Abstract
OBJECTIVE:
The purpose of this study was to confirm whether Black and White women with endometrial cancer are equally tolerant of chemotherapy and identify factors that impact survival.
METHODS:
A retrospective review of 169 Black women and 982 White women with FIGO Stage III/IV or recurrent endometrial carcinoma was performed. All patients received doxorubicin combined with cisplatin. Chemotherapy parameters that were reviewed included relative dose (RD), relative time (RT), and relative dose intensity (RDI). Treatment cycles ≥ 7 were defined as treatment completion.
RESULTS:
Although Black patients were more likely to experience grade 3-4 anemia (20% vs. 14%) and genitourinary (5% vs. 1%) toxicity, and less likely to experience severe GI toxicity (10% vs. 17%), the overall incidence of grade 3-4 treatment-related chemotoxicity was the same between the two groups (82% vs. 82%). There were no differences in the number of cycles received, RD (0.57 vs. 0.58), RT (0.77 vs. 0.78), or RDI (0.76 vs. 0.76) for Black and White patients.
CONCLUSION:
Black patients with advanced stage or recurrent endometrial cancer, treated on four GOG protocols, had similar dose intensity and severe chemotherapy-related toxicity compared to White patients, suggesting that previously described racial disparities in survival among patients in GOG trials may have an novel etiology.
Keywords: Endometrial Cancer, Chemotherapy, GOG
INTRODUCTION
In the United States, approximately 39,000 women will be diagnosed with endometrial cancer and over 7,400 deaths will be attributable to this disease 1. Although the incidence of endometrial cancer is 30% lower among Black women compared to White women, mortality among Blacks with endometrial cancer is 60% higher 2. The underlying cause of this racial disparity in endometrial cancer outcome among Black patients with endometrial cancer is felt to be multifactorial 3-5. Population-based studies have suggested that inequalities in treatment may contribute to poor survival observed among Black women with endometrial cancer 6-8. In an effort to further evaluate the racial disparity in endometrial cancer using a clinical setting in which patients received similar care, our group evaluated data from four clinical trials performed by the GOG 9. This analysis showed that Black patients had a 26% greater chance of mortality compared to Whites, controlling for performance status, stage, histology, grade, and treatment. The overall response to platinum- and adriamycin-based chemotherapy for Blacks was 35% compared to 43% for Whites (p<0.016). Although the proportion of Black and White patients completing the prescribed therapy (46% versus 42%) as well as the proportion of treatment related deaths (1.9% versus 1.6%) was similar, data regarding the dose intensity and duration of chemotherapy or treatment related toxicities was not evaluated 9. The purpose of the current study was to further evaluate these chemotherapy parameters to determine if Black patients are treated less aggressively with adjuvant chemotherapy compared to Whites and whether any differences in dosing parameters affect survival outcome. Racial differences in chemotherapy response and ultimately in survival among endometrial cancer patients would suggest that a biologic etiology may be in part be associated with the poor outcome observed among Black women with advanced stage or recurrent endometrial cancer.
MATERIALS AND METHODS
We reviewed patient data from participants in four GOG randomized treatment trials for International Federation of Gynecologic Oncology (FIGO) Stage III, Stage IV, or recurrent endometrial cancer 7: GOG Protocols 107, 139, 163, and 177 10-13. All treatment arms of the individual protocols were included. All eligible patients had histologically confirmed endometrial cancer with measurable disease and a GOG performance status (PS) of 0 to 2. Prior radiation or hormone therapy, but not prior chemotherapy, was allowed. The treatment regimen for each trial is listed in Table 1. Further details regarding eligibility, treatment, and outcomes have been published previously 10-13. Data on patient demographics, tumor characteristics, and course of treatment were collected. Racial designation as Black or White reflected a non-uniform collection of both self-described and investigator-reported methods. Patients from other racial groups were excluded from this analysis secondary to too few numbers for analysis.
TABLE 1.
