Abstract
Pneumatic dilation (PD) is considered to be the first line nonsurgical therapy for achalasia. The principle of the procedure is to weaken the lower esophageal sphincter by tearing its muscle fibers by generating radial force. The endoscope-guided procedure is done without fluoroscopic control. Clinicians usually use a low-compliance balloon such as Rigiflex dilator to perform endoscope-guided PD for the treatment of esophageal achalasia. It has the advantage of determining mucosal injury during the dilation process, so that a repeat endoscopy is not needed to assess the mucosal tearing. Previous studies have shown that endoscope-guided PD is an efficient and safe nonsurgical therapy with results that compare well with other treatment modalities. Although the results may be promising, long-term follow-up is required in the near future.
Keywords: Esophagoscopy, Dilatation, Esophageal achalasia
INTRODUCTION
Achalasia occurs at all ages; the mean occurrence is in middle age and affects both sexes and all races equally. The diagnosis is made by examinations such as barium esophagography, and esophageal manometry and endoscopy, after it has been distinguished from secondary achalasia caused by malignancy[1-5]. The truth we are facing in dealing with esophageal achalasia is that there is so far no cure for the disease. There are currently three main treatment modalities: pneumatic dilation (PD), surgery and botulinum toxin (BT) injection therapy. All therapeutic approaches are to loosen the lower esophageal sphincter (LES), because LES dysfunction leads to obstruction of the esophagus[6-20]. The goals are to relieve symptoms, improve esophageal emptying and avoid megaesophagus[21]. Traditional smooth muscle relaxants in the form of nitrates and calcium channel antagonists play little role. Besides, there are many intolerable side effects such as headaches, hypotension, and eventual tachyphylaxis[22,23].
No doubt, PD is considered to be the first line nonsurgical therapy for achalasia. The principle of the procedure is to weaken the LES by tearing its muscle fibers by generating radial force. For many decades, there have been many reports about long-term efficacy of PD in the treatment of achalasia under the guidance of fluoroscopy[16-20]. However, some factors may hinder the practice of PD by some gastroenterologists. Some may have fear of the misplaced risk of perforation, and the overall decreased immediate morbidity from laparoscopic myotomy. It is the responsibility of gastroenterologists to continue the tradition of PD as a generally available technique, or otherwise, myotomy may become the routine available therapy for achalasia.
Another reason may be concern about expose to the X-rays during the procedure under fluoroscopic guidance[24-26]. Some of the highest doses to both patients and medical workers only arise from some other interventional radiology procedures[12,26]. Potential high doses during interventional procedures have been reached despite the procedure being carried out on equipment that normally has effective local shielding, so that the dose outside the lead coat is relatively low during fluoroscopy[12,27]. However, fluoroscopic-guided PD requires positioning of the balloon, which may need longer time, thus increasing the radiation exposure. Besides, the entire endoscope-guided PD procedure is done under direct visual control. It is easy to determine the mucosal injury during the dilation. Unlike fluoroscopic-guided PD, a repeat endoscopy to assess the mucosal tearing is not needed. The issue of the endoscope-guided PD for the treatment of esophageal achalasia is reviewed and discussed in this paper.
ENDOSCOPE-GUIDED PD
Levine et al[25] first proposed a safe and convenient PD technique under endoscopic guidance without the use of fluoroscopy. Since then, this technique has been used by many physicians[11,12,23,28-35]. There are many types of pneumatic dilators commercially available. The high-compliance balloons are the Rider-Moeller device and the Brown-McHardy dilator (Narco Scientifics, Piling Division, Fort Washington, PA, USA), Witzel dilator (ABS, par d’ Activite Saint Michel, France) while the low-compliance balloons such as Gruntzig-type dilator (Rigiflex dilator; Microvasive, Watertown, MA, USA). Clinicians usually prefer using the low-compliance balloon (Gruntzig-type, Rigiflex dilator), because it has various theoretical advantages over a high-compliance balloon[34]. Rigiflex dilator is designed so that it can be inflated to a desired maximum diameter. Further inflation can only result in the increase of the pressure but not the diameter[35]. Therefore, the wall tension is increased maximally at the stenotic zone. In contrast, an inflated high-compliance balloon may lead to an increase in the esophageal wall tension more proximal to the stenotic zone more than the stenotic zone itself, which may lead to perforation, by the Laplace law[36-38]. Such speculation is supported theoretically by the fact that the most common site of esophageal perforation is proximal to the cardia on the left lateral side of the esophagus in clinical practice[34,35].
TECHNIQUE OF ENDOSCOPE-GUIDED PD
There is so far no clear consensus on the optimal method for performing PD with regard to balloon diameter, and the amount and rate of inflation pressure. It has been shown that the risk of perforation increases with the size of the balloon[39]. Mikaeli’s[39] and Karamanolis’ groups[14] have reported that graded pneumatic balloon dilatation with a 30-mm diameter and slower rate of balloon inflation is an effective and safe initial method of therapy for achalasia. According to our experience, we recommend that a 3.0-cm dilator with a smaller average inflation pressure of 10-12 psi is sufficient to attain satisfactory clinical remission, at least for oriental populations[14].
