Abstract
AIM: To evaluate the natural history of subepithelial lesions.
METHODS: We reviewed the medical records of 104 159 patients who underwent upper gastrointestinal endoscopy at the Center for Health Promotion of Samsung Medical Center between 1996 and 2003. Subepithelial lesions were detected in 795 patients (0.76%); 252 patients were followed using upper gastrointestinal endoscopy for 82.5 ± 29.2 mo (range, 12-160 mo; median, 84 mo; 1st quartile, 60 mo; 3rd quartile, 105 mo). The median interval of follow-up endoscopy was 12 mo (range, 6-105 mo; 1st quartile, 12 mo; 3rd quartile, 24 mo).
RESULTS: The mean patient age was 53 years (range, 22-80 years), and the male-to-female ratio was 2.36:1 (177/75). The lesion size at initial measurement averaged 8.9 mm (range, 2-25 mm; median, 8 mm; 1st quartile, 5 mm; 3rd quartile, 10 mm). Of the 252 lesions, 244 (96.8%) were unchanged and 8 (3.2%) were significantly increased in size (from 12.9 ± 6.0 to 21.2 ± 12.2 mm) after a mean interval of 59.1 ± 27.5 mo (range, 12-86 mo). Surgical resection of lesions was performed when the lesions were ≥ 3 cm in diameter. Two lesions were diagnosed as gastrointestinal stromal tumors with an intermediate or high risk of malignancy and one lesion was classified as a schwannoma.
CONCLUSION: Most small subepithelial lesions do not change as shown by endoscopic examination, and regular follow-up with endoscopy may be considered in small, subepithelial lesions, especially lesions < 1 cm in size.
Keywords: Subepithelial tumor, Ultrasonography, Gastrointestinal diseases, Gastrointestinal endoscopy, Time factors
INTRODUCTION
Upper endoscopy is commonly performed for the evaluation of symptoms, and for screening and surveillance of neoplasias. Subepithelial masses or bulges covered with normal-appearing mucosa are frequently encountered during endoscopy. Although a previous study has reported that the incidence of gastric subepithelial lesions is approximately 0.3%[1], Kawanowa et al[2] reported 50 microscopic gastrointestinal stromal tumors (GISTs) in 100 stomachs entirely resected from patients with gastric cancer. With more widespread use of endoscopy for screening, it is likely that subepithelial lesions will be detected more frequently. The optimal management of incidentally-detected, subepithelial lesions has not been determined.
When clinicians are faced with a subepithelial lesion, they must decide to remove or follow up the lesion. Endosonographic or endoscopic surveillance is common in patients with asymptomatic, subepithelial lesions without signs of malignancy, such as large size, rapid growth, or ulceration, although such an approach has not been formally validated[3]. Because little is known about the natural course of subepithelial lesions, the appropriate strategy for management is still controversial. This imposes a tremendous emotional burden on patients who can become preoccupied with the possibility that the tumor is malignant.
The aim of this study was to determine the natural history and provide a basis of surveillance of incidentally-detected, asymptomatic subepithelial lesions.
MATERIALS AND METHODS
We used computerized medical records and a database of disease codes to study 104 159 patients who underwent upper gastrointestinal endoscopy at the Center for Health Promotion of Samsung Medical Center in Seoul, Korea between March 1996 and March 2003. A computer search over a 7-year period revealed 795 patients (0.76%) with the diagnostic code for submucosal tumors of the esophagus, stomach, or duodenum. Within this group, 252 patients had been followed with upper gastrointestinal endoscopy. Thirty-seven of 61 patients who had lesions > 1 cm in size were further evaluated with endoscopic ultrasonography (EUS). Seven of 8 lesions with a significant increase in size were evaluated using EUS. The duration of follow-up was determined by the last known visit for endoscopic examination at our hospital. All examinations were performed by experienced endoscopists (> 1000 endoscopic examinations each). Endoscopists approximated the size of lesions by using an open biopsy forceps for comparison (6 mm).
Statistical analysis
Statistical evaluation was performed with SPSS (Statistical Program for the Social Sciences; SPSS, Inc., Chicago, IL, USA). Descriptive statistics were used when appropriate.
