Abstract
Inhibin, which is important for normal gonadal function, acts on the pituitary gonadotropins to suppress follicle-stimulating hormone (FSH) secretion. The level and cellular localization of the inhibin isotypes, α, βA and βB, in the testis of mice were examined during postnatal development in order to determine if inhibin expression is related to testicular maturation. Mouse testes were sampled on postnatal days (PNDs) 1, 3, 6, 18, 48 and 120, and analyzed by Western blotting and immunofluorescence. Western blot analysis showed very low levels of inhibin α, βA and βB expression in the testes at days 1 to 6 after birth. The levels then increased gradually from PND 18 to 48-120, and there were significant peaks at PND 48. Inhibin α, βA and βB were detected in testicular cells during postnatal development using immunohistochemistry. The immunoreactivity of inhibin α was rarely observed in testicular cells during PND 1 to 6, or in the cytoplasmic process of Sertoli cells surrounding the germ cells and interstitial cells during PND 18 to 120. Inhibin βA and βB immunoreactivity was rarely observed in the testis from PND 1 to 6. On the other hand, it was observed in some spermatogonial cells, as well as in the interstitial space between PND 48 and PND 120. We conclude that the expression of inhibin isotypes increases progressively in the testis of mice with increasing postnatal age, suggesting that inhibin is associated with a negative feedback signal for FSH in testicular maturation.
Keywords: inhibin, mouse, postnatal development, Sertoli cell, testis
Introduction
Inhibin is a glycoprotein hormone that is produced principally by the gonads. It is a disulfide linked dimer of two different subunits, a common α isotype and a βA isotype forming inhibin A subunit or a βB isotype forming inhibin B subunit [21]. Although five distinct β isotypes have been isolated, which are termed βA to βE, only the biological activity of βA and βB has been demonstrated [11]. Inhibin belongs to the transforming growth factor β superfamily of growth and differentiation factors, which are important for normal gonadal function. Previous studies reported expression of inhibin in the testis of various mammals including humans [7], primates [20], rats [26], mice [23], hamsters [9], and pigs [8]. Inhibin acts on pituitary gonadotropins to suppress follicle-stimulating hormone (FSH) secretion [5] and to reduce spermatogonial numbers [25].
The pattern of inhibin expression is associated with the two distinct phases of rat Sertoli cells [10]. The first phase is related to an increase in circulating FSH levels [10], which induce Sertoli cell proliferation. The second phase is related to the increasing levels of FSH that are present during pubertal maturation [2,10,24]. Inhibin provides a negative feedback signal that downregulates the secretion of FSH [5,17]. In addition, inhibin α isotype knockout mice show testicular stromal tumors and arrest of gametogenesis [12,18]. On the other hand, transgenic mice overexpressing the inhibin A subunit or the inhibin α isotype have small testes and a reduced level of spermatogenesis [13]. This suggests that inhibin isotypes may regulate testicular maturation along with FSH. The secretion of inhibin is restricted primarily to Sertoli cells in rat testis [16]. Spermatogenic cells in the seminiferous tubules are capable of modulating the expression of inhibin in Sertoli cells both in vitro [4,19] and in vivo [1,6]. Therefore, differential expression of inhibin isotypes might be observed in seminiferous tubules in mice during testicular development.
This study examined the level and cellular localization of inhibin isotypes, α, βA and βB, in the testis of mice during postnatal development in order to determine if inhibin is associated with testicular maturation.
Materials and Methods
Animals and tissue preparation
ICR mice used in this experiment were obtained from the animal center at the Korea Research Institute of Bioscience and Biotechnology. Mice were housed in a room maintained under the following conditions: a temperature of 23 ± 2℃, a relative humidity of 50 ± 5%, with artificial lighting from 08:00 to 20:00 and 13-18 air changes per h. The mice were fed a standard animal diet. Three mice at postnatal days (PNDs) 1, 3, 6, 18, 48 and 120 were obtained from the same litters.
Mice were sacrificed and testes were immediately removed (n = 3). A sample of the testes was embedded in paraffin wax after routine fixation in 10% buffered formalin. Paraffin sections (5 µm thick) were used in all immunostaining experiments. The opposite testis was snap-frozen and stored for immunoblot analysis. All experiments were carried out in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals (USA).