Gynecologic Oncology Group Treatment Protocols of the Study Group
| Treatment | Number of Patients | |||
|---|---|---|---|---|
| GOG protocol | Arm A | Arm B | White | Black |
| GOG 107 | Doxorubicin 60 mg/m2 | Doxorubicin 60 mg/m2 and cisplatin 50 mg/m2 |
237 | 34 |
| GOG 139 | Doxorubicin 60 mg/m2 over 30 min and cisplatin 60 mg/m2 over 30 min |
Doxorubicin 60 mg/m2 over 30 min at 6 a.m. and cisplatin 60 mg/m2 over 30 min at 6 p.m. |
271 | 53 |
| GOG 163 | Doxorubicin 60 mg/m2 and cisplatin 50 mg/m2 |
Doxorubicin 60 mg/m2, 24-h infusion paclitaxel at a dose of 150 mg/m2, and G-CSF at a dose of 5 mcg/kg on Days 3- 12 |
258 | 45 |
| GOG 177 | Doxorubicin 60 mg/m2 and cisplatin 50 mg/m2 |
Doxorubicin 45 mg/m2 on Day 1, cisplatin, 50 mg/m2 on Day 1, 3-h infusion paclitaxel at a dose of 160 mg/m2, on Day 2, and G-CSF at a dose of 5 mcg/kg on Days 3-12 |
216 | 37 |
Several characteristics of chemotherapy were evaluated in relation to race and outcome. Treatment parameters were defined according to Griggs, in their study of breast cancer 16. Relative dose (RD) was the ratio of actual: expected dose of chemotherapy in standard chemotherapy regimens. This was calculated for each drug in the chemotherapy. Relative time (RT) was defined as the ratio of actual: expected duration of chemotherapy. Relative dose intensity (RDI) was defined as the ratio of relative dose to relative time. We defined patient completing at least 7 cycles as treatment completion since this was the maximal numbers cycles involving adriamycin received in three of the four GOG studies included in our analysis and was based solely on precedent at the time of protocol development 13. The relative time was only calculated for up to 7 cycles (prescribed by protocol) and the expected duration of time on chemotherapy was 21 (i.e. chemotherapy every 3 weeks) × 7 (i.e. total cycles) days. The expected dose was the standard dose required by protocol × 7 (Table 1). Although the protocols required dose adjustment for old age, prior RT or Body Surface Area (BSA) > 2, these adjustments were treated as dose reductions. The average RD or RDI calculated based on combination regimens was used as a summarized parameter of the combined regimen, with a ratio < 1.0 indicating patient receiving less intensity of chemotherapy than planned.
Statistical Analysis
The mean RD, RT and RDI between Blacks and Whites were compared using student-T test. The number of treatment cycles, cause of treatment incompletion and toxicity were compared by Pearson chi-square or Fisher exact test. The factors associated with treatment incompletion were identified using a logistic regression model. The association of RD, RT or RDI with survival was estimated using a Cox regression model, adjusted for established prognostic factors (PS, stage, tumor grade, histology, prior hysterectomy, type of chemotherapy and race). The interaction between race and treatment parameters was also assessed. Kaplan-Meier procedure was used to estimate the cumulative probability of survival. Since patients with disease were more likely to drop the chemotherapy, resulting in dose reduction, and to avoid the possible “outcome to cause bias” or “outcome by outcome analysis”, the survival analysis based on treatment parameters was restricted to patients who completed all 7 cycles of chemotherapy and the survival was recalculated as the time from the completion of chemotherapy until death, regardless of causes. All statistical analyses were performed using Statistical Analysis System software (SAS version 9.1; SAS Inc., Cary, NC).