Endoscope-guided PD is carried out by choosing a desirable diameter of balloon dilator, under conscious sedation, with informed consent after an overnight fast. Nowadays, the low-compliance Rigiflex dilator is preferred by most doctors. The endoscope is inserted down to the duodenum (Figure 1A). A guide-wire is placed into the duodenum under endoscopic guidance and then the endoscope is removed (Figure 1B). A Regiflex balloon dilator of a desirable diameter, which is marked with a thick colored marker at the mid-section of the balloon, is passed over the guide wire to the stomach. The endoscope is reinserted to serve as a guide to control the position of the balloon in the esophagus. The balloon is withdrawn to the esophagus, until the mark reaches the gastroesophageal junction. The balloon is inflated up to 12 psi and maintained for 60 s, until an ischemic ring at the LES can be seen by the endoscope through the transparent balloon (Figure 1C). The same inflation procedure is repeated once more and held for another 30 s. The balloon is flattened completely and removed together with the endoscope (Figure 1D). Gastrograffin ingestion is performed immediately after the dilation to determine possible esophageal perforation. Chest pains and vital signs are monitored closely. Chest X-rays or computer tomography are carried out should severity of the chest pains imply any possibility of rupture.
Figure 1.
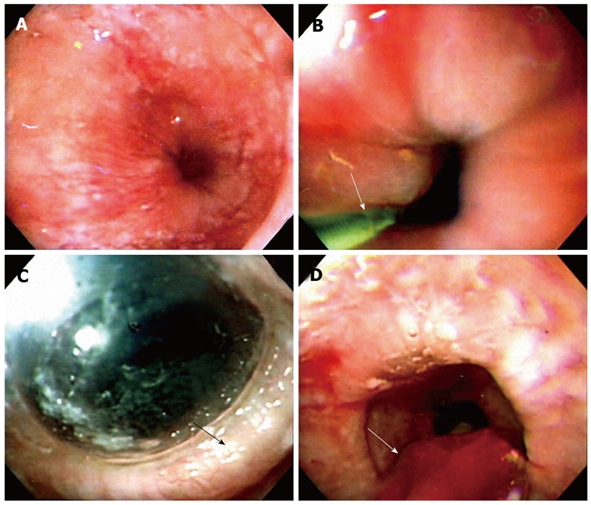
Technique of endoscope-guided pneumatic dilation (PD). A: A dilated low esophageal lumen with tight gastroesophageal junction under endoscope-guided PD; B: The endoscope is inserted down to the duodenum. A guide-wire (arrow) is placed into the duodenum under endoscopic guidance and the endoscope is removed. A Regiflex balloon dilator, which is marked with a thick colored marker at the mid-section of the balloon, is passed over the guide wire to the stomach; C: The endoscope is reinserted to serve as a guide to control the position of the balloon in the esophagus. The balloon is withdrawn to the esophagus, until the mark reaches the gastroesophageal junction. The balloon is then inflated up to 12 psi and maintained for 60 s, until an ischemic ring (arrow) at the LES is seen by the endoscope through the transparent balloon; D: The balloon is flattened completely and removed together with the endoscope (arrow).
Studies on the techniques of Rigiflex balloon dilation of achalasia by positioning the endoscope above the balloon without fluoroscopy have shown results comparable with studies when using fluoroscopy (Figure 2). The advantages are performing this procedure without any extra equipment in a time and cost-effective manner. However, Rai’s group[28] has introduced a novel technique by the presence of the endoscope across the gastroesophageal junction during the dilation procedure, with good efficacy reported after dilation. However, despite the safety report from Rai’s group, the potential danger of increased perforation is a concern of many other clinicians. Some have argued that such a technique is likely to interfere with the application of uniform radial force on the spastic sphincter. The effect of dilation toward the side of the endoscope can be compromising, and lead to a decrease in overall efficacy of the procedure and the possibility of generating an unequal radial force on the sphincter[40,41].
Figure 2.
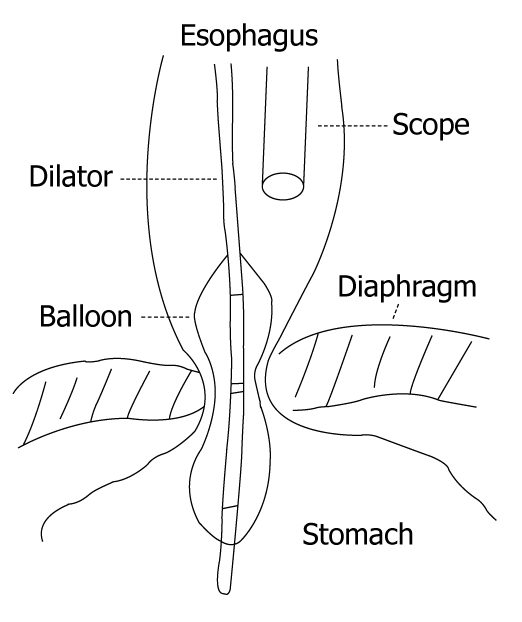
Almost all endoscope-guided PD are performed by using a low compliance Rigiflex balloon dilator by positioning the endoscope above the balloon without fluoroscopy. The advantages are that the procedure can be performed without any extra equipment in a time and cost-effective manner.