Antisera
Rabbit polyclonal anti-inhibin α (H-134), βA (H-120) and βB (H-110) antibodies were obtained from Santa Cruz Biotechnology (USA). Mouse monoclonal anti-beta-actin and vimentin antibodies were purchased from Sigma (USA) and Neomarkers (USA), respectively.
Western blot analysis
Testes tissues were immersed quickly in buffer H (50 mM β-glycerophosphate, 1.5 mM EGTA, 0.1 mM Na3VO4, 1 mM DTT, 10 µg/ml aprotinin, 2 µg/ml pepstatin, 10 µg/ml leupeptin, 1 mM PMSF, pH 7.4), and sonicated for 10 sec. The homogenate was transferred to microtubes and centrifuged at 19,340 × g for 10 min. The supernatant was then harvested. For the immunoblot assay, the supernatant was loaded into individual lanes of 10% sodium dodecyl (lauryl) sulfate-polyacrylamide gels, electrophoresed and immunoblotted onto polyvinylidene difluoride membranes (Immobilon-P; Millipore, USA). The residual binding sites on the membrane were blocked by incubation with 5% nonfat milk in phosphate-buffered saline (PBS, pH 7.4) for 1 h. Subsequently, the membrane was incubated overnight at 4℃ with rabbit polyclonal anti-inhibin-α, βA and βB antibodies (1 : 1,000 dilution). After extensive washing and incubation with horseradish peroxidase-conjugated goat anti-rabbit antibody (1 : 20,000 dilution; Pierce, USA), signals were visualized using chemiluminescence (Super Signal West Pico; Pierce, USA). For normalization purposes, membranes were re-probed with antibodies against beta-actin (1 : 20,000 dilution; Sigma, USA). Several exposure times were used to obtain signals in the linear range. The bands were quantified using Scion Image Beta 4.0.2 for Windows XP software (Scion, USA). The data were analyzed using one-way ANOVA followed by a Student-Newman-Keuls post hoc test for multiple comparisons. In all cases, a p value < 0.05 was considered significant.
Immunofluorescence
Paraffin-embedded sections of testes (5 µm) were deparaffinized, treated with a citrate buffer (0.01 M, pH 6.0) in a microwave for 20 min, and then treated with 0.3% hydrogen peroxide in methyl alcohol for 20 min to block endogenous peroxidase activity. After three washes with PBS, sections were incubated with 10% normal goat serum, and then incubated with rabbit monoclonal inhibin α, βA and βB (1 : 100 dilution) for 1 h at room temperature. The immunoreactivity was visualized using fluorescein isothiocyanate (FITC)-labeled goat anti-rabbit IgG (1 : 50 dilution; Sigma, USA). Cell phenotypes of inhibin α, βA and βB expression were examined by double label immunofluorescence using cell-type-specific markers, including vimentin (1 : 500 dilution) for the Sertoli and interstitial cells. First, the paraffin sections were reacted with primary rabbit anti-inhibin α, βA and βB followed by FITC-labeled goat anti-rabbit IgG (1 : 50 dilution; Sigma, USA). Slides were then incubated with mouse vimentin followed by tetramethyl rhodamine isothiocyanate-labeled goat anti-mouse IgG (1 : 50 dilution; Sigma, USA).
Results
Histological finding of the mouse testis during postnatal development
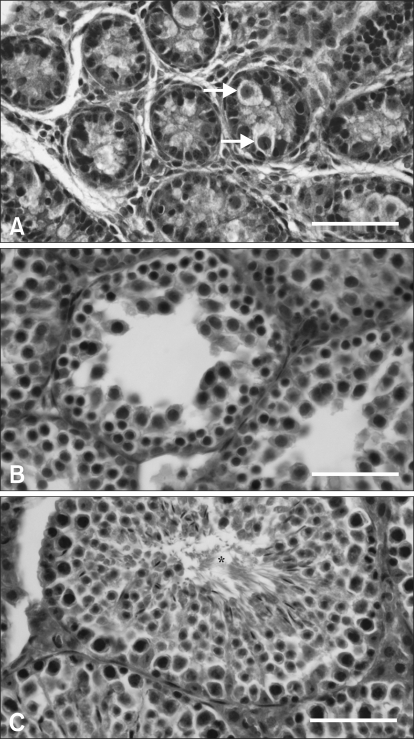
The testis at PND 48-120 showed an increase in the height of the seminiferous epithelium and the defined lumens of the tubules including mature sperm cells (Fig. 1C), while the tubules at PND 1-18 were largely undifferentiated (Figs. 1A and B). As shown in Fig. 1C, there was an abundant population of interstitial cells in the testis at PND 48. The seminiferous tubules contained primary spermatocytes, spermatids and Sertoli cells at various stages. This suggests that sexual maturation in this experimental animal occurs between PND 18 and 48.
