) and Fluoro-Jade C-stained (b) sections demonstrating hippocampal subfields. The CA1, CA3, and hilus regions are shown at higher magnification in c. There was no significant difference in the number of the irreversibly degenerating cells between the group that was administered diazepam (KA +DZP group) 3 h after the onset of SE, and the group that was not treated with diazepam (KA group). Staining in this example was performed 7 days after SE. Scale bar 300 μm
Amygdala
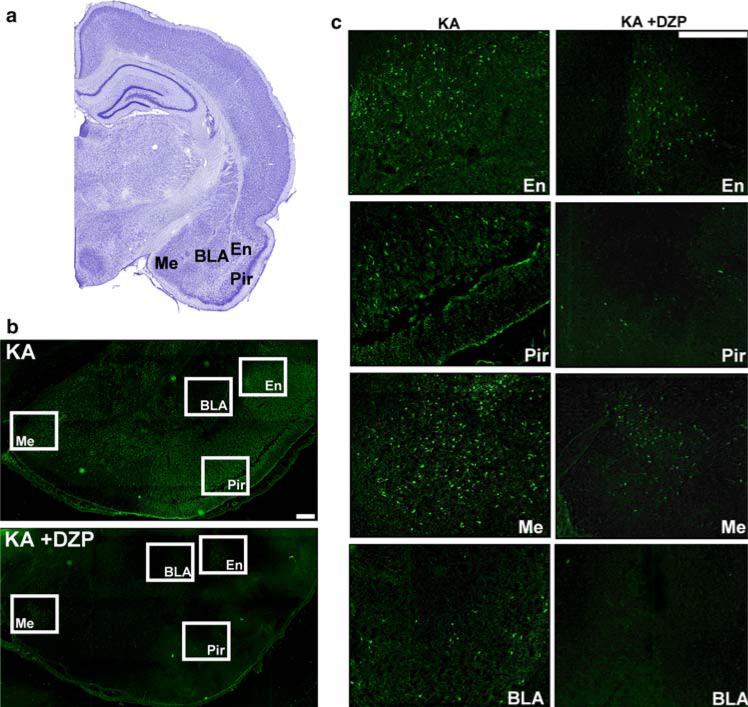
The amygdala plays a central role in the generation and spread of seizure activity in animal models of epilepto-genesis (White and Price 1993a, b; Mohapel et al. 1996). Benzodiazepine-sensitive GABAA receptors are present in the amygdala (Sieghart and Sperk 2002). In the present study, we found extensive neurodegeneration in the amygdala, 7−9 days after SE, which was significantly reduced by diazepam. The qualitative analysis revealed a score of 2.88 ± 0.50 for the KA group, which was significantly higher than the score of 1.23 ± 0.34 in the KA +DZP group (P < 0.05). In the quantitative analysis, we counted 105.27 ± 4.29 FJ+ cells/mm2 in the amygdala of the KA rats. This number was significantly reduced (by 63%) in the KA +DZP group, in which 39.29 ± 11.26 FJ+ cells were counted (Figs. 2, 3; Table 1; P < 0.005). Thus, diazepam administered after prolonged SE reduces neuronal degeneration in the amygdala, 7−9 days after SE.
Fig. 2.