RESULTS
The mean age of the 252 patients with subepithelial lesions was 53 years (range, 22-80 years), and the male-to-female ratio was 2.36:1 (177/75). The mean lesion size was 8.9 mm (range, 2-25 mm; median, 8 mm; 1st quartile, 5 mm; 3rd quartile, 10 mm). The stomach [130 patients (51.6%)] was the most common site for a subepithelial lesion, followed by the esophagus [104 patients (41.3%)], and duodenum [18 patients (7.1%)].
Biopsies were obtained from 191 of the 252 patients at the time of the initial endoscopy. Endoscopy and biopsy were sufficient for diagnosis in 3 patients. Of these 3 patients, 1 had an esophageal leiomyoma, and 2 had Brunner gland hyperplasia of the duodenum.
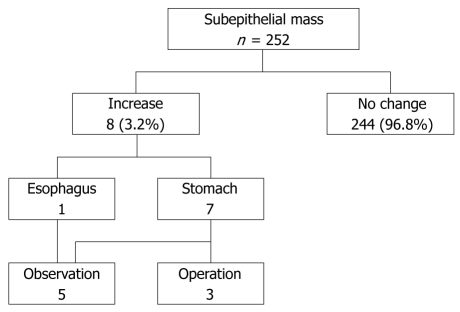
Of 252 lesions, 244 (96.8%) were unchanged and 8 (3.2%) significantly increased in size (from 12.9 ± 6.0 to 21.2 ± 12.2 mm) during a mean interval of 59.1 ± 27.5 mo (range, 12-86 mo, Figure 1, Table 1). In these 8 lesions, there was an increase of over 25% and more than 5 mm in diameter at surveillance. Six of the 8 lesions arose from the 4th layer, corresponding to the muscularis propria, and appeared hypoechoic; they were considered to be GISTs. One lesion arose from the 3rd layer and appeared hyperechoic; it was considered to be a lipoma. The other lesion was further evaluated by stomach computed tomography (CT) probably because of the patient’s rejection of an EUS examination; however, the lesion was not observed on CT. Surgical resection was performed in 3 lesions ≥ 3 cm in size, which were diagnosed as GISTs with an intermediate and a high risk of malignancy, and a schwannoma (Figures 2, 3, 4). The 5 patients who did not undergo surgery were followed by means of upper gastrointestinal endoscopy or EUS. No further change was observed in size, shape and EUS finding such as echo pattern or regularity of the outer margin over a period of 1-5 years.
Figure 1.
Clinical course of subepithelial masses.
Table 1.
Characteristics of 8 patients with increased subepithelial masses
| Patients | Age (yr) | Gender | Location | Initial size (mm) | Increased size(mm) | Follow-up interval (mo) | EUS | Treatment | Surgical gross pathology |
| 1 | 60 | F | Stomach | 22 | 30 | 40 | GIST | Operation | Schwannoma |
| 2 | 63 | M | Stomach | 20 | 30 | 86 | GIST | Observation | |
| 3 | 65 | M | Stomach | 15 | 40 | 12 | GIST | Operation | GIST |
| 4 | 37 | F | Stomach | 12 | 18 | 36 | GIST | Observation | |
| 5 | 53 | F | Stomach | 12 | 20 | 56 | Lipoma | Observation | |
| 6 | 44 | F | Stomach | 12 | 30 | 84 | GIST | Operation | GIST |
| 7 | 54 | M | Stomach | 4 | 10 | 81 | Not performed | Observation | |
| 8 | 37 | F | Esophagus | 8 | 15 | 78 | GIST | Observation |
EUS: Endoscopic ultrasound; GIST: Gastrointestinal stromal tumor.
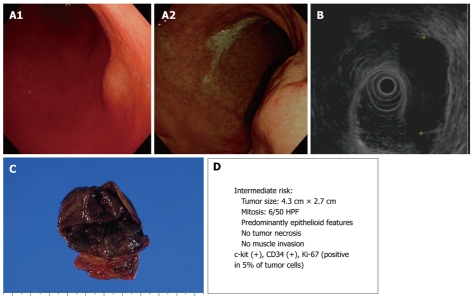
Figure 2.
Endoscopic, endoscopic ultrasonography (EUS), and gross findings of gastrointestinal stromal tumors (GISTs). A: Endoscopic view of a round subepithelial mass with a significant interval change; B: EUS shows an ovoid, homogeneous, hypoechoic mass in the fourth gastric wall layer; C: Gross findings of wedge resection reveal a soft, well-defined mass measuring 4.3 cm × 2.7 cm; D: Malignant potential.