RESULTS
A total of 982 White, and 169 Black women with FIGO stage III, IV or recurrent endometrial cancer were assigned to receive doxorubicin and cisplatin chemotherapy (Table 1). Clinical characteristics of this cohort of patients have previously been published. 9 Blacks were more likely to have advanced stage disease (p< 0.001), poorly differentiated tumors (p< 0.001), papillary serous histology (p≤ 0.001) and poor performance status (p=0.008) compared with Whites. After adjusting for clinical and treatment factors, Black patients were at an increased risk for death (hazard ratio (HR): 1.26, 95% confidence interval (CI): 1.06-1.51, p=0.010)9.
Dose Intensity
There were no differences in treatment parameters between Black and White patients (Table 2). The RD was similar for the two populations (0.56 and 0.58 respectively), as was RT (0.77 and 0.78 respectively) and RDI (0.76 and 0.76 respectively). Forty five percent of Whites completed at least 7 cycles of chemotherapy compared to 40% among Blacks. The majority of patients who did not complete therapy stopped therapy because of disease progression (55% for Whites versus 53% for Blacks). Similar proportions, 29% of Black and 22.3% of White patients discontinued therapy because of toxicity. Multivariate analysis revealed that patients with abnormal PS (p<0.001), non-endometrioid cell type (p<0.001) or recurrent disease (p<0.001) were less likely to complete the treatment cycles required by protocol (Table 3). But, Black patients had the same likelihood of completing the treatment as White patients (p>0.05).
TABLE 2.
TREATMENT PARAMETERS BY RACE
| Treatment Parameter | White (n=982) |
Black (n=169) |
p-value |
|---|---|---|---|
| Relative Dose 1 | |||
| Mean (SD) | 0.58 (0.28) | 0.57 (0.29) | 0.55 |
| Median (25th-75th Pct) | 0.63 (0.32-0.81) | 0.63 (0.28-0.83) | |
| Relative Time 2 | |||
| Mean (SD) | 0.78 (0.36) | 0.77 (0.38) | 0.62 |
| Median (25th-75th Pct) | 0.93 (0.43-1.05) | 0.90 (0.42-1.08) | |
| Relative Dose Intensity (RDI) 3 | |||
| Mean (SD) | 0.76 (0.14) | 0.76 (0.16) | 0.91 |
| Median (25th-75th Pct) | 0.76 (0.66-0.88) | 0.76 (0.63-0.88) | |
| No. Treatment Cycles (%) | 0.56 | ||
| 0 | 1.0 | 2.4 | |
| 1 | 8.8 | 10.1 | |
| 2 | 12.9 | 15.4 | |
| 3 | 8.3 | 9.5 | |
| 4 | 8.6 | 5.9 | |
| 5 | 6.7 | 8.3 | |
| 6 | 9.0 | 8.3 | |
| 7 | 44.8 | 40.2 | |
| Cause for < 7 cycles (%) 4 | 0.34 | ||
| Disease progression | 55.2 | 52.5 | |
| Toxicity | 22.3 | 28.7 | |
| Other/unknown | 22.5 | 18.8 |
ratio of actual to expected dose of chemotherapy.
ratio of actual to expected duration of chemotherapy.
relative dose / relative time.
n=542 for white patients and n=101 for black patients
TABLE 3.
SIGNIFICANT FACTORS ASSOCIATED WITH TREATMENT INCOMPLETION
| Characteristic | Incompletion (%) | Odds Ratio (95% C.I.) | p-value |
|---|---|---|---|
| Performance Status | <0.001 | ||
| 0 | 47.1 | Referent | |
| 1 | 57.0 | 1.56 (1.21-2.02) | |
| 2 | 79.1 | 4.44 (2.92-6.90) | |
| Stage | <0.001 | ||
| III/IV | 47.9 | Referent | |
| Recurrence | 59.4 | 1.87 (1.43-2.45) | |
| Histology | <0.001 | ||
| Endometrioid | 50.9 | Referent | |
| Clear cell | 66.7 | 2.23 (1.15-4.54) | |
| Serous | 61.3 | 1.70 (1.22-2.39) | |
| Other | 60.3 | 1.59 (1.19-2.13) |
Odds ratio estimated by Logistic regression model. Race and other patient characteristics (age, tumor grade, prior RT, prior hysterectomy and type of chemotherapy regimen) not correlated with treatment incompletion (p>0.05 for all).