Termination of KA-induced SE by diazepam, 3 h after its onset, reduces neuronal neurodegeneration in the amygdala, piriform cortex, and endopiriform nucleus during epileptogenesis. Nissl-stained (a) and Fluoro-Jade C-stained (b) sections demonstrating the medial (Me), basolateral (BLA) nuclei of the amygdala, the piriform cortex (Pir), and endopiriform nucleus (En). The Fluoro-Jade C-stained sections are shown at higher magnification in c. The number of irreversibly degenerating cells in the rats that were administered diazepam (KA +DZP group), 3 h after the onset of SE, was significantly lower than the number of degenerating cells in the group that was not treated with diazepam (KA group). Staining in this example was performed 7 days after SE. Scale bar 300 μm
Piriform cortex and endopiriform nucleus
The piriform cortex and the adjacent endopiriform nucleus are also susceptible to damage after SE (Ben-Ari 1985; Covolan and Mello 2000; Druga et al. 2003; Chen and Buckmaster 2005). In the present study, we determined if terminating SE after 3 h with diazepam reduces neurode-generation in these two brain regions. At 7−9 days after SE, the qualitative evaluation score for neurodegeneration in the piriform cortex was 3.63 ± 0.10 for the KA group, compared to 1.30 ± 0.37 for the KA +DZP group, indicating that diazepam significantly (P < 0.001) reduced the extent of neurodegeneration (Fig. 3; Table 1). Similarly, we found a significant reduction (77%; P < 0.05) in the number of degenerating cells in the piriform cortex of the KA +DZP rats (Figs. 2, 3; Table 1). Thus, in the KA group we counted 42.06 ± 9.91 FJ+ cells, while the number of FJ+ cells in the KA +DZP group was 9.73 ± 3.04.
The endopiriform nucleus had the densest FJ+ cells compared to the other structures (Figs. 2, 3; Table 1). The qualitative evaluation score for neurodegenerating cells in the endopiriform nucleus of rats in the KA group was 3.50 ± 0.40, whereas in the KA +DZP group it was significantly lower (1.77 ± 0.28; P < 0.01). Similarly, the number of FJ+ cells in the KA group was 194.19 ± 21.67, while the number of FJ+ cells in the KA +DZP group was 102.29 ± 27.85, a 47% reduction (P < 0.05; Figs. 2, 3; Table 1).
Discussion
In the present study, we found that neurodegeneration occurring at 7−9 days after KA-induced SE is more extensive in the amygdala and the endopiriform nucleus compared to the piriform cortex and the hippocampus, and that SE termination after 3 h by administration of diazepam results in significant protection from neurodegeneration in the amygdala, endopiriform nucleus, and piriform cortex, but not in the hippocampus.
Atrophy of temporal lobe structures, which implies primarily neuronal loss, is a frequently reported histopathology of TLE patients (Babb and Brown 1989; Babb 1991; Cendes et al. 1993; Hudson et al. 1993; Williamson et al. 1993; Cendes et al. 1994). Similarly, in animal models of TLE, neuronal damage of limbic structures is evident after an epileptogenic insult (Schwob et al.1980; Heggli and Malthe-Sørensson 1982; Tuunanen et al. 1996, 1999; Covolan and Mello 2000; Chen and Buckmaster 2005). The amygdala appears to be particularly vulnerable to seizure-induced neuronal damage, as seen either acutely after SE (Schwob et al. 1980; Tuunanen et al. 1999; Covolan and Mello 2000), during the epileptogenic latent period (Heggli and Malthe-Sørensson 1982; Tuunanen et al. 1996, Qashu et al. 2008), or after spontaneous seizures have developed (Chen and Buckmaster 2005). By using a marker specific for irreversibly degenerating cells (Schmued et al. 1997), we show here that the extent of ongoing neurodegeneration during epileptogenesis is greater in the amygdala and the endopiriform nucleus compared to the hippocampus and piriform cortex. A higher susceptibility of the amygdala to seizure-induced brain damage compared to the hippocampus has also been observed in previous studies after KA-induced SE (Schwob et al. 1980; Heggli and Malthe-Sørensson 1982; Riba-Bosch and Pérez-Claussell 2004; Chen and Buckmaster 2005), but also after SE induced by other mechanisms, such as administration of nerve agents (Shih et al. 2003). There is evidence suggesting that the amygdala is even more prone to generating seizure activity than the hippocampus (Goddard 1967; Kairiss et al. 1984; Racine et al. 1988), and, therefore, during SE the amygdala perhaps suffers more intense seizures than the hippocampus, resulting in greater neuropathological damage. It is not clear at present if there are also other mechanisms related to the physiology and biochemistry of the amygdala that make this structure more vulnerable to seizure-induced neuronal damage than other brain regions, even when the intensity and duration of the seizures are not higher in the amygdala. The endopiriform nucleus is another brain region that is highly susceptible to seizure-induced neuronal damage, as revealed in the present study, as well as in previous studies (Covolan and Mello 2000; Druga et al. 2003; Chen and Buckmaster 2005).