POST-DILATION INVESTIGATIONS
Assessment by symptom scores vs esophagography
Usually, structured interviews are performed using validated symptom score methods[10,28,42] at the initial investigation, 6 wk later, and every year thereafter. Depending on whether dysphagia, regurgitation, and chest pain occur occasionally, daily, or several times during the day, a symptom score can be determined. In the system validated by Eckardt[42], a symptom score of 0-3 was assigned to the degree of weight loss. Thus a completely asymptomatic patient would have a symptom score of 0, whereas a severely affected patient could have a high symptom score. In most of these symptom scoring systems, patients were considered to have reached clinical remission if symptoms had totally disappeared or if they had improved by attaining a certain drop in score. Patients who requested further therapy despite having a certain drop in score were considered treatment failures.
A discrepancy between objective parameters and the subjective symptomatic improvement after PD exists in clinical practice. Radiographic findings do not reliably correlate the symptoms to improved esophageal emptying after PD in some large studies[43-45]. However, Vaezi et al[46-49] have reported that there was a significant association between improvement in patient symptoms and barium height. They believe that radiographic findings can reliably predict clinical remission and have suggested strongly the need for further treatment in those patients with poor esophageal clearance after each dilation, to avoid possible future complications such as sigmoid-type achalasia.
Large-scale, long-term follow-up investigations[15,50,51] have reported unfavorable recurrence in patients who have undergone fluoroscopic-guided PD. During the prolonged observation period (median, 13.8 years) in a prospective follow-up investigation study conducted by Eckardt et al[15], only 40% of patients treated with a single round of PD remained in remission at 5 years. We used endoscope-guided PD to treat achalasia and attained cumulative remissions of 86.7% in first 2 years, which had dropped to 72.9% after 5 years, but it remained at 61.7% in years 6 and 7, but patients were assessed by clinical symptom scores[11]. Bias probably existed when using subjective symptomatic scoring assessment to determine clinical remission. We agree with Vaezi’s group who claimed that underscores may occur by only using an objective assessment. However, we believe that esophagography can only offer an additional objective assessment to the response to achalasia treatment, especially in patients who report symptomatic improvement, but the evidence is not strong enough to overthrown the traditional assessment of clinical remission by using subjective symptom score assessment[52]. Although an additional objective parameter such as esophagography to the subjective symptom scores should be more optimal in assessing clinical remission, further investigations that include larger sample sizes and longer follow-up periods are required for clarification of this issue.
Manometric studies
Manometry is an important predictor of treatment failure with balloon dilation, other than younger age (< 40 years), male sex, pulmonary symptoms, and failed response to one or two initial dilations[15,41,53,54]. It has been demonstrated previously that post-dilated LES pressure is relevant to better remission. In general, decreases in LES pressure of > 50% after PD, or an absolute end-expiratory LES pressure of < 10 mmHg, are more indicative of clinical success[15,52,53]. Therefore, it is suggested strongly that manometry be performed routinely before and after PD. One recent advance in the diagnosis of esophageal achalasia is the use of updated high resolution manometry (HRM) with pressure topography plotting[55]. We are optimistic that more promising evidence may emerge on the use of HRM in the near future.
EFFECTIVENESS AND POST-DILATION SAFETY OF ENDOSCOPE-GUIDED PNEUMATIC DILATORS FOR ACHALASIA
Table 1 summarizes studies on the effectiveness of graded endoscope-guided pneumatic dilators for achalasia. Most studies were retrospective except for three prospective, longitudinal cohort studies with a mean follow-up period of 2-4.5 years[11,24,25,28,29]. All studies attained an acceptable clinical remission rate of 54%-91%, which was comparable to those reported by using fluoroscopy-guided PD[32,50,56-59]. Although the existing mid-term follow-up results are encouraging, further long-term follow-up is required in the near future.
Table 1.
Cumulative effectiveness of endoscope-guided pneumatic dilators for the treatment of achalasia by using low compliance Regiflex dilators
| Author (yr) | n | Study design | Dilator size (cm) | Improvement (%) | Follow-up (yr) | Perforation (%) |
| (excellent/good) | mean (range) | |||||
| Levine et al[25] (1987) | 62 | Retrospective | 3.0-3.5 | 85/88 | - | 0 |
| Lambroza et al[24] (1995) | 27 | Retrospective | 3.0 | 67 | 1.8 (0.1-4.8) | 0 |
| Dobrucali et al[28] (2004) | 43 | Prospective | 3.0-3.5 | 54/79 | 2.4 (0.5-5) | 0 |
| Rai et al[29] (2005) | 56 | Prospective | 3.5 | 92.9/89.3 | 2 | 0 |
| Chuah et al[11] (2009) | 32 | Prospective | 3.0 | 69/91 | 4.5 (2.5-7) | 3.3 |
The major adverse event caused by PD is esophageal perforation, with a 2% overall cumulative rate, and may occur in up to as many as 5% of all the reported cases of fluoroscope-guided PD[32,49,56-59]. As shown in Table 1, the reported perforation rates was 0%-3.3% for endoscope-guided PD[11,24,25,28,29]. This implies the relative safety of endoscope-guided PD compared to fluoroscope-guided PD. However, esophageal perforation is a potential hazard after PD[60,61]. Usually, gastrograffin is ingested immediately after each PD to detect extravasation, which implies the presence of perforation. However, on rare occasions, immediate gastrograffin ingestion may not always detect perforation, which can become clinically evident several hours later after delayed presentation (> 24 h)[62,63]. Therefore, we must observe the clinical symptoms and signs closely, such as severe chest pain and fever, which imply the potential presence of perforation after PD.