Fig. 1.
Light micrographs of the mouse testes at postnatal day (PND) 1 (A), PND 18 (B), and PND 48 (C). The arrows in A indicate gonocytes in undifferentiated seminiferous epithelium. The asterisk in C indicates the defined lumens of the tubules including mature sperm cells. H&E stain. Scale bars = 40 µm.
Temporal expression pattern of the inhibin isotypes α, βA and βB during the postnatal development of mouse testis
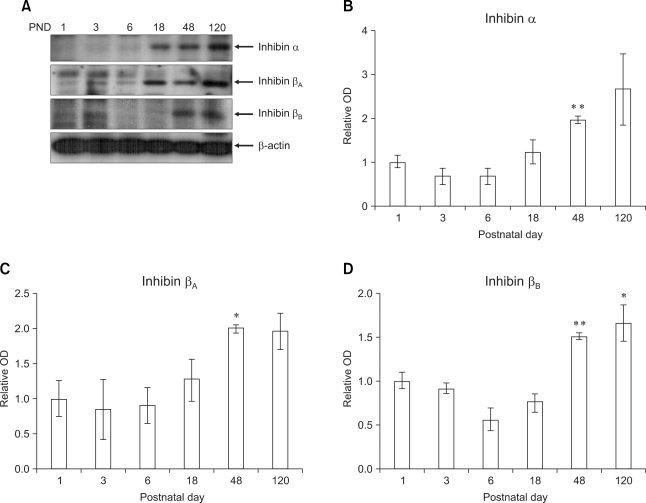
The protein levels of the inhibin α, βA and βB isotypes in the testes during postnatal development were analyzed semiquantitatively by Western blotting to determine the developmental changes in the inhibin isotypes.
As shown in Fig. 2, a low intensity signal for inhibin α expression was detected in the testis at days 1-6 after birth. The level gradually increased at PND 18 to 120, and there was a significant peak (approximately 2 fold, p < 0.01 vs. PND 1-6) at PND 48 (Figs. 2A and B). A low level of inhibin βA expression was observed in the early phase of development (PND 1-6). The level increased and showed a significant peak (approximately 2 fold, p < 0.05 vs. PND 1 and 6) at day 48 after birth (Figs. 2A and C). A low intensity signal for inhibin βB expression was detected in the testis at PND 1-6. The level increased at PND 48-120, and there were substantial levels at both PND 48 (approximately 1.5 fold, p < 0.01 vs. PND 1-18) and PND 120 (approximately 1.6 fold, p < 0.05 vs. PND 1-18) (Figs. 2A and D).
Fig. 2.
Expression of inhibin isotypes α, βA and βB in mouse testis increased progressively with postnatal age. Photographs: Representative photographs of Western blots for inhibin isotypes α, βA and βB and beta-actin (A). Arrowheads indicate the positions of the inhibin isotypes (40~47 kDa) and beta-actin (45 kDa). Minor bands at various molecular weights were detected on the immunoblots for the inhibin isotypes α, βA and βB. Bar graph: The results of densitometric data analysis (mean ± SE, n = 3 mice/group). The relative expression levels of the inhibin isotypes were calculated after normalization to the beta-actin band from three different samples. The value for the testis at postnatal day (PND) 1 was arbitrarily defined as 1 (B, C and D, graphs). *p < 0.05, **p < 0.01 vs. PND 1-6.