GABAergic interneurons in the amygdala are the most vulnerable to SE-induced damage (Tuunanen et al. 1996). We have recently found that in the basolateral amygdala, the amygdala nucleus that plays the most central role in the initiation and propagation of seizure activity (White and Price 1993a, b; Mohapel et al. 1996), the loss of GABAergic neurons is significantly greater than the loss of other cells, 7−10 days after SE, resulting in a dramatic reduction of inhibitory activity (Qashu et al. 2008). Thus, because it is GABAergic neurons that are primarily lost in the amygdala after SE, the amygdala circuitry becomes hyperexcitable, which may contribute significantly to the progression of epileptogenesis and the development of epilepsy. It is important therefore to protect against SE-induced neuronal loss in the amygdala.
We found in the present study that diazepam administration to terminate SE reduced neuronal degeneration in the amygdala, endopiriform cortex and piriform cortex, but not in the hippocampus. Benzodiazepine (BZ)-sensitive receptors are densely located in limbic structures (Niehoff and Kuhar 1983; Fritschy and Mohler 1995), and BZs are the first-line treatment for termination of SE in humans (Chen and Wasterlain 2006), and reduction of mortality after prolonged SE in animal models of TLE (Mello et al. 1993). As SE duration increases, however, BZs are less potent as anticonvulsants (Walton and Treiman 1988; Jones et al. 2002) and neuroprotectants (Pitkänen et al. 2005), and recent data suggest that this could be due to an internalization of GABAA receptors, as described in the hippocampus, beginning in the first hour of SE (Naylor et al. 2005; Goodkin et al. 2005). The failure of neuro-protection in the hippocampus by diazepam after 3 h of SE reported previously (Pitkänen et al. 2005) is consistent with our results that diazepam administration after 3 h of SE did not reduce neurodegeneration in the hippocampus, 1 week after SE. However, the number of degenerating cells in the amygdala, piriform cortex, and endopiriform nucleus was reduced by diazepam. Thus, the impaired function and internalization of GABAA receptors in the hippocampus (Goodkin et al. 2005; Naylor et al. 2005; Feng et al. 2008) that diminish the efficacy of diazepam may not occur in other limbic structures that retain responsiveness to BZs after prolonged SE.
A reduction of GABAA receptor expression has not been reported acutely after SE in the amygdala, piriform cortex, or endopiriform nucleus. In fact, Kish et al. (1983) have shown no change in radio-labeled BZ- or GABA-binding 2 h after SE in the amygdala/piriform cortex area; however, this study also reported no change in the hippocampus. Radio-labeled ligands can bind non-neuronal cell types such as glia; this may account for the difference in the results of Kish et al. (1983) and those by Goodkin et al. (2005), and Naylor et al. (2005), which used electrophysiology and immunohistochemistry to show SE-induced intracellular accumulation/internalization of GABAA receptors in the hippocampus. Thus, it is unclear at present whether GABAA receptors are not downregulated in the amygdala, piriform cortex, and endopiriform nucleus during prolonged SE, which would account for the neuroprotective effect of diazepam in these brain regions.
A direct neuroprotective effect by diazepam administration after prolonged SE may be difficult to tease apart from its anticonvulsant effect. Because the severity of neuronal loss corresponds to the duration of SE (Lemos and Cavalheiro 1995; Gorter et al. 2003), the termination of SE by diazepam alone may be sufficient to reduce the subsequent neuronal loss without a direct neuroprotective effect of diazepam. The dose of diazepam used in the present study (25 mg/kg) is effective at terminating SE, as determined by disruption of electrographic cortical seizure activity (not shown here). However, the use of deep electrodes would be necessary to determine if subcortical electrographic seizure activity was also attenuated or terminated with diazepam. Because seizure or spiking activity in subcortical structures can persist even after attenuation of cortical seizure activity (unpublished observations), and, in the hippocampus, GABAA receptors are internalized within the first hour of SE (Goodkin et al. 2005; Naylor et al. 2005; Feng et al. 2008), the lack of a neuroprotective effect of diazepam in the hippocampus may be explained by a reduced anticonvulsant efficacy of diazepam within this region during prolonged SE.