Reflux symptoms after PD are usually mild and transient and should be easily controlled with proton-pump inhibitors[19]. However, objective assessment of gastroesophageal reflux after PD has rarely been studied. Other complications are usually minor, and include intramural hematoma, diverticula at the gastric cardia, mucosal tears, prolonged post-procedure chest pain, hematemesis without change in hematocrit, fever and angina.
LAPAROSCOPIC MYOTOMY VS ENDOSCOPE-GUIDED PD
Like every other treatment of achalasia, the goal of surgery is to assuage the esophageal obstruction by myotomy of the LES. Minimally invasive laparoscopic myotomy with a variety of fundoplication procedures has evolved to be a primary approach for many surgeons and gastroenterologists in a majority of patients with achalasia[64-67]. However, there are only limited systematic reviews and meta-analyses that have compared existing treatment methods for achalasia and all favor surgery to PD[64,68,69]. With overall success rates of 47%-82% at 10 years, laparoscopic Heller myotomy with partial fundoplication appears to have evolved into the surgical procedure of choice[64,65]. Despite this, the major concern for myotomy is still that it can be complicated by severe acid reflux disease, and the role of fundoplication with myotomy continues to be controversial[21,69-72]. Hence, it is generally accepted that myotomy is usually suggested for younger male patients (< 40 years), those with pulmonary symptoms, and those who have failed to respond to one or two initial dilations; older age appears to be associated with favorable outcomes of PD[70,71].
BT INJECTION THERAPY VS ENDOSCOPE-GUIDED PD
As a result of its wider safety margin and fewer complications, BT injections have been practiced widely in past decades, with excellent immediate responses (success rates of > 90%). Unfortunately, the duration of response for BT injections is relatively discouraging (6-9 mo on average) in most patients, and only half of all patients benefit for > 1 year[6,10,73]. The effect of BT injections vanishes with time in elderly patients, which necessitates repeated injections to keep the patients symptom-free. As a result of the number of repeated injections required, this procedure is more expensive than PD by ≥ 50%. However, it has been reported that the long-term success is highest among elderly patients and in those with an LES pressure that did not exceed the upper normal level before treatment[6,10,74,75]. Also, younger patients (< 55 years) with a severe increase in LES pressure do not seem to benefit from BT injections, and PD or minimally invasive myotomy are more advantageous[10]. Generally, minimally invasive myotomy is recommended in younger patients.
In short, PD is more efficacious than BT injections for sustained symptomatic relief in patients with achalasia. BT is as good as PD in achieving a short-term improvement in achalasia. It is also effective in patients with tortuous megaesophagus and previous failed PD. However, as mentioned earlier, recurrence is high during 1-year follow-up[76]. Furthermore, some surgeons may be concerned that previous BT injections make subsequent minimally invasive myotomy riskier and more difficult[77]. Therefore, BT injections are recommended as a suitable alternative only for a minority of older or high-risk patients.
CONCLUSION
Endoscope-guided PD is an efficient and safe nonsurgical therapy with results comparable to other treatment modalities. Besides, it has the advantage that the entire procedure is done without fluoroscopic control, and the mucosal injury during the dilation can be determined by direct visual observation. Long-term follow-up studies are required in the near future.
Footnotes
Peer reviewers: Kyoichi Adachi, MD, Department of Gastroenterology and Hepatology, Shimane University, School of Medicine Shimane, 89-1 Enya-cho, Izumo-shi Shimane 693-8501, Japan; Fabio Pace, Professor, Division of Gastroenterology, “L. Sacco” University Hospital, University of Milan, Via G. B. Grassi, 74, Milano 20157, Italy
S- Editor Tian L L- Editor Kerr C E- Editor Zheng XM
References
- 1.Chuah SK, Kuo CM, Wu KL, Changchien CS, Hu TH, Wang CC, Chiu YC, Chou YP, Hsu PI, Chiu KW, et al. Pseudoachalasia in a patient after truncal vagotomy surgery successfully treated by subsequent pneumatic dilations. World J Gastroenterol. 2006;12:5087–5090. doi: 10.3748/wjg.v12.i31.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gockel I, Eckardt VF, Schmitt T, Junginger T. Pseudoachalasia: a case series and analysis of the literature. Scand J Gastroenterol. 2005;40:378–385. doi: 10.1080/00365520510012118. [DOI] [PubMed] [Google Scholar]