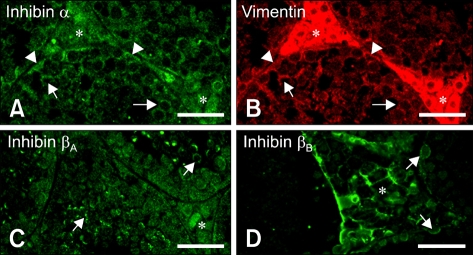
Immunofluorescent detection of inhibin α, βA and βB in mice testis
At PND 1-6, there was little immunoreactivity for inhibin α, βA and βB subunits in testicular cells (data not shown). Inhibin α expression (Fig. 3A) was observed in cytoplasmic processes of vimentin-positive Sertoli cells surrounding spermatogenic cells (Fig. 3B) at PND 18-120. Immunoreactivity for inhibin βA was observed in the interstitial and spermatogenic cells (Fig. 3C) during PND 48-120. Inhibin βB immunoreactivity was observed mainly in cell membranes of some spermatogonia in the seminiferous tubules as well as in the interstitial cells after PND 48 (Fig. 3D).
Fig. 3.
Immunofluorescent localization of inhibin α, βA, and βB isotypes in mouse testis at postnatal days 48. (A and B) Double-immunofluorescent staining in the same section showed the co-localization of inhibin α with vimentin in cell bodies of Sertoli cells (arrowheads), the cytoplasmic process of Sertoli cells (arrows) and in interstitial spaces (asterisks). (C) Immunofluorescent localization of the inhibin βA subunit was observed in the cell membrane of some spermatogenic cells (arrows) as well as in the interstitial cells (asterisk). (D) Immunofluorescent localization of the inhibin βB subunit was observed mainly in cell membranes of interstitial cells (asterisk) as well as in some spermatogonia (arrows). Scale bars = 30 µm.
Discussion
This study shows a gradual increase in the expression of inhibin isotypes, α, βA and βB, in the testis of mice during postnatal development. Each inhibin isotype was localized differentially in testicular cells of the testes between PNDs 18-120. However, expression of these isotypes were rarely observed in testes during the early phase of postnatal development (PND 1-6).
In this study, histological examination of the development of mouse testis showed that sexual maturation is acquired between PND 18 and 48. This suggests that the two major functions of the sexually matured testis, spermatogenesis and generation of sexual hormones, were accomplished between PND 18 and 48. During this phase, protein levels of the three isotypes of inhibin in the testis also increased. The histological findings in the sexual maturation of developing mouse testis are consistent with those of a previous report [23].
In this study, protein levels of the inhibin isotypes (α, βA and βB), were analyzed by western blotting. Low intensities of the isotypes were detected in the early phase, but the levels increased gradually during sexual maturation (PND 18 to 48). Immunohistochemical results showed that expression of inhibin isotypes increased gradually during postnatal development of mouse testis, mainly in the Sertoli and interstitial cells. Previously, it had not been reported that mRNAs for the α, βA and βB isotypes were closely associated with testicular maturation [14,22,23]. The level of FSH increased in rats during pubertal maturation [2,10,24]. Inhibin provides a negative feedback signal that regulates FSH secretion [5,17]. Therefore, the maturation of Sertoli cells by FSH stimulation promotes the expression of inhibin isotypes. Hence, inhibin regulates the development of Sertoli cells and spermatogenesis in mouse testis.
In this study, inhibin α immunoreactivity was detected mainly in Sertoli cells from puberty to adulthood, as previously indicated for rat testis [16]. In addition, expression of inhibin βA and βB subunits was detected in interstitial and spermatogenic cells in the testes of mice from puberty to adulthood. Several studies have reported that the differential expression in various types of testicular cells depends on the animal species [3,8,9,15,17]. Therefore, further studies will be needed to determine the functional role of inhibin via local or paracrine secretion among testicular cells.
In conclusion, expression of the inhibin isotypes α, βA and βB, in the testes of mice gradually increased during postnatal development. Each isotype was localized differentially in testicular cells during maturation. The expression of inhibin isotypes in the testis of mice increased progressively with postnatal age, which suggests that inhibin is associated with a negative feedback signal for FSH during testicular maturation.
Acknowledgments
This work was supported by the Grant of the Korean Ministry of Education, Science and Technology (The Regional Core Research Program/Biohousing Research Institute). This work was supported by the Biohousing Research Center.