Toxicity
For selected common toxicity criteria categories, more grade 3-4 anemia (20.1% vs. 14.2%, p=0.05) and severe genitourinary toxicity (4.7% vs. 1.4%, p=0.009), and less severe GI toxicity (10.1% vs. 16.7%, p=0.03) was observed among Black patients compared to White patients (Table 4). There is also a suggestion that Blacks experienced less leukopenia (55.6% vs. 61.6%, p=0.14) than Whites. However, there was no difference for overall severe adverse effects involving Whites and Blacks when considering any grade 3-4 toxicity event that occurred during participation in these clinical trials (82.3% vs. 82.1%, p=0.96) (Table 4). These results were consistent from multivariate analysis adjusted for age, PS, BMI and prior radiotherapy,
TABLE 4.
TREATMENT ADVERSE EFFECTS BY RACE
| Adverse Effect | White % (n=982) |
Black % (n=169) |
p-value |
|---|---|---|---|
| Selected Grade 3 or 4 | |||
| Leukopenia | 61.6 | 55.6 | 0.14 |
| Thrombocytopenia | 10.3 | 9.5 | 0.75 |
| Neutropenia | 67.9 | 63.9 | 0.30 |
| Anemia | 14.2 | 20.1 | 0.05 |
| Gastrointestinal | 16.7 | 10.1 | 0.03 |
| Genitourinary | 1.4 | 4.7 | 0.009 |
| Neurologic | 3.0 | 3.6 | 0.68 |
| Cardiovascular | 5.3 | 4.1 | 0.53 |
| Any Grade 3 or 4 | 82.1 | 82.3 | 0.96 |
Pearson chi-square or Fisher exact test used to compare the group-difference.
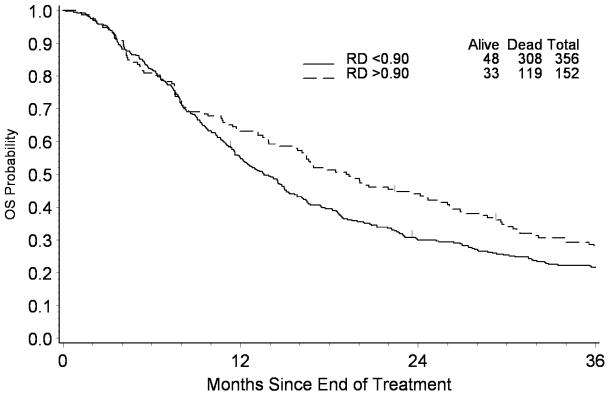
The median RD, RT and RDI were 0.83, 1.05 and 0.75, respectively, for patients who completed at least 7 cycles of chemotherapy. When analysis was restricted to this population, RD in this restricted population appeared to be associated with survival, whereas both RT and RDI were not. Based on current data, every 10% increase of RD is associated with a 9% reduction in the risk of death (HR: 0.91, 95% CI: 0.84-0.99). Figure 1 presents the survival from the completion of chemotherapy stratified by RD for the group. For patients with RD ≥ 0.9, the median survival was estimated to be 19.2 months compared to 13.6 months for those with RD < 0.9 (Log-rank test: p=0.02). The association of RD with survival was not modified by Race (p>0.05 for test of interaction).
Figure 1.
Kaplan-Meier Estimate of Survival According to Relative Dose
DISCUSSION
In our assessment of chemotherapy parameters and potential racial disparities in treatment of patient with advanced or recurrent endometrial cancer, we evaluated both the treatment related toxicity and dose intensity of therapy. Toxicity related to chemotherapy may be an underlying cause for dose adjustments in some patients, and if different among certain minorities could account for observed differences in cancer outcome. Dose intensity, the amount of drug delivered per unit of time, is an important predictor of outcome in adjuvant chemotherapy for cancer 14 which can be increased by either using higher doses of chemotherapy (RD) or by shortening the interval between cycles (RT) 15. In a MedLine search of the terms “endometrial cancer” and “chemotherapy”, we did not find any previous reports of these chemotherapy parameters in relation to endometrial cancer, particularly not in the context of racial disparities.
