Abstract
Injectable scaffolds are promising substrates for regenerative medicine applications. In this study, multi-arm amino-terminated poly(ethylene glycol) (PEG) hydrogels were cross-linked with genipin, a compound naturally derived from the gardenia fruit. In this study, 4-arm and 8-arm amino-terminated PEG hydrogels crosslinked with varying concentrations of genipin were characterized. Both surface and cross-sectional structures of PEG-based hydrogels were observed by scanning electron microscopy. In vitro gelation time, water uptake, swelling and weight loss of PEG hydrogels in phosphate buffered saline at 37°C were studied. Results showed that the 8-arm PEG demonstrated a much slower gelation time compared to the 4-arm PEG, which may be due to the differing structures of the multi-arm PEG hydrogels, which in turn affects the ability of genipin to react with the amine groups. Human adipose-derived stem cells (ASCs) were seeded onto the 4-arm and 8-arm PEG hydrogels in vitro to assess the biological performance and applicability of the gels as cell carriers. The 4-arm PEG hydrogel resulted in enhanced cell adhesion as compared to the 8-arm PEG hydrogel. Overall, these characteristics provide a potential opportunity for multi-arm PEG hydrogels as injectable scaffolds in a variety of tissue engineering applications.
Keywords: multi-arm poly(ethylene glycol), genipin, biodegradable hydrogel, adipose tissue engineering
INTRODUCTION
Various biomaterials including hydrogels and microspheres have been employed as injectable scaffolds for a variety of tissue engineering applications [1–5]. Biodegradable, injectable hydrogels could be utilized as delivery systems, cell carriers, and scaffolds for tissue engineering [6–8]. Hydrogels allow easy and homogenous drug or cell distribution within any defect size or shape. Although in many of the natural hydrogels (e.g., fibrin glue and collagen) that have been used to tissue engineering applications, the physical mechanisms have been difficult to control and, therefore, limit the final network structure and properties. In contrast, covalently crosslinked synthetic hydrogels formed from a chain polymerization of multivinyl macromers offer many advantages.
Although many variations of synthetic biocompatible, biodegradable polymers can form hydrogels via chemical crosslinking, poly(ethylene glycol) (PEG) remains one of the most widely investigated systems [9–13]. Biodegradable PEG hydrogels can be obtained via copolymerization with degradable polymers such as poly(lactic acid), poly(glycolic acid) and poly(propylene fumarate) [14–16]. Furthermore, many naturally occurring biopolymers, such as hyaluronic acid, fibrinogen, chitosan, and heparin [17–21], are also generally examined in combination with biodegradable PEG hydrogels. PEG hydrogels have been used as cell scaffolds, adhesive medical applications, and delivery vehicles with promising results [22–25]. Particularly, the ability to control the crosslinking density provides the flexibility and tailorability to PEG-based hydrogels for cell encapsulation and tissue growth.
Our laboratory has recently examined the synthesis of biodegradable PEG hydrogels using genipin, a non-toxic cross-linking agent derived from the gardenia fruit [4–5, 26–28]. Recent studies have identified that genipin can be utilized to crosslink functional amine groups present in natural tissues and biomaterials with very minimal cytotoxic effects compared to studies performed with glutaraldehyde, a commonly used crosslinker [29–31]. The utilization of genipin (0.5~3.5 wt%) to crosslink natural biocompatible polymers, such as chitosan and gelatin, to form biodegradable hydrogels has the potential to produce novel scaffolds for various tissue engineering applications.
In this study, novel amino-terminated multi-arm PEG based hydrogels utilizing genipin as a crosslinking agent were examined. We examined 2 PEG structures: 4-arm PEG, a molecule with 4 PEG chains attached at a central point, and 8-arm PEG, a molecule with 8 PEG chains attached to a central molecule. Gelation time, morphology, swelling, water uptake and weight loss of 4-arm and 8-arm PEG hydrogels were examined. The hydrogels were then used to culture human adipose derived stem cells (ASCs) in vitro to assess their biological performance and applicability as cell carriers, as the ASCs represent a mesenchymal stem cell population that will be clinically useful in bone, cartilage, muscle, and soft tissue regeneration.
MATERIALS AND METHODS
Materials
4-arm and 8-arm poly(ethylene glycol) amine (Mw, 10KDa) were purchased from Jenken Technology CO., Ltd., Beijing, China. Genipin was purchased from Wako Pure Chemical Industries, Ltd., Japan. Phosphate buffered saline without Calcium and Magnesium (GIBCO 70011, pH 7.4) and CyQuant Cell Proliferation Assay Kit were purchased from Invitrogen, Eugene, Oregon, USA. All chemicals and reagents were used as received.
Preparation of multi-arm PEG hydrogels
4-arm PEG (Figure 1a) and 8-arm PEG (Figure 1b) 10% (w/v) solutions were prepared by dissolving 0.6g of PEG powder in 6mL of Nanopure water. The PEG solutions were then crosslinked with various volumes of an 88mM genipin solutions (Scheme 1) for final concentrations of 15mM, 17.6mM, 25mM and 35.2mM. The solutions reacted in glass vials with stirring at room temperature. Once the solutions became dark blue, the crosslinked solutions were transferred into 6.5mm diameter Teflon Petri dishes. Water was evaporated from the samples at room temperature for 48h.