- 3.Mohamed A. Education and Imaging. Gastrointestinal: pseudoachalasia caused by a lower esophageal stromal tumor. J Gastroenterol Hepatol. 2009;24:1152. doi: 10.1111/j.1440-1746.2009.05883.x. [DOI] [PubMed] [Google Scholar]
- 4.Saino G, Bona D, Nencioni M, Rubino B, Bonavina L. Laparoscopic diagnosis of pleural mesothelioma presenting with pseudoachalasia. World J Gastroenterol. 2009;15:3569–3572. doi: 10.3748/wjg.15.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moorman AJ, Oelschlager BK, Rulyak SJ. Pseudoachalasia caused by retroperitoneal B-cell lymphoma. Clin Gastroenterol Hepatol. 2008;6:A32. doi: 10.1016/j.cgh.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Zárate N, Mearin F, Baldovino F, Armengol JR, Malagelada JR. Achalasia treatment in the elderly: is botulinum toxin injection the best option? Eur J Gastroenterol Hepatol. 2002;14:285–290. doi: 10.1097/00042737-200203000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Chumpitazi BP, Fishman SJ, Nurko S. Long-term clinical outcome after botulinum toxin injection in children with nonrelaxing internal anal sphincter. Am J Gastroenterol. 2009;104:976–983. doi: 10.1038/ajg.2008.110. [DOI] [PubMed] [Google Scholar]
- 8.Bassotti G, D'Onofrio V, Battaglia E, Fiorella S, Dughera L, Iaquinto G, Mazzocchi A, Morelli A, Annese V. Treatment with botulinum toxin of octo-nonagerians with oesophageal achalasia: a two-year follow-up study. Aliment Pharmacol Ther. 2006;23:1615–1619. doi: 10.1111/j.1365-2036.2006.02907.x. [DOI] [PubMed] [Google Scholar]
- 9.Pasricha PJ, Rai R, Ravich WJ, Hendrix TR, Kalloo AN. Botulinum toxin for achalasia: long-term outcome and predictors of response. Gastroenterology. 1996;110:1410–1415. doi: 10.1053/gast.1996.v110.pm8613045. [DOI] [PubMed] [Google Scholar]
- 10.Pasricha PJ, Ravich WJ, Hendrix TR, Sostre S, Jones B, Kalloo AN. Intrasphincteric botulinum toxin for the treatment of achalasia. N Engl J Med. 1995;332:774–778. doi: 10.1056/NEJM199503233321203. [DOI] [PubMed] [Google Scholar]
- 11.Chuah SK, Hu TH, Wu KL, Hsu PI, Tai WC, Chiu YC, Lee CM, Changchien CS. Clinical remission in endoscope-guided pneumatic dilation for the treatment of esophageal achalasia: 7-year follow-up results of a prospective investigation. J Gastrointest Surg. 2009;13:862–867. doi: 10.1007/s11605-009-0804-z. [DOI] [PubMed] [Google Scholar]
- 12.Chuah SK, Hu TH, Wu KL, Kuo CM, Fong TV, Lee CM, Changchien CS. Endoscope-guided pneumatic dilatation of esophageal achalasia without fluoroscopy is another safe and effective treatment option: a report of Taiwan. Surg Laparosc Endosc Percutan Tech. 2008;18:8–12. doi: 10.1097/SLE.0b013e31815c1ba2. [DOI] [PubMed] [Google Scholar]
- 13.Tutuian R, Castell DO, Katz PO. Pneumatic dilatations for achalasia: a safe and effective choice for most patients. Am J Gastroenterol. 2006;101:2441–2442. doi: 10.1111/j.1572-0241.2006.00742_9.x. [DOI] [PubMed] [Google Scholar]
- 14.Karamanolis G, Sgouros S, Karatzias G, Papadopoulou E, Vasiliadis K, Stefanidis G, Mantides A. Long-term outcome of pneumatic dilation in the treatment of achalasia. Am J Gastroenterol. 2005;100:270–274. doi: 10.1111/j.1572-0241.2005.40093.x. [DOI] [PubMed] [Google Scholar]
- 15.Eckardt VF, Gockel I, Bernhard G. Pneumatic dilation for achalasia: late results of a prospective follow up investigation. Gut. 2004;53:629–633. doi: 10.1136/gut.2003.029298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Portale G, Costantini M, Rizzetto C, Guirroli E, Ceolin M, Salvador R, Ancona E, Zaninotto G. Long-term outcome of laparoscopic Heller-Dor surgery for esophageal achalasia: possible detrimental role of previous endoscopic treatment. J Gastrointest Surg. 2005;9:1332–1339. doi: 10.1016/j.gassur.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Diamantis T, Pikoulis E, Felekouras E, Tsigris C, Arvelakis A, Karavokyros I, Bastounis E. Laparoscopic esophagomyotomy for achalasia without a complementary antireflux procedure. J Laparoendosc Adv Surg Tech A. 2006;16:345–349. doi: 10.1089/lap.2006.16.345. [DOI] [PubMed] [Google Scholar]
- 18.Pasricha PJ. Quality versus quantity: outcomes after achalasia surgery. Dig Liver Dis. 2006;38:551–553. doi: 10.1016/j.dld.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Campos GM, Vittinghoff E, Rabl C, Takata M, Gadenstätter M, Lin F, Ciovica R. Endoscopic and surgical treatments for achalasia: a systematic review and meta-analysis. Ann Surg. 2009;249:45–57. doi: 10.1097/SLA.0b013e31818e43ab. [DOI] [PubMed] [Google Scholar]