Figure 3.
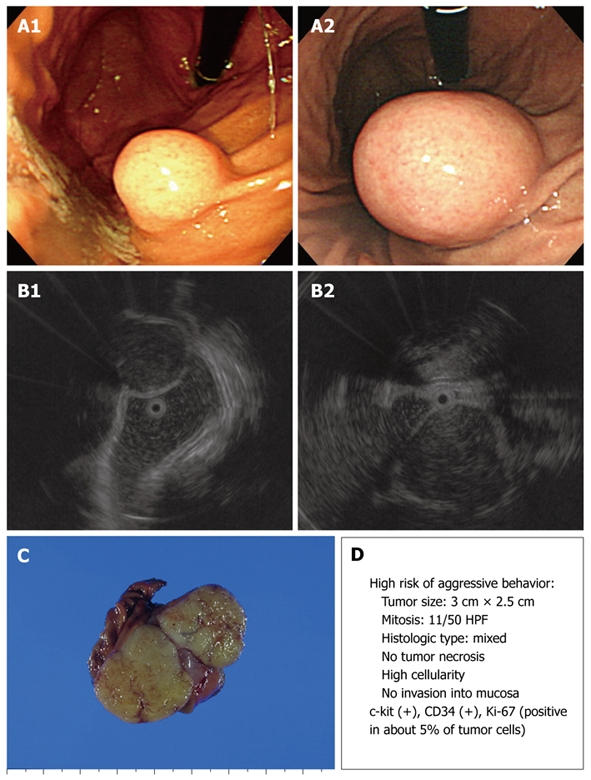
Endoscopic, EUS, and gross findings of GISTs. A: Endoscopic view of a round subepithelial mass with a significant interval change; B: EUS shows an ovoid, homogeneous, hypoechoic mass in the fourth gastric wall layer; C: Gross findings of wedge resection reveal a soft, well-defined mass measuring 3.0 cm × 2.5 cm; D: Malignant potential.
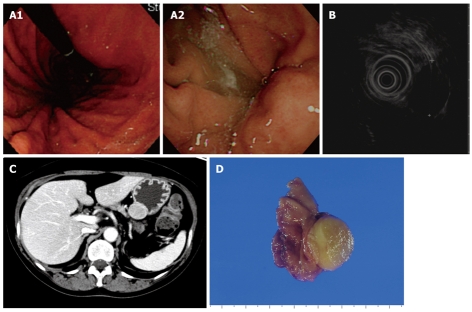
Figure 4.
Endoscopic, EUS, abdominal computed tomography (CT), and gross findings of a schwannoma. A: Endoscopic view of an ovoid subepithelial mass with a significant interval change; B: EUS shows a round, homogeneous, hypoechoic mass in the third gastric wall layer; C: CT scan shows a 2.8 cm × 2.2 cm homogeneous, well-defined, soft tissue mass on the upper body of the stomach; D: Gross findings of wedge resection reveal a 3 cm × 2.5 cm well-demarcated, round, firm, yellow mass.
DISCUSSION
We demonstrated that during a mean period of 82.5 ± 29.2 mo (range, 12-160 mo; median, 84 mo; 1st quartile, 60 mo; 3rd quartile, 105 mo), there was no significant change in the size of small (< 30 mm) subepithelial lesions detected incidentally during upper gastrointestinal endoscopy in 244 of 252 patients (96.8%). Eight lesions (3.2%) were significantly increased in size (from 12.9 ± 6.0 to 21.2 ± 12.2 mm) during a mean interval of 59.1 ± 27.5 mo (range, 12-86 mo), four patients had subepithelial lesions ≥ 3 cm in size on follow-up endoscopic examination. Three patients of them underwent tumor resection and were diagnosed with intermediate or high risk GISTs and a schwannoma.