Pitkänen et al. (2005) reported disease modifying effects of diazepam administered after 3 h of SE, such as reduced frequency of spontaneous seizures; this was attributed to reduced SE duration compared to that of vehicle-treated rats. Although not analyzed here, we would have expected to find similar results, not only because of SE termination, but also because of the significant reduction of neurode-generation in all limbic structures studied, apart from the hippocampus.
Because the efficacy of many of the proposed anti-epileptogenic and/or neuroprotective treatments has been analyzed in a limited number of brain areas, we underscore here the need to evaluate structures beyond the hippocampus for neuroprotective efficacy during epilepto-genesis. The amygdala, a limbic structure that undergoes extensive neurodegeneration after SE-induced epilepto-genesis, responds to benzodiazepine treatment after prolonged SE, when the hippocampus does not. As the mechanisms underlying pharmacoresistance during prolonged SE are beginning to be unraveled in the hippocampus (Goodkin et al. 2005; Naylor et al. 2005; Feng et al. 2008), it is essential to understand the cellular changes that occur in other limbic structures for assessment of the full potential of anticonvulsant and neuroprotective therapies in animal models of TLE.
Acknowledgments
This work was funded by the National Institutes of Health CounterACT Program through the National Institute of Neurological Disorders and Stroke (award # U01 NS058162-01). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the federal government. This work was also supported by the Defense Threat Reduction Agency-Joint Science and Technology Office, Medical S&T Division (grant 1.E0021_07_US_C).
Contributor Information
Felicia Qashu, Neuroscience Program, Uniformed Services University of the Health Sciences, Bethesda, MD 20814, USA.
Taiza H. Figueiredo, Department of Anatomy, Physiology and Genetics, F. Edward Hébert School of Medicine, Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Bethesda, MD 20814, USA
Vassiliki Aroniadou-Anderjaska, Department of Anatomy, Physiology and Genetics, F. Edward Hébert School of Medicine, Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Bethesda, MD 20814, USA; Neuroscience Program, Uniformed Services University of the Health Sciences, Bethesda, MD 20814, USA.
James P. Apland, Neurotoxicology Branch, USAMRICD, Aberdeen Proving Ground, MD 21010, USA
Maria F. M. Braga, Department of Anatomy, Physiology and Genetics, F. Edward Hébert School of Medicine, Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Bethesda, MD 20814, USA Neuroscience Program, Uniformed Services University of the Health Sciences, Bethesda, MD 20814, USA.