- 20.Williams VA, Peters JH. Achalasia of the esophagus: a surgical disease. J Am Coll Surg. 2009;208:151–162. doi: 10.1016/j.jamcollsurg.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 21.Richter JE. A young man with a new diagnosis of achalasia. Clin Gastroenterol Hepatol. 2008;6:859–863. doi: 10.1016/j.cgh.2008.02.061. [DOI] [PubMed] [Google Scholar]
- 22.Hoogerwerf WA, Pasricha PJ. Pharmacologic therapy in treating achalasia. Gastrointest Endosc Clin N Am. 2001;11:311–324, vii. [PubMed] [Google Scholar]
- 23.Gelfond M, Rozen P, Gilat T. Isosorbide dinitrate and nifedipine treatment of achalasia: a clinical, manometric and radionuclide evaluation. Gastroenterology. 1982;83:963–969. [PubMed] [Google Scholar]
- 24.Lambroza A, Schuman RW. Pneumatic dilation for achalasia without fluoroscopic guidance: safety and efficacy. Am J Gastroenterol. 1995;90:1226–1229. [PubMed] [Google Scholar]
- 25.Levine ML, Dorf BS, Moskowitz G, Bank S. Pneumatic dilatation in achalasia under endoscopic guidance: correlation pre- and postdilatation by radionuclide scintiscan. Am J Gastroenterol. 1987;82:311–314. [PubMed] [Google Scholar]
- 26.Williams JR. The interdependence of staff and patient doses in interventional radiology. Br J Radiol. 1997;70:498–503. doi: 10.1259/bjr.70.833.9227232. [DOI] [PubMed] [Google Scholar]
- 27.Faulkner K, Moores BM. An assessment of the radiation dose received by staff using fluoroscopic equipment. Br J Radiol. 1982;55:272–276. doi: 10.1259/0007-1285-55-652-272. [DOI] [PubMed] [Google Scholar]
- 28.Dobrucali A, Erzin Y, Tuncer M, Dirican A. Long-term results of graded pneumatic dilatation under endoscopic guidance in patients with primary esophageal achalasia. World J Gastroenterol. 2004;10:3322–3327. doi: 10.3748/wjg.v10.i22.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rai RR, Shende A, Joshi A, Mathur A, Nijhawan S. Rigiflex pneumatic dilation of achalasia without fluoroscopy: a novel office procedure. Gastrointest Endosc. 2005;62:427–431. doi: 10.1016/j.gie.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Cox J, Buckton GK, Bennett JR. Balloon dilatation in achalasia: a new dilator. Gut. 1986;27:986–989. doi: 10.1136/gut.27.8.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neumann G, Samani U, Nas S. A new endoscopic technique for introducing pneumatic dilators in patients with achalasia. Gastrointest Endosc. 1992;38:598–601. doi: 10.1016/s0016-5107(92)70527-6. [DOI] [PubMed] [Google Scholar]
- 32.Bhatnagar MS, Nanivadekar SA, Sawant P, Rathi PM. Achalasia cardia dilatation using polyethylene balloon (Rigiflex) dilators. Indian J Gastroenterol. 1996;15:49–51. [PubMed] [Google Scholar]
- 33.Witzel L. Treatment of achalasia with a pneumatic dilator attached to a gastroscope. Endoscopy. 1981;13:176–177. doi: 10.1055/s-2007-1021677. [DOI] [PubMed] [Google Scholar]
- 34.Stark GA, Castell DO, Richter JE, Wu WC. Prospective randomized comparison of Brown-McHardy and microvasive balloon dilators in treatment of achalasia. Am J Gastroenterol. 1990;85:1322–1326. [PubMed] [Google Scholar]
- 35.Muehldorfer SM, Hahn EG, Ell C. High- and low-compliance balloon dilators in patients with achalasia: a randomized prospective comparative trial. Gastrointest Endosc. 1996;44:398–403. doi: 10.1016/s0016-5107(96)70088-3. [DOI] [PubMed] [Google Scholar]
- 36.Bittinger M, Wienbeck M. Pneumatic dilation in achalasia. Can J Gastroenterol. 2001;15:195–199. doi: 10.1155/2001/593657. [DOI] [PubMed] [Google Scholar]
- 37.Rabinovici R, Katz E, Goldin E, Kluger Y, Ayalon A. The danger of high compliance balloons for esophageal dilatation in achalasia. Endoscopy. 1990;22:63–64. doi: 10.1055/s-2007-1012793. [DOI] [PubMed] [Google Scholar]
- 38.Kadakia SC, Parker A, Carrougher JG, Shaffer RT. Esophageal dilation with polyvinyl bougies, using a marked guidewire without the aid of fluoroscopy: an update. Am J Gastroenterol. 1993;88:1381–1386. [PubMed] [Google Scholar]
- 39.Mikaeli J, Bishehsari F, Montazeri G, Yaghoobi M, Malekzadeh R. Pneumatic balloon dilatation in achalasia: a prospective comparison of safety and efficacy with different balloon diameters. Aliment Pharmacol Ther. 2004;20:431–436. doi: 10.1111/j.1365-2036.2004.02080.x. [DOI] [PubMed] [Google Scholar]