Race was not a predictive factor for early discontinuation of chemotherapy among the patients evaluated. We previously reported that 46.2% of Blacks and 42.2% of Whites completed the required number of cycles (p=0.37) 9. In our current analysis, we established that the majority of patients had discontinuation of chemotherapy because of disease progression, which was similar between Black and White patients (55.2% and 52.5% respectively). The second most frequent reason for shortening of therapy in Blacks and Whites was treatment related toxicity that prompted protocol related changes in chemotherapy administration (22.3 versus 28.7% respectively). The remaining patients in each of the two groups had chemotherapy stopped because of poor performance status; a proportion that was similar in the two groups.
Blacks experienced a slightly increased incidence of severe (any grade 3 or 4) anemia and severe genitourinary toxicity and a lower frequency of leukopenia and severe gastrointestinal toxicity than White patients receiving chemotherapy. This increased incidence of anemia could be related to a generally higher incidence of baseline anemia found in the Black population5. Nonetheless, we found no evidence that these severe clinical toxicities accounted for any difference in RD or RDI among White and Black patients. Overall, 82% of both Black and White patients experienced any grade 3 or 4 toxicity while on chemotherapy.
We identified three factors associated with discontinuation of chemotherapy in advanced and recurrent endometrial cancer patients when other clinical factors were evaluated using regression analysis. Patients with PS > 0 (P<0.001), recurrent disease (p<0.001) and non endometrioid histology (P<0.001) were all less likely to complete the number of cycles of intravenous chemotherapy required by each of the protocols. When analysis was restricted to patients who successfully completed seven cycles of chemotherapy or women who received fewer cycles secondary to toxicity issues, RD, RT and RDI were all similar between Blacks and Whites.
Although there are no previous reports in endometrial cancer for comparison, Griggs et al 16 found systematic differences in the administration of chemotherapy given to Black women with breast cancer. Blacks received lower RD (0.80 vs 0.85, p = 0.03) and RDI (0.76 vs. 0.80, p=0.01) than Whites. The authors speculated that these differences in chemotherapy administration could potentially contribute to racial disparities in breast cancer survival 16. Although differences in chemotherapy administration could possibly be a contributor to racial disparities in endometrial cancer outcome on a population basis, our data revealed that racial disparities in survival can persist even when Black and White women receive chemotherapy similarly in clinical trial setting.
In our analysis, we found that almost half of the patients enrolled on these protocols were not able to maintain the dosage prescribed (i.e. RD 0.56 for Blacks and 0.58 for Whites). The main reasons for dose adjustment were progression, toxicity, and poor performance status which were equivalent among Blacks and Whites. For those patients able to maintain dosage, every 10% increase in RD was associated with a 9% reduction in the risk of death.
Previous investigations involving racial disparities in chemotherapy toxicity are limited particularly in regards to gynecologic cancer. In a study of another disease site, Black patients with stage II and III colon cancer experienced less overall (defined as toxicity events ≥ grade 1) GI treatment-related toxicity (i.e. diarrhea, nausea, vomiting, and stomatitis) compared to Whites 17. The only racial difference in severe toxicity observed was specifically for diarrhea with 23% of Whites experiencing severe diarrhea compared to 8% of Blacks. The clinical relevance of this discrepancy in overall toxicity can be scrutinized however, as the two groups derived similar benefits from the adjuvant chemotherapy administered. Significantly lower rates of gastrointestinal toxicity also have been observed among Black cervix cancer patients treated on GOG clinical trials (Unpublished correspondence 18) suggesting that Black patients may have more tolerance to the emetogenic effects of chemotherapy. Ethnic hematologic tolerance associated with chemotherapy may also be different. Lower basal white blood cell and platelet levels have been reported among Black women in population based studies. Ethnic neutropenia has been described in women receiving chemotherapy with reduced white blood cells noted both at baseline and throughout treatment 19. Hershman et al 19 found in a study of 472 stage I and II breast cancer patients that lower levels of white blood cells were observed in Blacks versus Whites and that this was associated with a prolongation of treatment (19 versus 15 weeks) resulting in less dose intensity treatment. Other studies have shown that although leukopenia is more common in Blacks with colon cancer, severe leukopenia is less frequent when compared to Whites 17. We observed no difference in severe thrombocytopenia or leukopenia between Black and White patients.