Figure 1.
Structures of 4-arm aminated PEG (a) and 8-arm aminated PEG (b).
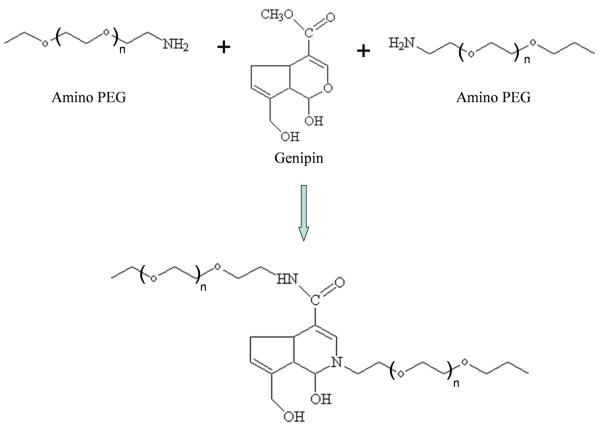
Scheme 1.
The reaction scheme of amino PEG-genipin hydrogel.
The gelation rate of multi-arm PEG hydrogels was monitored under sealed reaction conditions. 600μL of the PEG/genipin mixtures was injected into 2mL polypropylene test tube and incubated at 37°C. The gelation time was recorded when the solution lost its fluidity.
Characterization of PEG hydrogel
Morphologies
The morphologies of PEG hydrogels were characterized by utilizing scanning electron microscopy (SEM) after gelation and subsequent freeze-drying. Dried PEG hydrogels were swelled in Nanopure water at 37°C for 2h, frozen at −20°C for 2h, and lyophilized at −50°C for 24h (Freezone 4.5, Labconco, US). The hydrogels were then gold-coated using a Cressington 108 Auto (Cressington, Watford, UK). The surface and cross-sectional morphologies of hydrogels were viewed using a JSM-6330F SEM (JEOL, Peabody, US) operated at 10kV accelerating.
Swelling and PBS Uptake Studies
Dehydrated PEG hydrogel disks were punched out using a 6mm dermal biopsy punch. Initial diameter were measured and recorded. The disks were then placed in 1mL PBS in microcentrifuge tubes. At specified time intervals, the diameter of the hydrated disks at 37°C were measured. The percent increase in diameter was calculated as:
Where Dt and D0 are the diameter of the final and initial diameters, respectively.
To determine PBS uptake, dehydrated hydrogel disks were weighed and incubated in 1mL PBS in microcentrifuge tubes at 37°C. The hydrated hydrogel disks were removed from PBS at specified time intervals, blotted with a filter paper to remove the surface water, weighed (Wwt) and then air-dried (Wdt) at room temperature. The PBS uptake ratio was calculated utilizing the following equations:
Where Wwt and Wdt are the wet and dry weight of the samples at time t, respectively.
Weight loss
The weight loss of PEG hydrogels were followed as a function of incubation time in the PBS at 37°C. The 6mm PEG hydrogel disks were weighed initially in their air-dried condition (W0). Weight loss of the hydrogel was monitored as a function of incubation time in PBS at 37°C. The scaffolds were removed from the PBS at a specified time intervals, washed with water, air-dried and weighed (Wt). The weight loss ratio was defined as 100%×(W0−Wt)/W0. The weight remaining ratio was defined as 1−100%×(W0−Wt)/W0.
Cell isolation
Human adipose-derived stem cells (ASCs) were isolated from human adipose tissue obtained from elective cosmetic surgery procedures performed at the University of Pittsburgh [32–33]. The fat tissues were minced with scissors in the collagenase solution consisted of Hanks’ balanced salt solution (3.0 mL/g of fat) (Sigma-Aldrich, St. Louis, MO), bovine serum albumin (fatty acid free, pH 7.0, 3.5 g/100 mL Hanks’) (Intergen Company, Purchase, NY) and 1% type II collagenase (3.0 mg/g of fat) (Worthington Biochemical Corporation, Lakewood, NJ). The centrifuge tubes were shaken at 100 rpm for 50 minutes at 37°C. Following digestion, the content of each tube was filtered through a double-layered sterile gauze. The filtrates were then centrifuged at 1000 rpm for 10 minutes at 37°C, and a 3-layer suspension, consisting of a fatty layer on the top, a serum layer in the middle, and a cellular pellet at the bottom of each tube, was obtained. The fatty layer and most of the supernatant was aspirated off, leaving the pellet intact at the bottom. The pellet in each tube was then suspended in 10 mL of erythrocyte lysis buffer (pH 7.4), vortexed, and centrifuged again at 1000 rpm for 10 minutes at 37°C. The pellets were suspended in the plating medium consisted of DMEM/F-12 with 10% heat-inactivated fetal bovine serum, 1% penicillin/streptomycin and 1% Fungizone (all products obtained from Gibco, Invitrogen Corporation, Carlsbad, CA). Adherent ASCs were expanded for a period of 5–8 days at 37°C, and the medium was changed every other day until the cells achieved 80% confluence.