References
- Aroniadou-Anderjaska V, Fritsch B, Qashu F, Braga MF. Pathology and pathophysiology of the amygdala in epileptogenesis and epilepsy. Epilepsy Res. 2008;78:102–116. doi: 10.1016/j.eplepsyres.2007.11.011. doi:10.1016/j.eplepsyres.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb TL. Research on the anatomy and pathology of epileptic tissue. In: Luders H, editor. Epilepsy surgery. Raven Press; New York: 1991. pp. 719–727. [Google Scholar]
- Babb TL, Brown WJ. Pathological findings in epilepsy. In: Engel J, editor. Surgical treatment of the epilepsies. Raven Press; New York: 1989. pp. 511–540. [Google Scholar]
- Ben-Ari Y. Limbic seizures and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14:375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- Bleck TP. Management approaches to prolonged seizures and status epilepticus. Epilepsia. 1999;40(S1):59–63. doi: 10.1111/j.1528-1157.1999.tb00880.x. doi:10.1111/j.1528−1157.1999.tb00880.x. [DOI] [PubMed] [Google Scholar]
- Cascino GD, Hesdorffer D, Logroscino G, Hauser WA. Morbidity of nonfebrile status epilepticus in Rochester, Minnesota, 1965−1984. Epilepsia. 1998;39:829–832. doi: 10.1111/j.1528-1157.1998.tb01176.x. doi:10.1111/j.1528−1157.1998.tb01176.x. [DOI] [PubMed] [Google Scholar]
- Cendes F, Andermann F, Gloor P, Evans A, Jones-Gotman M, Watson C, Melanson D, Olivier A, Peters T, Lopes-Cendes I, Leroux RT. MRI volumetric measurement of amygdala and hippocampus in temporal lobe epilepsy. Neurology. 1993;43:719–725. doi: 10.1212/wnl.43.4.719. [DOI] [PubMed] [Google Scholar]
- Cendes F, Andermann F, Gloor P, et al. Relationship between atrophy of the amygdala and ictal fear in temporal lobe epilepsy. Brain. 1994;117:739–746. doi: 10.1093/brain/117.4.739. doi:10.1093/brain/117.4.739. [DOI] [PubMed] [Google Scholar]
- Chen S, Buckmaster PS. Stereological analysis of forebrain regions in kainate-treated epileptic rats. Brain Res. 2005;1057:141–152. doi: 10.1016/j.brainres.2005.07.058. doi:10.1016/j.brainres.2005.07.058. [DOI] [PubMed] [Google Scholar]
- Chen JW, Wasterlain CG. Status epilepticus: pathophysiology and management in adults. Lancet Neurol. 2006;5:246–256. doi: 10.1016/S1474-4422(06)70374-X. doi:10.1016/S1474−4422(06)70374-X. [DOI] [PubMed] [Google Scholar]
- Covolan L, Mello LE. Temporal profile of neuronal injury following pilocarpine or kainic acid-induced status epilepticus. Epilepsy Res. 2000;39:133–152. doi: 10.1016/s0920-1211(99)00119-9. doi:10.1016/S0920−1211(99)00119−9. [DOI] [PubMed] [Google Scholar]
- Druga R, Kubová H, Suchomelová L, Haugvicová R. Lithium/pilocarpine status epilepticus-induced neuropathology of piriform cortex and adjoining structures in rats is age-dependent. Physiol Res. 2003;52:251–264. [PubMed] [Google Scholar]
- Feng HJ, Mathews GC, Kao C, Macdonald RL. Alterations of GABA A-receptor function and allosteric modulation during development of status epilepticus. J Neurophysiol. 2008;99:1285–1293. doi: 10.1152/jn.01180.2007. doi:10.1152/jn.01180.2007. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. doi:10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- Goddard GV. Development of epileptic seizures through brain stimulation at low intensity. Nature. 1967;214:1020–1021. doi: 10.1038/2141020a0. doi:10.1038/2141020a0. [DOI] [PubMed] [Google Scholar]