The data that we have observed in this study and our previous analysis8 suggest that a racial disparity in outcome persists in a setting in which patients receive similar treatment (i.e. surgery, radiotherapy and chemotherapy) for advanced stage or recurrent endometrial cancer. In our current study, Blacks were found to have a lower response rate to platinum- and adriamycin-based chemotherapy suggesting that there may be unconventional differences in the endometrial cancers that develop in Blacks compared to Whites. Previous mutational analysis has shown a racial disparity in the two most commonly altered tumor suppressor genes found in endometrial cancers. The PTEN tumor suppressor gene which is associated with endometrial cancer that have a more favorable prognosis is found more often among Whites compared to Blacks 20. Conversely, the mutations in the p53 tumor suppressor gene are more often found in Blacks and associated with a poor prognosis 21, 22. Recent microarray studies have attempted to identify additional racial disparities in global gene expression that could in part explain differences in outcome 23, 24. If biologic differences in the endometrial cancers from Black and Women exist, future pharmaco-genomic or more selective contemporary chemotherapeutic therapy may be used to provide individualized treatment that is targeted to the molecular profile of the patient 25, 26.
It should be mentioned that this study was a retrospective analysis. Although the total sample size is large, the number of Black patients was still small. We consider the results from this data as exploratory. More data from endometrial cancer or other cancer patients treated with cisplatin-based will be needed to validate if Black patients may be less likely to develop some specific type of adverse effects.
ACKNOWLEDGEMENTS
The following Gynecologic Oncology Group member institutions participated in the primary treatment studies: Wake Forest University School of Medicine, Columbus Cancer Council, University of California Medical Center at Irvine, Duke University Medical Center, Wayne State University, Indiana University Medical Center, University of Kentucky, Washington University School of Medicine, Abington Memorial Hospital, University of Iowa Hospitals and Clinics, Tufts-New England Medical Center, University of Mississippi Medical Center, Milton S. Hershey Medical Center, Rush-Presbyterian-St. Luke's Medical Center, Albany Medical College, University of Texas Southwestern Medical Center at Dallas, Community Clinical Oncology Program, University of Oklahoma, University of Minnesota Medical School, University of Virginia, The Cleveland Clinic Foundation, University of Alabama at Birmingham, Colorado Gynecologic Oncology Group, University of North Carolina School of Medicine, University of Massachusetts Medical School, Fox Chase Cancer Center, Women's Cancer Center, SUNY at Stony Brook, Medical University of South Carolina, Johns Hopkins Oncology Center, Cooper Hospital/University Medical Center, University of Chicago, Walter Reed Army Medical Center, Tampa Bay Cancer Consortium, University of Rochester Medical Center, Eastern Pennsylvania GYN/ONC Center, Tacoma General Hospital, Thomas Jefferson University Hospital, Case Western Reserve University, University of Cincinnati, University of Pennsylvania Cancer Center, Gynecologic Oncology Network, Georgetown University Hospital, Mayo Clinic, Oregon Health Sciences University, University of Miami School of Medicine, University of California at Los Angeles, SUNY Upstate Medical Center, Eastern Virginia Medical School, M.D. Anderson Cancer Center, Ellis Fischel Cancer Center, SUNY Downstate Medical Center, Emory University Clinic, University of Southern California at Los Angeles, Stanford University Medical Center, Memorial Sloan-Kettering Cancer Center, University of Washington, North Shore University Hospital, and Long Island Jewish Medical Center.