Cell adhesion
ASC adhesion to the PEG hydrogels was assessed. Hydrated PEG hydrogel disks (6mm diameter) were sterilized with 70% ethanol and UV irradiation. ASCs were plated at a density of 20,000 cells per well (96 well plate) in DMEM/F12 with 10% FBS and 1% penicillin/streptomycin on PEG hydrogel disks and control wells (polystyrene tissue culture treated wells, TCP). After 6 hours, the number of cells attached to disks was quantified using a CyQuant Cell Proliferation assay [33–34].
Statistical analysis
Using Origin 7.0 Software (OriginLab Corp., USA), the experimental data from all the studies were analyzed using Analysis of Variance (ANOVA). Statistical significance was set to p value ≤ 0.05. Results were presented as mean ± standard deviation.
RESULTS
Gelation time
The gelation rate of multi-arm PEG hydrogels was monitored under sealed reaction conditions. Increasing the temperature and concentration of genipin decreased the gelation time. The gelation rate of the 4-arm hydrogels was quicker than the 8-arm hydrogel (Figure 2). At 37°C, gelation time of 4-arm-25mM and 8-arm-25mM PEG hydrogels were 1.7h and 6.2h, respectively. Increasing the concentration of genipin, gelation time of 4-arm-35.2mM and 8-arm-35.2mM PEG hydrogels decreased to 1.3h and 4.4h, respectively. The extent of crosslinking was not 100% as there were ~0.7% and ~0.3% free amino groups remaining on the 4-arm-35.2mM and 8-arm-35.2mM gels, respectively (determined by ninhydrin assay, data not shown).
Figure. 2.
Gelation time of 4-arm and 8-arm PEG hydrogel at 37°C. Values reported are an average n=5, ± standard deviation.
Morphologies
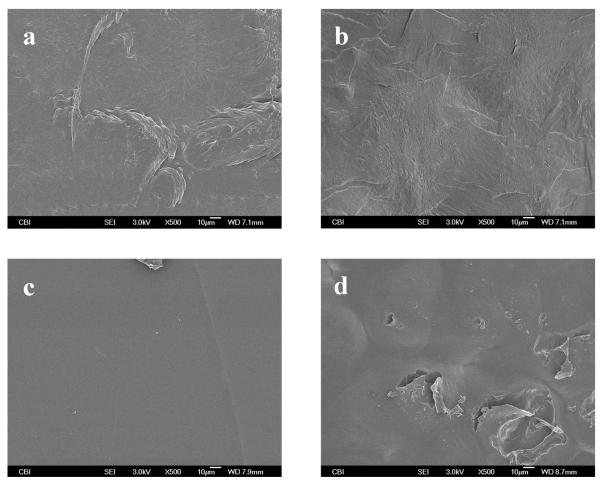
The surface and cross-sectional images of the hydrogels are shown in Figure 3 and Figure 4, respectively. The surface of 4-arm hydrogel (Figure 3a and Figure 3b) appears rough compared to the 8-arm PEG hydrogel (Figure 3c and Figure 3d), regardless of the concentration of genipin. The 8-arm-35.2mM PEG hydrogel showed a comparatively smooth surface (Figure 3c). However, decreasing the concentration of genipin, in the 8-arm PEG hydrogel resulted in a rougher surface (Figure 3d).
Figure 3.
Surface morphologies of 4-arm 35.2mM hydrogel (a), 4-arm 17.6mM hydrogel (b), 8-arm 35.2mM hydrogel (c) and 8-arm 17.6mM PEG (d).
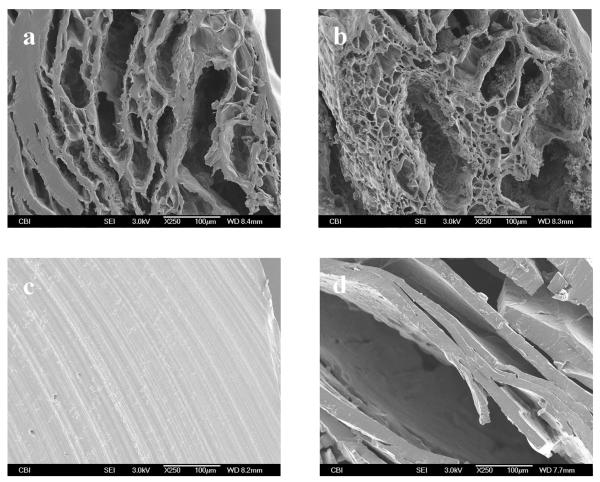
Figure 4.
Cross-sectional morphologies of 4-arm 35.2mM hydrogel (a), 4-arm 17.6mM hydrogel (b), 8-arm 35.2mM hydrogel (c) and 8-arm 17.6mM PEG (d).