- 40.Thomas V, Harish K, Sunilkumar K. Pneumatic dilation of achalasia cardia under direct endoscopy: the debate continues. Gastrointest Endosc. 2006;63:734. doi: 10.1016/j.gie.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 41.Abid S, Lindberg G. Rigiflex pneumatic dilation of achalasia without fluoroscopy: a novel office procedure. Gastrointest Endosc. 2006;63:537. doi: 10.1016/j.gie.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 42.Eckardt VF. Clinical presentations and complications of achalasia. Gastrointest Endosc Clin N Am. 2001;11:281–292, vi. [PubMed] [Google Scholar]
- 43.Eckardt VF, Aignherr C, Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology. 1992;103:1732–1738. doi: 10.1016/0016-5085(92)91428-7. [DOI] [PubMed] [Google Scholar]
- 44.Kim CH, Cameron AJ, Hsu JJ, Talley NJ, Trastek VF, Pairolero PC, O'Connor MK, Colwell LJ, Zinsmeister AR. Achalasia: prospective evaluation of relationship between lower esophageal sphincter pressure, esophageal transit, and esophageal diameter and symptoms in response to pneumatic dilation. Mayo Clin Proc. 1993;68:1067–1073. doi: 10.1016/s0025-6196(12)60900-8. [DOI] [PubMed] [Google Scholar]
- 45.Gross R, Johnson LF, Kaminski RJ. Esophageal emptying in achalasia quantitated by a radioisotope technique. Dig Dis Sci. 1979;24:945–949. doi: 10.1007/BF01311951. [DOI] [PubMed] [Google Scholar]
- 46.Vaezi MF, Baker ME, Achkar E, Richter JE. Timed barium oesophagram: better predictor of long term success after pneumatic dilation in achalasia than symptom assessment. Gut. 2002;50:765–770. doi: 10.1136/gut.50.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaezi MF, Baker ME, Richter JE. Assessment of esophageal emptying post-pneumatic dilation: use of the timed barium esophagram. Am J Gastroenterol. 1999;94:1802–1807. doi: 10.1111/j.1572-0241.1999.01209.x. [DOI] [PubMed] [Google Scholar]
- 48.Vaezi MF, Richter JE, Wilcox CM, Schroeder PL, Birgisson S, Slaughter RL, Koehler RE, Baker ME. Botulinum toxin versus pneumatic dilatation in the treatment of achalasia: a randomised trial. Gut. 1999;44:231–239. doi: 10.1136/gut.44.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Oliveira JM, Birgisson S, Doinoff C, Einstein D, Herts B, Davros W, Obuchowski N, Koehler RE, Richter J, Baker ME. Timed barium swallow: a simple technique for evaluating esophageal emptying in patients with achalasia. AJR Am J Roentgenol. 1997;169:473–479. doi: 10.2214/ajr.169.2.9242756. [DOI] [PubMed] [Google Scholar]
- 50.West RL, Hirsch DP, Bartelsman JF, de Borst J, Ferwerda G, Tytgat GN, Boeckxstaens GE. Long term results of pneumatic dilation in achalasia followed for more than 5 years. Am J Gastroenterol. 2002;97:1346–1351. doi: 10.1111/j.1572-0241.2002.05771.x. [DOI] [PubMed] [Google Scholar]
- 51.Vela MF, Richter JE, Khandwala F, Blackstone EH, Wachsberger D, Baker ME, Rice TW. The long-term efficacy of pneumatic dilatation and Heller myotomy for the treatment of achalasia. Clin Gastroenterol Hepatol. 2006;4:580–587. doi: 10.1016/s1542-3565(05)00986-9. [DOI] [PubMed] [Google Scholar]
- 52.Chuah SK, Hu TH, Wu KL, Chen TY, Changchien CS, Lee CM. The role of barium esophagogram measurements in assessing achalasia patients after endoscope-guided pneumatic dilation. Dis Esophagus. 2009;22:163–168. doi: 10.1111/j.1442-2050.2008.00888.x. [DOI] [PubMed] [Google Scholar]
- 53.Farhoomand K, Connor JT, Richter JE, Achkar E, Vaezi MF. Predictors of outcome of pneumatic dilation in achalasia. Clin Gastroenterol Hepatol. 2004;2:389–394. doi: 10.1016/s1542-3565(04)00123-5. [DOI] [PubMed] [Google Scholar]
- 54.Dağli U, Kuran S, Savaş N, Ozin Y, Alkim C, Atalay F, Sahin B. Factors predicting outcome of balloon dilatation in achalasia. Dig Dis Sci. 2009;54:1237–1242. doi: 10.1007/s10620-008-0493-6. [DOI] [PubMed] [Google Scholar]
- 55.Pandolfino JE, Kwiatek MA, Nealis T, Bulsiewicz W, Post J, Kahrilas PJ. Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology. 2008;135:1526–1533. doi: 10.1053/j.gastro.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghoshal UC, Kumar S, Saraswat VA, Aggarwal R, Misra A, Choudhuri G. Long-term follow-up after pneumatic dilation for achalasia cardia: factors associated with treatment failure and recurrence. Am J Gastroenterol. 2004;99:2304–2310. doi: 10.1111/j.1572-0241.2004.40099.x. [DOI] [PubMed] [Google Scholar]
- 57.Surgery of the Alimentary Tract. SSAT patient care guidelines. Esophageal achalasia. J Gastrointest Surg. 2007;11:1210–1212. doi: 10.1007/s11605-007-0207-y. [DOI] [PubMed] [Google Scholar]
- 58.Zerbib F, Thétiot V, Richy F, Benajah DA, Message L, Lamouliatte H. Repeated pneumatic dilations as long-term maintenance therapy for esophageal achalasia. Am J Gastroenterol. 2006;101:692–697. doi: 10.1111/j.1572-0241.2006.00385.x. [DOI] [PubMed] [Google Scholar]