- Goodkin HP, Yeh JL, Kapur J. Status epilepticus increases the intracellular accumulation of GABAA receptors. J Neurosci. 2005;25:5511–5520. doi: 10.1523/JNEUROSCI.0900-05.2005. doi:10.1523/JNEUROSCI.0900−05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorter JA, Goncalves Pereira PM, van Vliet EA, Aronica E, Lopes da Silva FH, Lucassen PJ. Neuronal cell death in a rat model for mesial temporal lobe epilepsy is induced by the initial status epilepticus and not by later repeated spontaneous seizures. Epilepsia. 2003;44:647–658. doi: 10.1046/j.1528-1157.2003.53902.x. doi:10.1046/j.1528−1157.2003.53902.x. [DOI] [PubMed] [Google Scholar]
- Heggli DE, Malthe-Sørensson D. Systemic injection of kainic acid: effect on neurotransmitter markers in piriform cortex, amygdaloid complex, and hippocampus and protection by cortical lesioning and anticonvulsants. Neuroscience. 1982;5:1257–1264. doi: 10.1016/0306-4522(82)91132-0. doi:10.1016/0306−4522(82)91132−0. [DOI] [PubMed] [Google Scholar]
- Hellier JL, Patrylo PR, Buckmaster PS, Dudek FE. Recurrent spontaneous motor seizures after repeated low-dose systemic treatment with kainate: assessment of a rat model of temporal lobe epilepsy. Epilepsy Res. 1998;31:73–84. doi: 10.1016/s0920-1211(98)00017-5. doi:10.1016/S0920−1211(98)00017−5. [DOI] [PubMed] [Google Scholar]
- Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA. Risk of unprovoked seizure after acute symptomatic seizure: effect of status epilepticus. Ann Neurol. 1998;44:908–912. doi: 10.1002/ana.410440609. doi:10.1002/ana.410440609. [DOI] [PubMed] [Google Scholar]
- Hsieh PF. Neuropathology of limbic status epilepticus by electrical stimulation of naïve rats. Neurol Res. 1999;21:399–403. doi: 10.1080/01616412.1999.11740950. [DOI] [PubMed] [Google Scholar]
- Hudson LP, Munoz DG, Miller L, McLachlan RS, Girvin JP, Blume WT. Amygdaloid sclerosis in temporal lobe epilepsy. Ann Neurol. 1993;33:622–631. doi: 10.1002/ana.410330611. doi:10.1002/ana.410330611. [DOI] [PubMed] [Google Scholar]
- Jones DM, Esmaeil N, Maren S, Macdonald RL. Characterization of pharmacoresistance to benzodiazepines in the rat Li-pilocarpine model of status epilepticus. Epilepsy Res. 2002;50:301–312. doi: 10.1016/s0920-1211(02)00085-2. doi:10.1016/S0920−1211(02)00085−2. [DOI] [PubMed] [Google Scholar]
- Kairiss EW, Racine RJ, Smith GK. The development of the interictal spike during kindling in the rat. Brain Res. 1984;322:101–110. doi: 10.1016/0006-8993(84)91185-5. doi:10.1016/0006−8993(84)91185−5. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Sperk G, Hornykiewicz O. Alterations in benzodiazepine and GABA receptor binding in rat brain following systemic injection of kainic acid. Neuropharmacology. 1983;22:1303–1309. doi: 10.1016/0028-3908(83)90204-6. doi:10.1016/0028−3908(83)90204−6. [DOI] [PubMed] [Google Scholar]
- Krumholz A, Sung GY, Fisher RS, Barry E, Bergey GK, Grattan LM. Complex partial status epilepticus accompanied by serious morbidity and mortality. Neurology. 1995;45:1499–1504. doi: 10.1212/wnl.45.8.1499. [DOI] [PubMed] [Google Scholar]
- Lemos T, Cavalheiro EA. Suppression of pilocarpine-induced status epilepticus and the late development of epilepsy in rats. Exp Brain Res. 1995;102:423–428. doi: 10.1007/BF00230647. doi:10.1007/BF00230647. [DOI] [PubMed] [Google Scholar]
- Logroscino G, Hesdorffer DC, Cascino GD, Annegers JF, Bagiella E, Hauser WA. Long-term mortality after a first episode of status epilepticus. Neurology. 2002;58:537–541. doi: 10.1212/wnl.58.4.537. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Majak K, Moryś J. Endopiriform nucleus connectivities: the implication for epileptogenesis and epilepsy. Folia Morphol. 2007;66:267–271. [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. doi:10.1016/S0166−2236(96)80023−3. [DOI] [PubMed] [Google Scholar]