During hydrogel fabrication, porous structures were obtained in the 4-arm-35.2mM and 4-arm-17.6mM PEG hydrogel (Figure 4a and Figure 4b). Pores appeared evenly distributed throughout the 4-arm-35.2mM PEG hydrogel scaffolds. However, a similar porous structure was not observed with the 8-arm PEG hydrogel (Figure 4c and Figure 4d). On the contrary, the 8-arm-35.2mM PEG hydrogel showed a comparatively compact structure (Figure 4c). Interestingly, a layered structure was observed in the 8-arm-17.6mM PEG hydrogel (Figure 4d), which may due to less crosslinking.
Swelling and PBS Uptake
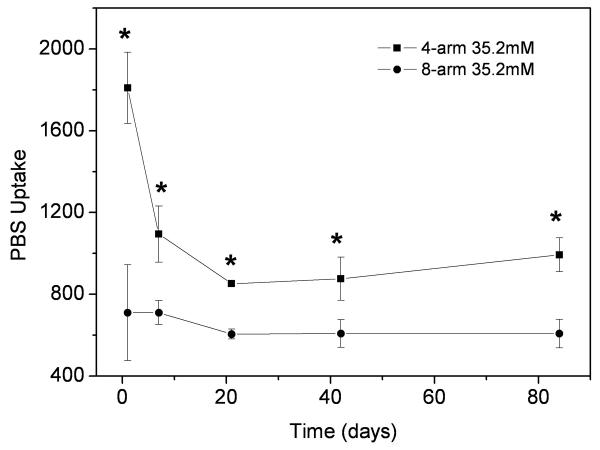
The swelling of PEG hydrogels was determined. The swelling of 4-arm-35.2mM and 8-arm-35.2mM PEG hydrogels was increased ~ 4-22% after incubation in PBS, whereas no difference was found between 4-arm-35.2mM and 8-arm-35.2mM PEG hydrogels, except for day 21 and day 84 (Figure 5).
Figure 5.
Percent increase in diameter versus time of the PEG hydrogels. Values reported are an average n=3, ± standard deviation.
The water uptake of the 4-arm-35.2mM and 8-arm-35.2mM PEG hydrogels was determined by examination of mass increases after immersion in PBS at various time points. As shown in Figure 6, both hydrogels lost their PBS uptake ability quickly before 21 days, then the values changed steadily up to 84 days checked so far. However, for all incubation times, the 4-arm PEG dydrogels demonstrated a significant increase in water uptake compared to the 8-arm PEG hydrogels. The water uptake was also dependent upon the genipin concentration. A lower genipin concentration resulted in an increased water uptake, likely due to a lower degree of crosslinking.
Figure 6.
PBS uptake of 4-arm 35.2mM hydrogel (a), 4-arm 17.6mM hydrogel (b), 8-arm 35.2mM hydrogel (c) and 8-arm 17.6mM PEG (d) after they were incubated in PBS at 37°C for 7d. Values reported are an average n=3, ± standard deviation.
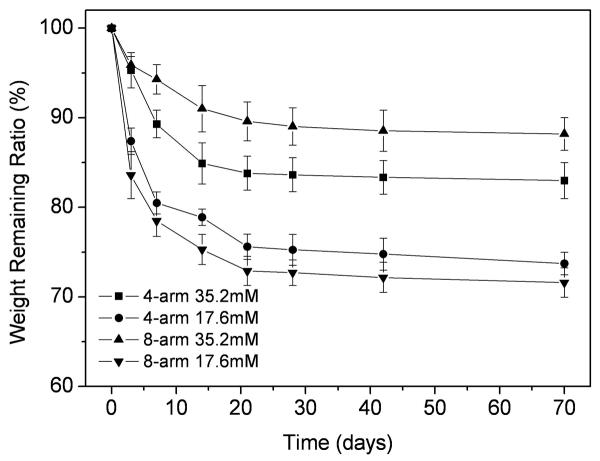
Weight loss
Weight loss of PEG hydrogels was monitored as a function of time in PBS at 37°C, as shown in Figure 7. The weight loss of hydrogels in PBS increased along with the incubation time, with a faster rate of degradation at the initial stage, followed by a slower degradation. The genipin concentration also significantly influenced weight loss. The hydrogels with the lower concentration of genipin showed a significantly faster weight loss. This was as expected since the hydrogels with lower genipin concentration are less crosslinked, therefore, the weight loss should be faster.
Figure 7.
Weight loss of 4-arm 35.2mM hydrogel (a), 4-arm 17.6mM hydrogel (b), 8-arm 35.2mM hydrogel (c) and 8-arm 17.6mM PEG (d). Values reported are an average n=5, ± standard deviation.
The 8-arm-35.2 PEG hydrogel showed a slower weight loss when compared to the 4-arm-35.2 PEG hydrogel. By contrast, with lower concentration of genipin, the weight loss of the 8-arm-17.6 PEG was faster than that of the 4-arm-17.6 PEG, but no significant difference (p>0.05) was observed at any time point, except for week 2 (Figure 7). At week 10, the weight remaining ratio of 4-arm-35.2, 4-arm-17.6, 8-arm-35.2, and 8-arm-17.6 PEG hydrogel were 83.0%, 73.7%, 88.1% and 71.6%, respectively.