- 59.Barkin JS, Guelrud M, Reiner DK, Goldberg RI, Phillips RS. Forceful balloon dilation: an outpatient procedure for achalasia. Gastrointest Endosc. 1990;36:123–126. doi: 10.1016/s0016-5107(90)70964-9. [DOI] [PubMed] [Google Scholar]
- 60.Kiev J, Amendola M, Bouhaidar D, Sandhu BS, Zhao X, Maher J. A management algorithm for esophageal perforation. Am J Surg. 2007;194:103–106. doi: 10.1016/j.amjsurg.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 61.Bernard AW, Ben-David K, Pritts T. Delayed presentation of thoracic esophageal perforation after blunt trauma. J Emerg Med. 2008;34:49–53. doi: 10.1016/j.jemermed.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 62.Lin MT, Tai WC, Chiu KW, Chou YP, Tsai MC, Hu TH, Lee CM, Changchien CS, Chuah SK. Delayed presentation of intrathoracic esophageal perforation after pneumatic dilation for achalasia. World J Gastroenterol. 2009;15:4461–4463. doi: 10.3748/wjg.15.4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dunaway PM, Wong RK. Achalasia. Curr Treat Options Gastroenterol. 2001;4:89–100. doi: 10.1007/s11938-001-0051-1. [DOI] [PubMed] [Google Scholar]
- 64.Kilic A, Schuchert MJ, Pennathur A, Gilbert S, Landreneau RJ, Luketich JD. Long-term outcomes of laparoscopic Heller myotomy for achalasia. Surgery. 2009;146:826–831; discussion 831-833. doi: 10.1016/j.surg.2009.06.049. [DOI] [PubMed] [Google Scholar]
- 65.Lopushinsky SR, Urbach DR. Pneumatic dilatation and surgical myotomy for achalasia. JAMA. 2006;296:2227–2233. doi: 10.1001/jama.296.18.2227. [DOI] [PubMed] [Google Scholar]
- 66.Cowgill SM, Villadolid D, Boyle R, Al-Saadi S, Ross S, Rosemurgy AS 2nd. Laparoscopic Heller myotomy for achalasia: results after 10 years. Surg Endosc. 2009:Epub ahead of print. doi: 10.1007/s00464-009-0508-1. [DOI] [PubMed] [Google Scholar]
- 67.Wang L, Li YM, Li L, Yu CH. A systematic review and meta-analysis of the Chinese literature for the treatment of achalasia. World J Gastroenterol. 2008;14:5900–5906. doi: 10.3748/wjg.14.5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang L, Li YM, Li L. Meta-analysis of randomized and controlled treatment trials for achalasia. Dig Dis Sci. 2009;54:2303–2311. doi: 10.1007/s10620-008-0637-8. [DOI] [PubMed] [Google Scholar]
- 69.Csendes A, Braghetto I, Burdiles P, Korn O, Csendes P, Henríquez A. Very late results of esophagomyotomy for patients with achalasia: clinical, endoscopic, histologic, manometric, and acid reflux studies in 67 patients for a mean follow-up of 190 months. Ann Surg. 2006;243:196–203. doi: 10.1097/01.sla.0000197469.12632.e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Richter JE. Update on the management of achalasia: balloons, surgery and drugs. Expert Rev Gastroenterol Hepatol. 2008;2:435–445. doi: 10.1586/17474124.2.3.435. [DOI] [PubMed] [Google Scholar]
- 71.Eckardt AJ, Eckardt VF. Current clinical approach to achalasia. World J Gastroenterol. 2009;15:3969–3975. doi: 10.3748/wjg.15.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Litle VR. Laparoscopic Heller myotomy for achalasia: a review of the controversies. Ann Thorac Surg. 2008;85:S743–S746. doi: 10.1016/j.athoracsur.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 73.Pasricha PJ, Ravich WJ, Hendrix TR, Sostre S, Jones B, Kalloo AN. Treatment of achalasia with intrasphincteric injection of botulinum toxin. A pilot trial. Ann Intern Med. 1994;121:590–591. doi: 10.7326/0003-4819-121-8-199410150-00006. [DOI] [PubMed] [Google Scholar]
- 74.Neubrand M, Scheurlen C, Schepke M, Sauerbruch T. Long-term results and prognostic factors in the treatment of achalasia with botulinum toxin. Endoscopy. 2002;34:519–523. doi: 10.1055/s-2002-33225. [DOI] [PubMed] [Google Scholar]
- 75.D'Onofrio V, Miletto P, Leandro G, Iaquinto G. Long-term follow-up of achalasia patients treated with botulinum toxin. Dig Liver Dis. 2002;34:105–110. doi: 10.1016/s1590-8658(02)80238-9. [DOI] [PubMed] [Google Scholar]
- 76.Ghoshal UC, Chaudhuri S, Pal BB, Dhar K, Ray G, Banerjee PK. Randomized controlled trial of intrasphincteric botulinum toxin A injection versus balloon dilatation in treatment of achalasia cardia. Dis Esophagus. 2001;14:227–231. doi: 10.1046/j.1442-2050.2001.00189.x. [DOI] [PubMed] [Google Scholar]
- 77.Patti MG, Pellegrini CA, Horgan S, Arcerito M, Omelanczuk P, Tamburini A, Diener U, Eubanks TR, Way LW. Minimally invasive surgery for achalasia: an 8-year experience with 168 patients. Ann Surg. 1999;230:587–593; discussion 593-594. doi: 10.1097/00000658-199910000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]