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469) and the Gynecologic Oncology Group Statistical Office (CA 37517).
Footnotes
The study was presented to and approved by local institutional review boards prior to activation, and all patients provided written consent prior to enrollment on the study.
The views expressed herein are those of the authors and do not reflect the official policy or opinion of the Department of Defense or the United States Army or Navy.
Portions of this report were presented at the 39th Annual Meeting of the Society of Gynecologic Oncologists, held in Tampa, FL 2008 and published in Gynecol Oncol 108(3):S1, S19, 2008.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Ghafoor A, Jemal A, Cokkinides V, et al. Cancer statistics for African Americans. CA Cancer J Clin. 2002;52:326–41. doi: 10.3322/canjclin.52.6.326. [DOI] [PubMed] [Google Scholar]
- 3.Farley J, Risinger JI, Rose GS, Maxwell GL. Racial disparities in blacks with gynecologic cancers. Cancer. 2007;110:234–43. doi: 10.1002/cncr.22797. [DOI] [PubMed] [Google Scholar]
- 4.Hill HA, Eley JW, Harlan LC, Greenberg RS, Barrett RJ, 2nd, Chen VW. Racial differences in endometrial cancer survival: the black/white cancer survival study. Obstet Gynecol. 1996;88:919–26. doi: 10.1016/s0029-7844(96)00341-9. [DOI] [PubMed] [Google Scholar]
- 5.Sherman ME, Devesa SS. Analysis of racial differences in incidence, survival, and mortality for malignant tumors of the uterine corpus. Cancer. 2003;98:176–86. doi: 10.1002/cncr.11484. [DOI] [PubMed] [Google Scholar]
- 6.Hicks ML, Phillips JL, Parham G, et al. The National Cancer Data Base report on endometrial carcinoma in African-American women. Cancer. 1998;83:2629–37. doi: 10.1002/(SICI)1097-0142(19981215)83:12<2629::AID-CNCR30>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 7.Madison T, Schottenfeld D, James SA, Schwartz AG, Gruber SB. Endometrial cancer: socioeconomic status and racial/ethnic differences in stage at diagnosis, treatment, and survival. Am J Public Health. 2004;94:2104–11. doi: 10.2105/ajph.94.12.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Randall TC, Armstrong K. Differences in treatment and outcome between African-American and white women with endometrial cancer. J Clin Oncol. 2003;21:4200–6. doi: 10.1200/JCO.2003.01.218. [DOI] [PubMed] [Google Scholar]
- 9.Maxwell GL, Tian C, Risinger J, et al. Racial disparity in survival among patients with advanced/recurrent endometrial adenocarcinoma: a Gynecologic Oncology Group study. Cancer. 2006;107:2197–205. doi: 10.1002/cncr.22232. [DOI] [PubMed] [Google Scholar]
- 10.Fleming GF, Brunetto VL, Cella D, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2004;22:2159–66. doi: 10.1200/JCO.2004.07.184. [DOI] [PubMed] [Google Scholar]
- 11.Fleming GF, Filiaci VL, Bentley RC, et al. Phase III randomized trial of doxorubicin + cisplatin versus doxorubicin + 24-h paclitaxel + filgrastim in endometrial carcinoma: a Gynecologic Oncology Group study. Ann Oncol. 2004;15:1173–8. doi: 10.1093/annonc/mdh316. [DOI] [PubMed] [Google Scholar]
- 12.Gallion HH, Brunetto VL, Cibull M, et al. Randomized phase III trial of standard timed doxorubicin plus cisplatin versus circadian timed doxorubicin plus cisplatin in stage III and IV or recurrent endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2003;21:3808–13. doi: 10.1200/JCO.2003.10.083. [DOI] [PubMed] [Google Scholar]