References
- 1.Allenby G, Foster PM, Sharpe RM. Evidence that secretion of immunoactive inhibin by seminiferous tubules from the adult rat testis is regulated by specific germ cell types: correlation between in vivo and in vitro studies. Endocrinology. 1991;128:467–476. doi: 10.1210/endo-128-1-467. [DOI] [PubMed] [Google Scholar]
- 2.Au CL, Robertson DM, de Kretser DM. Measurement of inhibin and an index of inhibin production by rat testes during postnatal development. Biol Reprod. 1986;35:37–43. doi: 10.1095/biolreprod35.1.37. [DOI] [PubMed] [Google Scholar]
- 3.Buzzard JJ, Loveland KL, O'Bryan MK, O'Connor AE, Bakker M, Hayashi T, Wreford NG, Morrison JR, de Kretser DM. Changes in circulating and testicular levels of inhibin A and B and activin A during postnatal development in the rat. Endocrinology. 2004;145:3532–3541. doi: 10.1210/en.2003-1036. [DOI] [PubMed] [Google Scholar]
- 4.Clifton RJ, O'Donnell L, Robertson DM. Pachytene spermatocytes in co-culture inhibit rat Sertoli cell synthesis of inhibin βB-subunit and inhibin B but not the inhibin α-subunit. J Endocrinol. 2002;172:565–574. doi: 10.1677/joe.0.1720565. [DOI] [PubMed] [Google Scholar]
- 5.de Kretser DM, Robertson DM. The isolation and physiology of inhibin and related proteins. Biol Reprod. 1989;40:33–47. doi: 10.1095/biolreprod40.1.33. [DOI] [PubMed] [Google Scholar]
- 6.Guitton N, Touzalin AM, Sharpe RM, Cheng CY, Pinon-Lataillade G, Méritte H, Chenal C, Jégou B. Regulatory influence of germ cells on Sertoli cell function in the pre-pubertal rat after acute irradiation of the testis. Int J Androl. 2000;23:332–339. doi: 10.1046/j.1365-2605.2000.00248.x. [DOI] [PubMed] [Google Scholar]
- 7.Illingworth PJ, Groome NP, Byrd W, Rainey WE, McNeilly AS, Mather JP, Bremner WJ. Inhibin-B: a likely candidate for the physiologically important form of inhibin in men. J Clin Endocrinol Metab. 1996;81:1321–1325. doi: 10.1210/jcem.81.4.8636325. [DOI] [PubMed] [Google Scholar]
- 8.Jin W, Arai KY, Herath CB, Kondo M, Ishi H, Tanioka Y, Watanabe G, Groome NP, Taya K. Inhibins in the male Göttingen miniature pig: Leydig cells are the predominant source of inhibin B. J Androl. 2001;22:953–960. doi: 10.1002/j.1939-4640.2001.tb03435.x. [DOI] [PubMed] [Google Scholar]
- 9.Jin W, Wada S, Arai KY, Kishi H, Herath CB, Watanabe G, Suzuki AK, Groome NP, Taya K. Testicular secretion of inhibin in the male golden hamster (Mesocricetus auratus) J Androl. 2001;22:207–211. [PubMed] [Google Scholar]
- 10.Lee VW, de Kretser DM, Hudson B, Wang C. Variations in serum FSH, LH and testosterone levels in male rats from birth to sexual maturity. J Reprod Fertil. 1975;42:121–126. doi: 10.1530/jrf.0.0420121. [DOI] [PubMed] [Google Scholar]
- 11.Mason AJ. Human inhibin and activin: Structure and recombinant expression in mammalian cells. In: Burger HG, de Kretser D, Findlay J, Igarashi M, editors. Inhibin-Non-Steroidal Regulation of Follicle Stimulating Hormone Secretion. New York: Raven Press; 1987. pp. 42–77. [Google Scholar]
- 12.Matzuk MM, Finegold MJ, Su JG, Hsueh AJ, Bradley A. α-inhibin is a tumour-suppressor gene with gonadal specificity in mice. Nature. 1992;360:313–319. doi: 10.1038/360313a0. [DOI] [PubMed] [Google Scholar]
- 13.McMullen ML, Cho BN, Yates CJ, Mayo KE. Gonadal pathologies in transgenic mice expressing the rat inhibin α-subunit. Endocrinology. 2001;142:5005–5014. doi: 10.1210/endo.142.11.8472. [DOI] [PubMed] [Google Scholar]