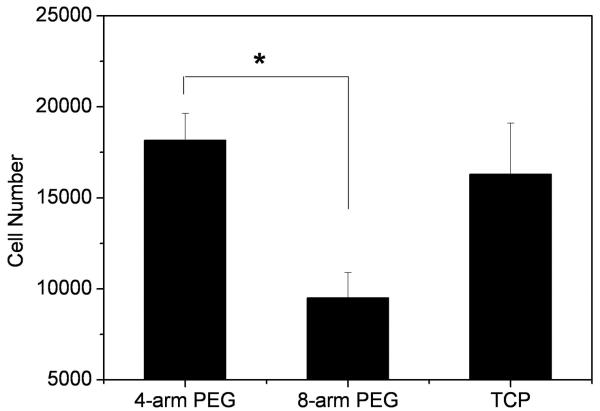
Cell adhesion
The adhesion of human ASCs to 4-arm-35.2 and 8-arm-35.2 PEG hydrogel disks was characterized. As shown in Figure 8, the results that were obtained from the ASCs adhesion study determined that after 6h of incubation, the cell number on the 4-arm-35.2 PEG surface was significantly greater than that of 8-arm-35.2 PEG hydrogel (p<0.05), with no significant difference between the 4-arm-35.2 PEG and the control TCP.
Figure 8.
Number of ASCs adhered to surface of 4-arm-35.2mM and 8-arm-35.2mM PEG hydrogels versus control wells. Values reported are an average n=5, ± standard deviation.
DISCUSSION
An injectable hydrogel is clinically desired as the system could result in minimally invasive surgeries. Hydrogels that gel in situ could fill a defect of any size or shape. The hydrogel can be utilized for cell delivery, as well as growth factor or drug delivery. Various surgeries will require hydrogels with differing gelation rates. A general goal is to develop a gel that gives the surgeon plenty of time to prepare the injury site for injection, while maintaining the integrity of the gel at room temperature. In this study, we developed a multi-branched PEG based hydrogel, which may be used as a injectable hydrogel scaffold for tissue repair.
During the reaction of the PEG diamine with genipin, the solution will change colors, beginning as a clear solution and resulting in a dark blue solution [5, 26]. Since many of the potential applications for multi-branched polymers depend directly on the location and accessibility of terminal end groups [35-36], our hypothesis was that differing structures of the multi-arm PEG hydrogels would result in significantly different gelation rates and degradation times, due to the structure of the gels in water. In our study, the gelation rate was significantly quicker with 4-arm PEG than the 8-arm PEG. While the 8-arm PEG contained more amino functionalities as compared to the 4-arm PEG, we believe the increase in gelation rate of the 4-arm is most likely due to the structure of multi-arm PEG, thus influencing the ability of genipin to react with the amine groups. This is supported by previous studies. For example, Park et al. examined the 4-arm and 8-arm PEG-vinylsulfone hydrogel by thiol-bearing peptide crosslinking for use as a chondrocyte carrier [37]. Similar to our result, the Park et al. study showed that the 8-arm PEG hydrogel was both stiffer and less swollen than the corresponding 4-arm hydrogel. The cross-link density was eight times higher in the 8-arm PEG hydrogel compared with the 4-arm hydrogel. The higher crosslink density resulted in a lower cell number per cluster and a lower expression level of extracellular matrix genes compared with less cross-linked gels.
In another study, Butler et al. examined the reaction mechanism of chitosan, bovine serum albumin (BSA), and gelatin crosslinked with genipin [38]. The results demonstrated that the structure of the molecule reacting with genipin was more important than the number of primary amines. The lower elastic modulus attained after a given time during crosslinking of the globular protein BSA as compared to the coiled protein gelatin, despite possessing more crosslinkable basic residues, demonstrated the importance of protein secondary and tertiary structures in determining the availability of sites for crosslinking with genipin in protein systems.
In another study examining the relationship of PEG-architecture and gelation time, Lee et al. reported the preparation of linear and branched 3,4-dihydroxyphenylalanine (DOPA) modified PEG (PEG-DOPA) hydrogels through chemically or enzymatically induced cross-linking of DOPA residues in the presence of oxidizing agents, such as mushroom tyrosinase, H2O2, NaIO4, and O2 [39]. The results suggested that PEG architecture played an important role in determining the gelation time. The gelation of 4-arm PEG amine was quicker than the linear bis-amine PEG.
Finally, Lin et al. examined the properties of copolymers of PEG and poly(N-isopropylacrylamide) (PNIPAAm) that self-assembles to form hydrogels in a thermoreversible fashion [40]. As the number of arms increased form one to two to four, the strength and deformability of PEG-PNIPAAm hydrogel increased; however, the mechanical properties decreased when 8-arm PEG structures were examined. Due to the intramolecular aggregation which prevented crosslinking of the chains, the 8-arm PEG has a more compact structure and thus gives rise to a smaller binding capacity.