- 13.Thigpen JT, Brady MF, Homesley HD, et al. Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma: a gynecologic oncology group study. J Clin Oncol. 2004;22:3902–8. doi: 10.1200/JCO.2004.02.088. [DOI] [PubMed] [Google Scholar]
- 14.Ziegler J, Citron M. Dose-dense adjuvant chemotherapy for breast cancer. Cancer Nurs. 2006;29:266–72. doi: 10.1097/00002820-200607000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Lambrou N, Trimble EL. Dose-intensive approaches to ovarian cancer. Curr Oncol Rep. 1999;1:54–8. doi: 10.1007/s11912-999-0010-x. [DOI] [PubMed] [Google Scholar]
- 16.Griggs JJ, Sorbero ME, Stark AT, Heininger SE, Dick AW. Racial disparity in the dose and dose intensity of breast cancer adjuvant chemotherapy. Breast Cancer Res Treat. 2003;81:21–31. doi: 10.1023/A:1025481505537. [DOI] [PubMed] [Google Scholar]
- 17.McCollum AD, Catalano PJ, Haller DG, et al. Outcomes and toxicity in African American and Caucasian patients in a randomized adjuvant chemotherapy trial for colon cancer. J Natl Cancer Inst. 2002;94:1160–7. doi: 10.1093/jnci/94.15.1160. [DOI] [PubMed] [Google Scholar]
- 18.Tian C. In: Gastrointestinal Toxicity in Gynecologic Oncology Group Trials. GL M, editor. Roswell Park; 2007. [Google Scholar]
- 19.Hershman D, McBride R, Jacobson JS, et al. Racial disparities in treatment and survival among women with early-stage breast cancer. J Clin Oncol. 2005;23:6639–46. doi: 10.1200/JCO.2005.12.633. [DOI] [PubMed] [Google Scholar]
- 20.Clifford SL, Kaminetsky CP, Cirisano FD, et al. Racial disparity in overexpression of the p53 tumor suppressor gene in stage I endometrial cancer. Am J Obstet Gynecol. 1997;176:S229–32. doi: 10.1016/s0002-9378(97)70380-6. [DOI] [PubMed] [Google Scholar]
- 21.Kohler MF, Carney P, Dodge R, et al. p53 overexpression in advanced-stage endometrial adenocarcinoma. Am J Obstet Gynecol. 1996;175:1246–52. doi: 10.1016/s0002-9378(96)70036-4. [DOI] [PubMed] [Google Scholar]
- 22.Maxwell GL, Risinger JI, Hayes KA, et al. Racial disparity in the frequency of PTEN mutations, but not microsatellite instability, in advanced endometrial cancers. Clin Cancer Res. 2000;6:2999–3005. [PubMed] [Google Scholar]
- 23.Ferguson SE, Olshen AB, Levine DA, Viale A, Barakat RR, Boyd J. Molecular profiling of endometrial cancers from African-American and Caucasian women. Gynecol Oncol. 2006;101:209–13. doi: 10.1016/j.ygyno.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 24.Maxwell GL, C G, Dainty L, Litzi T, Bidus M, Berchuck A, Barrett C, Risinger J. Racial disparity in global gene expression among patients with advanced endometrial adenocarcinoma. Gynecol Oncol. 2005;96:927. [Google Scholar]
- 25.Dressman HK, Berchuck A, Chan G, et al. An integrated genomic-based approach to individualized treatment of patients with advanced-stage ovarian cancer. J Clin Oncol. 2007;25:517–25. doi: 10.1200/JCO.2006.06.3743. [DOI] [PubMed] [Google Scholar]
- 26.Hsu DS, Balakumaran BS, Acharya CR, et al. Pharmacogenomic strategies provide a rational approach to the treatment of cisplatin-resistant patients with advanced cancer. J Clin Oncol. 2007;25:4350–7. doi: 10.1200/JCO.2007.11.0593. [DOI] [PubMed] [Google Scholar]