Our findings are consistent with prior studies of subepithelial lesions. Several prior studies have suggested that since most small subepithelial lesions do not exhibit changes that would raise the suspicion of malignant potential, a conservative policy (endoscopic follow-up without pathologic diagnosis) of surveillance is safe. Tio et al[4] showed that the size and echo pattern of 21 small (< 3 cm) subepithelial lesions did not change over a period of 1-3 years. Melzer et al[5] also showed no changes in size or echo pattern in the small subepithelial lesions (< 4 cm) of 24 of 25 patients over a mean period of 19 mo. However, one gastric lesion enlarged from 30 to 38 mm and changed from a hypoechoic to a non-homogeneous pattern. The patient underwent resection of a stromal tumor with high malignant potential. Lee et al[6] followed patients with 16 esophageal tumors, 9 gastric tumors, and one benign duodenal mesenchymal tumor (< 3 cm) for a mean period of 47.4 mo, and noted no change in 25 of 26 patients during EUS surveys. However, one gastric lesion enlarged from 26 to 34 mm without a change in the echo pattern or regularity of the outer margin. The patient underwent resection of a leiomyoma. Imaoka et al[7] followed 132 gastric subepithelial lesions for 5 years and found that only 2 lesions increased in size. These tumors were diagnosed as GISTs after surgical resection; one patient had liver metastasis. Lachter et al[8] found that the majority of small (< 17 mm) subepithelial tumors did not change in echogenicity or size during a median period of 5 years. The previous studies have been limited by small sample size and relatively short follow-up.
According to a stepwise approach to subepithelial tumors, EUS is recommended for subepithelial tumors > 1 cm in diameter, and histologic evaluation, such as EUS-guided fine needle aspiration biopsy (EUS-FNAB), is recommended for hypoechoic subepithelial tumors < 3 cm in diameter. Surgery is recommended for subepithelial tumors > 3 cm in diameter[9]. Although these procedures are helpful in categorizing a lesion, they cannot absolutely determine the type of lesion or determine if a lesion is benign or malignant[10,11]. Clinicians should consider if an invasive method, such as EUS-FNAB, is necessary or available. Furthermore, they should consider individual risk and patient preference.
The optimal management of subepithelial lesions remains controversial because the natural history of subepithelial lesions, such as GISTs, remains incompletely defined. GISTs are the most commonly identified intramural subepithelial tumors in the upper gastrointestinal tract[11]. Small GISTs (< 2 cm) have very low malignant potential according to the classification system proposed by the National Institutes of Health Consensus Conference[12]. The American Gastroenterological Association recommends periodic endoscopic or endosonographic follow-up or surgical resection for small, hypoechoic, 3rd- and 4th-layer (< 3 cm) masses, which are most likely GISTs[13]. Nishida et al[14] recommended that subepithelial tumors < 2 cm in size and without ulceration or surface depression can be followed with endoscopic examination once or twice per year.
Opinions concerning the duration of follow-up also vary. Brand et al[15] recommended follow-up for 6 mo after the initial diagnosis for subepithelial lesions with no EUS signs of malignancy. If there is no change during the initial follow-up period, annual follow-up is recommended. Hwang et al[16] suggested a 1-year follow-up interval and suggested that the interval between surveillance examinations be extended if the lesion remains unchanged for 2 consecutive follow-up examinations with EUS. Guidelines in Japan recommend endoscopic examination once or twice per year for subepithelial lesions < 2 cm in size[14].
Our finding must be interpreted in the context of the strength and weakness of this study. The high number of patients and a long follow-up study is its strength. However, there are several limitations in this study. First, there is a lack of accuracy in estimation of size. The open-biopsy forceps technique can underestimate or overestimate the size of submucosal lesion and shows inter-observer variation, but it is convenient to use in clinical practice. In our study, 2 submucosal lesions were estimated as 2 mm in size and we could not exclude the possibility of an under-estimation of the size. Second, we could not analyze all the patients with the diagnostic code for submucosal tumors, because only a proportion of the patients were followed up and analysis was performed only for them.
In conclusion, although the management strategy for small subepithelial lesions is still controversial, regular follow-up with endoscopy or EUS may be considered in small, asymptomatic, subepithelial lesions. Endoscopic surveillance can be an appropriate strategy for lesions < 1 cm. Further prospective, multicenter studies with long-term follow-up would help to validate these surveillance programs.
COMMENTS
Background
The natural history of subepithelial lesions has not been clearly elucidated, and the appropriate management strategy for small subepithelial tumors is still controversial.
Research frontiers
With more widespread use of endoscopy for screening, asymptomatic subepithelial lesions would be detected more frequently. However, the appropriate strategy for management is still controversial. In this study, the authors have determined the natural history and provided a basis for surveillance of incidentally-detected, asymptomatic subepithelial lesions.