Our results revealed that the structure of multi-arm PEG was an important factor in hydrogel properties. The swelling, water uptake and degradation rate were dependent on the structure of the PEG hydrogel. The 4-arm PEG readily formed hydrogels crosslinked with 15mM and 17.6mM genipin; however, the 8-arm PEG was difficult to form hydrogels with the same concentrations of genipin under sealed condition (Figure 2). Since the 8-arm PEG demonstrated a much slower gelation reaction and more compact structure than the 4-arm PEG, the 8-arm PEG required additional time to accumulate a more compact structure during gelation. The cross-sectional images of the 4-arm PEG hydrogels revealed a porous scaffold (Figure 4a and Figure 4b), which could be beneficial to the swelling and water uptake. The swelling of 8-arm PEG was more stable than 4-arm PEG hydrogel, especially after 7 days incubation in PBS at 37°C (Figure 5), whereas a significant decrease in water uptake was also found due to this compact structure (Figure 6). The 8-arm-35.2 PEG resulted a slower weight loss rate than the 4-arm-35.2 PEG hydrogel in PBS at 37°C (Figure 7), which is likely due to the structure of PEG and the ability of genipin to react with the amine groups. Both compact structure and the low swelling properties of 8-arm-35.2 PEG are beneficial to preservation of mass, and reducing degradation rates. Conversely, the 8-arm-17.6 PEG showed a rapid weight loss during incubation, as molecules were more easily released from the base structure due to the lower degree of crosslinking.
The ASC attachment study indicated that, after 6 hours of incubation, there was no significant difference in the number of cells adhering to the surface of the 4-arm-35.2mM PEG hydrogel compared to the positive control, TCP (Figure 8). However, the cell adhesion on the 4-arm-35.2mM hydrogel was nearly two times greater than those cultured on the 8-arm-35.2mM hydrogel, which may be due to the difference of surface morphologies. A rough surface is more favorable for cell attachment than a smooth one. Furthermore, gel matrices with lower elastic moduli demonstrated larger cell clusters and more diffuse, less cell surface-constrained cell-derived matrix for bovine primary chondrocyte culture [37]. Therefore, the 4-arm hydrogel may be more favorable for ASC attachment due to a lower elastic modulus. Our studies indicate the multi-arm PEG hydrogel crosslinked with genipin supports ASC adhesion, and may have potential uses in musculoskeletal tissue engineering applications.
CONCLUSIONS
The 4-arm and 8-arm PEG hydrogels were characterized by utilizing genipin as a crosslinking agent. The gelation time, swelling, water uptake and weight loss were dependent on the structure of the PEG hydrogel. Due to the molecular architecture, the 8-arm PEG hydrogel showed a much slower gelation reaction, more compact structure and lower water uptake than those of the 4-arm PEG hydrogel, as well as a better stability in vitro with a 35.2mM genipin. Furthermore, human adipose derived stem cell adhesion results indicated that both the 4-arm and 8-arm PEG hydrogels are able to support cell adhesion. This study represents the potential opportunity to use genipin cross-linked, multi-arm PEG-genipin hydrogels, especially the 4-arm PEG-genipin, as an injectable scaffold in a variety of tissue engineering applications.
Acknowledgements
We thankfully acknowledge the Center for Biologic Imaging for SEM, and the University of Pittsburgh CATER fellowship (NIH 5 T32 EB001026-03) (AJD), NIH R01051963 (CRC), and NIH R01CA114246-01A1 (JPR).