- 14.Mellor SL, Richards MG, Pedersen JS, Robertson DM, Risbridger GP. Loss of the expression and localization of inhibin α-subunit in high grade prostate cancer. J Clin Endocrinol Metab. 1998;83:969–975. doi: 10.1210/jcem.83.3.4640. [DOI] [PubMed] [Google Scholar]
- 15.Nagata S, Tsunoda N, Nagamine N, Tanaka Y, Taniyama H, Nambo Y, Watanabe G, Taya K. Testicular inhibin in the stallion: cellular source and seasonal changes in its secretion. Biol Reprod. 1998;59:62–68. doi: 10.1095/biolreprod59.1.62. [DOI] [PubMed] [Google Scholar]
- 16.Noguchi J, Hikono H, Sato S, Watanabe G, Taya K, Sasamoto S, Hasegawa Y. Ontogeny of inhibin secretion in the rat testis: secretion of inhibin-related proteins from fetal Leydig cells and of bioactive inhibin from Sertoli cells. J Endocrinol. 1997;155:27–34. doi: 10.1677/joe.0.1550027. [DOI] [PubMed] [Google Scholar]
- 17.O'Connor AE, de Kretser DM. Inhibins in normal male physiology. Semin Reprod Med. 2004;22:177–185. doi: 10.1055/s-2004-831893. [DOI] [PubMed] [Google Scholar]
- 18.Pierson TM, Wang Y, DeMayo FJ, Matzuk MM, Tsai SY, O'Malley BW. Regulable expression of inhibin A in wild-type and inhibin α null mice. Mol Endocrinol. 2000;14:1075–1085. doi: 10.1210/mend.14.7.0478. [DOI] [PubMed] [Google Scholar]
- 19.Pineau C, Sharpe RM, Saunders PT, Gérard N, Jégou B. Regulation of Sertoli cell inhibin production and of inhibin α-subunit mRNA levels by specific germ cell types. Mol Cell Endocrinol. 1990;72:13–22. doi: 10.1016/0303-7207(90)90235-z. [DOI] [PubMed] [Google Scholar]
- 20.Plant TM, Marshall GR. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocr Rev. 2001;22:764–786. doi: 10.1210/edrv.22.6.0446. [DOI] [PubMed] [Google Scholar]
- 21.Robertson DM, Cahir N, Findlay JK, Burger HG, Groome N. The biological and immunological characterization of inhibin A and B forms in human follicular fluid and plasma. J Clin Endocrinol Metab. 1997;82:889–896. doi: 10.1210/jcem.82.3.3801. [DOI] [PubMed] [Google Scholar]
- 22.Schmitt JF, Millar DS, Pedersen JS, Clark SL, Venter DJ, Frydenberg M, Molloy PL, Risbridger GP. Hypermethylation of the inhibin α-subunit gene in prostate carcinoma. Mol Endocrinol. 2002;16:213–220. doi: 10.1210/mend.16.2.0771. [DOI] [PubMed] [Google Scholar]
- 23.Seok OS, Ahn JM, Mayo KE, Cho BN. Developmental changes in inhibin-α gene expression in the mouse testis. Mol Cells. 2004;17:67–72. [PubMed] [Google Scholar]
- 24.Sharpe RM, Turner KJ, McKinnell C, Groome NP, Atanassova N, Millar MR, Buchanan DL, Cooke PS. Inhibin B levels in plasma of the male rat from birth to adulthood: effect of experimental manipulation of Sertoli cell number. J Androl. 1999;20:94–101. [PubMed] [Google Scholar]
- 25.van Dissel-Emiliani FM, Grootenhuis AJ, de Jong FH, de Rooij DG. Inhibin reduces spermatogonial numbers in testes of adult mice and Chinese hamsters. Endocrinology. 1989;125:1899–1903. doi: 10.1210/endo-125-4-1898. [DOI] [PubMed] [Google Scholar]
- 26.Woodruff TK, Besecke LM, Groome N, Draper LB, Schwartz NB, Weiss J. Inhibin A and inhibin B are inversely correlated to follicle-stimulating hormone, yet are discordant during the follicular phase of the rat estrous cycle, and inhibin A is expressed in a sexually dimorphic manner. Endocrinology. 1996;137:5463–5467. doi: 10.1210/endo.137.12.8940372. [DOI] [PubMed] [Google Scholar]