- Mello LE, Cavalheiro EA, Tan AM, Kupfer WR, Pretorious JK, Babb TL, Finch DM. Circuit mechanisms of seizures in the pilocarpine model of chronic epilepsy: cell loss and mossy fiber sprouting. Epilepsia. 1993;34:985–995. doi: 10.1111/j.1528-1157.1993.tb02123.x. doi:10.1111/j.1528−1157.1993.tb02123.x. [DOI] [PubMed] [Google Scholar]
- Mohapel P, Dufresne C, Kelly ME, McIntyre DC. Differential sensitivity of various temporal lobe structures in the rat to kindling and status epilepticus induction. Epilepsy Res. 1996;23:179–187. doi: 10.1016/0920-1211(95)00084-4. doi:10.1016/0920−1211(95)00084−4. [DOI] [PubMed] [Google Scholar]
- Naylor DE, Liu H, Wasterlain CG. Trafficking of GABA(A) receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci. 2005;25:7724–7733. doi: 10.1523/JNEUROSCI.4944-04.2005. doi:10.1523/JNEUROSCI.4944−04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehoff DL, Kuhar MJ. Benzodiazepine receptors: localization in rat amygdala. J Neurosci. 1983;3:2091–2097. doi: 10.1523/JNEUROSCI.03-10-02091.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen A, Tuunanen J, Kälviänen R, Partanen K, Salmenperä T. Amygdala damage in experimental and human temporal lobe epilepsy. Epilepsy Res. 1998;32:233–253. doi: 10.1016/s0920-1211(98)00055-2. doi:10.1016/S0920−1211(98)00055−2. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Kharatishvili I, Narkilahti S, Lukasiuk K, Nissinen J. Administration of diazepam during status epilepticus reduces development and severity of epilepsy in rat. Epilepsy Res. 2005;63:27–42. doi: 10.1016/j.eplepsyres.2004.10.003. doi:10.1016/j.eplepsyres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Kharatishvili I, Karhunen H, Lukasiuk K, Immonen R, Nairismägi J, Gröhn O, Nissinen J. Epileptogenesis in experimental models. Epilepsia. 2007;48(S2):13–20. doi: 10.1111/j.1528-1167.2007.01063.x. doi:10.1111/j.1528−1167.2007.01063.x. [DOI] [PubMed] [Google Scholar]
- Qashu F, Fritsch B, Figueiredo T, Aroniadou-Anderjaska V, Braga MF. Functional and molecular alterations of the GABAergic system in the basolateral amygdala during epileptogenesis triggered by status epilepticus. Soc Neurosci. 2008 doi: 10.1016/j.neuroscience.2009.06.034. 146.9 (abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesney LF. Clinical and EEG features of complex partial seizures of temporal lobe origin. Epilepsia. 1986;27(S2):27–45. doi: 10.1111/j.1528-1157.1986.tb05738.x. doi:10.1111/j.1528−1157.1986.tb05738.x. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. doi:10.1016/0013−4694(72)90177−0. [DOI] [PubMed] [Google Scholar]
- Racine RJ, Paxinos G, Mosher JM, Kairiss EW. The effects of various lesions and knife-cuts on septal and amygdala kindling in the rat. Brain Res. 1988;454:264–274. doi: 10.1016/0006-8993(88)90826-8. doi:10.1016/0006−8993(88)90826−8. [DOI] [PubMed] [Google Scholar]
- Riba-Bosch A, Pérez-Claussell J. Response to kainic acid injections: changes in staining for zinc, FOS, cell death and glial response in the rat forebrain. Neuroscience. 2004;125:803–818. doi: 10.1016/j.neuroscience.2004.02.017. doi:10.1016/j.neuroscience.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Riss J, Cloyd J, Collins S. Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand. 2008;118:69–86. doi: 10.1111/j.1600-0404.2008.01004.x. doi:10.1111/j.1600−0404.2008.01004.x. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Albertson C, Slikker W., Jr Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751:37–46. doi: 10.1016/s0006-8993(96)01387-x. doi:10.1016/S0006−8993(96)01387-X. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Fuller T, Price JL, Olney W. Widespread patterns of neuronal damage following systemic or intracerebral injections of kainic acid: a histological study. Neuroscience. 1980;5:991–1014. doi: 10.1016/0306-4522(80)90181-5. doi:10.1016/0306−4522(80)90181−5. [DOI] [PubMed] [Google Scholar]