References
- 1.Lee KY, Kong HJ, Larson RG, Mooney DJ. Hydrogel formation via cell crosslinking. Adv Mater. 2003;15:1828–1832. [Google Scholar]
- 2.Tememoff JS, Mikos AG. Injectable biodegradable materials for orthopedic tissue engineering. Biomaterials. 2000;21:2405–2412. doi: 10.1016/s0142-9612(00)00108-3. [DOI] [PubMed] [Google Scholar]
- 3.Hou QP, De Bank PA, Shakesheff KM. Injectable scaffolds for tissue regeneration. J Mater Chem. 2004;14:1915–1923. [Google Scholar]
- 4.DeFail AJ, Chu CR, Izzo N, Marra KG. Controlled release of bioactive TGF-β1 from microspheres embedded within biodegradable hydrogels. Biomaterials. 2006;27:1579–1585. doi: 10.1016/j.biomaterials.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Moffat KL, Marra KG. Biodegradable poly(ethylene glycol) hydrogels crosslinked with genipin for tissue engineering applications. J Biomed Mater Res. 2004;71B:181–187. doi: 10.1002/jbm.b.30070. [DOI] [PubMed] [Google Scholar]
- 6.Park YD, Tirelli N, Hubbell JA. Photopolymerized hyaluronic acid-based hydrogels and interpenetrating networks. Biomaterials. 2003;24(6):893–900. doi: 10.1016/s0142-9612(02)00420-9. [DOI] [PubMed] [Google Scholar]
- 7.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24(24):4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 8.McGlohorn JB, Grimes LW, Webster SS, Burg KJL. Characterization of cellular carriers for use in injectable tissue-engineering composites. J Biomed Mater Res. 2003;66A:441–449. doi: 10.1002/jbm.a.10546. [DOI] [PubMed] [Google Scholar]
- 9.Elisseeff J, McIntosh W, Anseth K, Riley S, Ragan P, Langer R. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. J Biomed Mater Res. 2000;51(2):164–171. doi: 10.1002/(sici)1097-4636(200008)51:2<164::aid-jbm4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 10.Behravesh E, Sikavitsas VI, Mikos AG. Quantification of ligand surface concentration of bulk-modified biomimetic hydrogels. Biomaterials. 2003;24(24):4365–4374. doi: 10.1016/s0142-9612(03)00338-7. [DOI] [PubMed] [Google Scholar]
- 11.Anseth KS, Metters AT, Bryant SJ, Martens PJ, Elisseeff JH, Bowman CN. In situ forming degradable networks and their application in tissue engineering and drug delivery. J Control Rel. 2002;78:199–209. doi: 10.1016/s0168-3659(01)00500-4. [DOI] [PubMed] [Google Scholar]
- 12.Guillaudeu SJ, Fox ME, Haidar YM, Dy EE, Szoka FC, Fréchet JMJ. PEGylated dendrimers with core functionality for biological applications. Bioconjugate Chem. 2008;19:461–469. doi: 10.1021/bc700264g. [DOI] [PubMed] [Google Scholar]
- 13.Feng X, Taton D, Chaikof EL, Gnanou Y. Bouquet-type dendrimer-like poly(ethylene Oxide)s with a focal aldehyde and peripheral hydroxyls. Biomacromolecules. 2007;8:2374–2378. doi: 10.1021/bm070146r. [DOI] [PubMed] [Google Scholar]
- 14.Hiemstra C, Zhong Z, Li L, Dijkstra PJ, Feijen J. In-situ formation of biodegradable hydrogels by stereocomplexation of PEG-(PLLA)8 and PEG-(PDLA)8 star block copolymers. Biomacromolecules. 2006;7:2790–2795. doi: 10.1021/bm060630e. [DOI] [PubMed] [Google Scholar]
- 15.Hiemstra C, Zhong Z, van Tomme SR, van Steenbergen MJ, Jacobs JJL, Otter WD, Hennink WE, Feijen J. In vitro and in vivo protein delivery from in situ forming poly(ethylene glycol)—poly(lactide) hydrogels. Journal of Controlled Release. 2007;119:320–327. doi: 10.1016/j.jconrel.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Holland TA, Tessmar JK, Tabata Y, Mikos AG. Transforming growth factor-beta1 release from oligo(poly(ethylene glycol) fumarate) hydrogels in conditions that model the cartilage wound healing environment. J Control Rel. 2004;94(1):101–114. doi: 10.1016/j.jconrel.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Wieland JA, Houchin-Ray TL, Shea LD. Non-viral vector delivery from PEG-hyaluronic acid hydrogels. Journal of Controlled Release. 2007;120:233–241. doi: 10.1016/j.jconrel.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leach JB, Bivens KA, Collins CN, Schmidt CE. Development of photocrosslinkable hyaluronic acidpolyethylene glycol-peptide composite hydrogels for soft tissue engineering. J Biomed Mater Res. 2004;70A:74–82. doi: 10.1002/jbm.a.30063. [DOI] [PubMed] [Google Scholar]
- 19.Almany L, Seliktar D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials. 2005;26:2467–2477. doi: 10.1016/j.biomaterials.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 20.Bhattarai N, Ramay HR, Gunn J, Matsen FA, Zhang M. PEG-grafted chitosan as an injectable thermosensitive hydrogel for sustained protein release. Journal of Controlled Release. 2005;103:609–624. doi: 10.1016/j.jconrel.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi N, Chae BS, Zhang L, Kiick KL, Furst EM. Rheological characterization of polysaccharide-poly(ethylene glycol) star copolymer hydrogels. Biomacromolecules. 2005;6:1931–1940. doi: 10.1021/bm0500042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicodemus GD, Villanueva I, Bryant SJ. Mechanical stimulation of TMJ condylar chondrocytes encapsulated in PEG hydrogels. J Biomed Mater Res. 2007;83A:323–331. doi: 10.1002/jbm.a.31251. [DOI] [PubMed] [Google Scholar]
- 23.Hudalla GA, Eng TS, Murphy WL. An approach to modulate degradation and mesenchymal stem cell behavior in poly(ethylene glycol) networks. Biomacromolecules. 2008;9:842–849. doi: 10.1021/bm701179s. [DOI] [PubMed] [Google Scholar]
- 24.Weber LM, Cheung CY, Anseth KS. Multifunctional pancreatic islet encapsulation barriers achieved via multilayer PEG hydrogels. Cell Transplantation. 2008;16:1049–1057. [PubMed] [Google Scholar]
- 25.Brown CD, Stayton PS, Hoffman AS. Semi-interpenetrating network of poly(ethylene glycol) and poly(D,L-lactide) for the controlled delivery of protein drugs. J. Biomater. Sci. Polymer Edn. 2005;16(2):189–201. doi: 10.1163/1568562053115471. [DOI] [PubMed] [Google Scholar]
- 26.Ferretti M, Marra KG, Kobayashi K, Defail AJ, Chu CR. Controlled in vivo degradation of genipin crosslinked polyethylene glycol hydrogels within osteochondral defects. Tissue Engineering. 2006;12(9):2657–2663. doi: 10.1089/ten.2006.12.2657. [DOI] [PubMed] [Google Scholar]
- 27.Chang Y, Tsai CC, Liang HC, Sung HW. In vivo evaluation of cellular and acellular bovine pericardia fixed with a naturally occurring crosslinking agent (genipin) Biomaterials. 2002;23(12):2447–2457. doi: 10.1016/s0142-9612(01)00379-9. [DOI] [PubMed] [Google Scholar]
- 28.Tsai CC, Huang RN, Sung HW, Liang HC. In vitro evaluation of the genotoxicity of a naturally occurring crosslinking agent (genipin) for biologic tissue fixation. J Biomed Mater Res. 2000;52(1):58–65. doi: 10.1002/1097-4636(200010)52:1<58::aid-jbm8>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 29.Liu BS, Yao CH, Chen YS, Hsu SH. In vitro evaluation of degradation and cytotoxicity of a novel composite as a bone substitute. J Biomed Mater Res. 2003;67A(4):1163–1169. doi: 10.1002/jbm.a.20017. [DOI] [PubMed] [Google Scholar]
- 30.Mi FL, Tan YC, Liang HC, Huang RN, Sung HW. In vitro evaluation of a chitosan membrane cross-linked with genipin. J Biomater Sci Polym Ed. 2001;12(8):835–850. doi: 10.1163/156856201753113051. [DOI] [PubMed] [Google Scholar]
- 31.Sung HW, Huang RN, Huang LLH, Tsai CC, Chiu CT. Feasibility study of a natural crosslinking reagent for biological tissue fixation. J Biomed Mater Res. 1998;42:560–567. doi: 10.1002/(sici)1097-4636(19981215)42:4<560::aid-jbm12>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 32.Aksu AE, Rubin JP, Dudas JR, Marra KG. Role of gender and anatomical region on induction of osteogenic differentiation of human adipose-derived stem cells. Ann Plast Surg. 2008;60:306–322. doi: 10.1097/SAP.0b013e3180621ff0. [DOI] [PubMed] [Google Scholar]
- 33.Marra KG, DeFail AJ, Clavijo-Alvarez JA, Badylak SF, Taieb A, Schipper B, Bennett J, Rubin JP. FGF-2 enhances vascularization for adipose tissue engineering. Plastic and Reconstructive Surgery. 2008;121(4):1153–1164. doi: 10.1097/01.prs.0000305517.93747.72. [DOI] [PubMed] [Google Scholar]
- 34.Santiago LY, Nowak RW, Rubin JP, Marra KG. Peptide-surface modification of poly(caprolactone) with laminin-derived sequences for adipose-derived stem cell applications. Biomaterials. 2006;27:2962–2969. doi: 10.1016/j.biomaterials.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Prosa TYJ, Bauer BJ, Amis EJ, Tomalia DA, Scherrenberg R. A SAXS study of the internal structure of dendritic polymer systems. J Polym Sci B: Polym Phys. 1997;35:2913–2924. [Google Scholar]
- 36.Tomalia DA, Fréchet JMJ. Discovery of dendrimers and dendritic polymers: A brief historical perspective. J Polym Sci B: Polym Chem. 2002;40:2719–2728. [Google Scholar]
- 37.Park Y, Lutolf MP, Hubbell JA, Hunziker EB, Wong M. Bovine primary chondrocyte culture in synthetic matrix metalloproteinase-sensitive poly(ethylene glycol)-based hydrogels as a scaffold for cartilage repair. Tissue Engineering. 2004;10:515–522. doi: 10.1089/107632704323061870. [DOI] [PubMed] [Google Scholar]
- 38.Butler MF, Yiu-Fai NG, Pudney DA. Mechanism and kinetics of crosslinking reaction between biopolymers containing primary amine groups and genipin. J Polym Sci Part A: Polym Chem. 2003;41:3941–3953. [Google Scholar]
- 39.Lee BP, Dalsin JL, Messersmith PB. Synthesis and gelation of DOPA-modified poly(ethylene glycol) hydrogels. Biomacromolecules. 2002;3:1038–1047. doi: 10.1021/bm025546n. [DOI] [PubMed] [Google Scholar]
- 40.Lin HH, Cheng YL. In-situ thermoreversible gelation of block and star copolymers of poly(ethylene glycol) and poly(N-isopropylacrylamide) of varying architectures. Macromolecules. 2001;34:3710–3715. [Google Scholar]