- Shih TM, Duniho SM, McDonough JH. Control of nerve agent-induced seizures is critical for neuroprotection and survival. Toxicol Appl Pharmacol. 2003;188:69–80. doi: 10.1016/s0041-008x(03)00019-x. doi:10.1016/S0041−008X(03)00019-X. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution, and function of GABA(A) receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. doi:10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Treiman DM. The role of benzodiazepines in the management of status epilepticus. Neurology. 1990;40(S5):32–42. [PubMed] [Google Scholar]
- Treiman DM. Treatment of convulsive status epilepticus. Int Rev Neurobiol. 2007;81:273–285. doi: 10.1016/S0074-7742(06)81018-4. doi:10.1016/S0074−7742(06)81018−4. [DOI] [PubMed] [Google Scholar]
- Treiman DM, Meyers PD, Walton NY, Collins JF, Colling C, Rowan AJ, Handforth A, Faught E, Calabrese VP, Uthman BM, Ramsay RE, Mamdani MB. A comparison of four treatments for generalized convulsive status epilepticus. Veterans Affairs Status Epilepticus Cooperative Study Group. N Engl J Med. 1998;339:792–798. doi: 10.1056/NEJM199809173391202. doi:10.1056/NEJM199809173391202. [DOI] [PubMed] [Google Scholar]
- Tuunanen J, Halonen T, Pitkänen A. Status epilepticus causes selective regional damage and loss of GABAergic neurons in the rat amygdaloid complex. Eur J Neurosci. 1996;8:2711–2725. doi: 10.1111/j.1460-9568.1996.tb01566.x. doi:10.1111/j.1460−9568.1996.tb01566.x. [DOI] [PubMed] [Google Scholar]
- Tuunanen J, Lukasiuk K, Halonen T, Pitkänen A. Status epilepticus-induced neuronal damage in the rat amygdaloid complex: distribution, time-course, and mechanisms. Neuroscience. 1999;94:473–495. doi: 10.1016/s0306-4522(99)00251-1. doi:10.1016/S0306−4522(99)00251−1. [DOI] [PubMed] [Google Scholar]
- Van Paesschen W, King MD, Duncan JS, Connelly A. The amygdala and temporal lobe simple partial seizures: a prospective and quantitative MRI study. Epilepsia. 2001;42:857–862. doi: 10.1046/j.1528-1157.2001.042007857.x. doi:10.1046/j.1528−1157.2001.042007857.x. [DOI] [PubMed] [Google Scholar]
- Walton NY, Treiman DM. Response of status epilepticus induced by lithium pilocarpine to treatment with diazepam. Exp Neurol. 1988;101:267–275. doi: 10.1016/0014-4886(88)90010-6. doi:10.1016/0014−4886(88)90010−6. [DOI] [PubMed] [Google Scholar]
- White LE, Price JL. The functional anatomy of limbic status epilepticus in the rat. I. Patterns of 14C-2-deoxyglucose uptake and Fos immunocytochemistry. J Neurosci. 1993a;13:4787–4809. doi: 10.1523/JNEUROSCI.13-11-04787.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LE, Price JL. The functional anatomy of limbic status epilepticus in the rat. II. The effects of focal deactivation. J Neurosci. 1993b;13:4810–4830. doi: 10.1523/JNEUROSCI.13-11-04810.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson PD, French JA, Thadani VM, Kim JH, Novelly RA, Spencer SS, Spencer DD, Mattson RH. Characteristics of medial temporal lobe epilepsy: II. Interictal and ictal scalp electroencephalography, neuropsychological testing, neuroimaging, surgical results, and pathology. Ann Neurol. 1993;34:781–787. doi: 10.1002/ana.410340605. doi:10.1002/ana.410340605. [DOI] [PubMed] [Google Scholar]
- Wolf HK, Aliashkevich AF, Blümke I, Wiestler OD, Zentner J. Neuronal loss and gliosis of the amygdaloid nucleus in temporal lobe epilepsy. A quantitative analysis of 70 surgical specimens. Acta Neuropathol. 1997;93:606–610. doi: 10.1007/s004010050658. doi:10.1007/s004010050658. [DOI] [PubMed] [Google Scholar]