Innovations and breakthroughs
Although several studies pertaining to the natural history of subepithelial lesions, including gastrointestinal tumors, have been published, they have been limited by small sample size and relatively short follow-up. In Korea, many subjects have regular endoscopic examinations because of the high incidence of gastric cancer.
Applications
The authors have provided the basis for surveillance of incidentally-detected, small subepithelial lesions by means of this study.
Peer review
Lim et al performed a retrospective 3-year follow p study of subepithelial gastrointestinal lesions in 252 patients. This is a high number of patients and a long follow-up, which increases the value of the work.
Footnotes
Peer reviewers: Dr. Mitsuhiro Fujishiro, Department of Gastroenterology, Faculty of Medicine, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, Japan; Dr. Justin MM Cates, MD, PhD, Department of Pathology, Vanderbilt University Medical Center, Medical Center North, C-3322, 1161 21st Avenue South, Nashville, TN 37232, United States
S- Editor Tian L L- Editor Cant MR E- Editor Zheng XM
References
- 1.Hedenbro JL, Ekelund M, Wetterberg P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg Endosc. 1991;5:20–23. doi: 10.1007/BF00591381. [DOI] [PubMed] [Google Scholar]
- 2.Kawanowa K, Sakuma Y, Sakurai S, Hishima T, Iwasaki Y, Saito K, Hosoya Y, Nakajima T, Funata N. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol. 2006;37:1527–1535. doi: 10.1016/j.humpath.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Polkowski M, Butruk E. Submucosal lesions. Gastrointest Endosc Clin N Am. 2005;15:33–54, viii. doi: 10.1016/j.giec.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Tio TL, Tytgat GN, den Hartog Jager FC. Endoscopic ultrasonography for the evaluation of smooth muscle tumors in the upper gastrointestinal tract: an experience with 42 cases. Gastrointest Endosc. 1990;36:342–350. doi: 10.1016/s0016-5107(90)71061-9. [DOI] [PubMed] [Google Scholar]
- 5.Melzer E, Fidder H. The natural course of upper gastrointestinal submucosal tumors: an endoscopic ultrasound survey. Isr Med Assoc J. 2000;2:430–432. [PubMed] [Google Scholar]
- 6.Lee SJ, Kim JO, Eun SH, Choi IS, Jung IS, Ko BM, Hong SJ, Ryu CB, Cho JY, Lee JS, et al. The endoscopic ultrasonographic survey of benign mesenchymal tumor in upper gastrointestinal tract. Korean J Gastrointest Endosc. 2007;35:140–145. [Google Scholar]
- 7.Imaoka H, Sawaki A, Mizuno N, Takahashi K, Nakamura T, Tajika M, Kawai H, Isaka T, Okamoto Y, Inoue H, et al. Incidence and clinical course of submucosal lesions of the stomach. Gastrointesc Endosc. 2005;61:AB16. [Google Scholar]
- 8.Lachter J, Bishara N, Rahimi E, Shiller M, Cohen H, Reshef R. EUS clarifies the natural history and ideal management of GISTs. Hepatogastroenterology. 2008;55:1653–1656. [PubMed] [Google Scholar]
- 9.Eckardt AJ, Wassef W. Diagnosis of subepithelial tumors in the GI tract. Endoscopy, EUS, and histology: bronze, silver, and gold standard? Gastrointest Endosc. 2005;62:209–212. doi: 10.1016/j.gie.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Hwang JH, Saunders MD, Rulyak SJ, Shaw S, Nietsch H, Kimmey MB. A prospective study comparing endoscopy and EUS in the evaluation of GI subepithelial masses. Gastrointest Endosc. 2005;62:202–208. doi: 10.1016/s0016-5107(05)01567-1. [DOI] [PubMed] [Google Scholar]
- 11.Chak A. EUS in submucosal tumors. Gastrointest Endosc. 2002;56:S43–S48. doi: 10.1016/s0016-5107(02)70085-0. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 13.Hwang JH, Rulyak SD, Kimmey MB. American Gastroenterological Association Institute technical review on the management of gastric subepithelial masses. Gastroenterology. 2006;130:2217–2228. doi: 10.1053/j.gastro.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 14.Nishida T, Hirota S, Yanagisawa A, Sugino Y, Minami M, Yamamura Y, Otani Y, Shimada Y, Takahashi F, Kubota T. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol. 2008;13:416–430. doi: 10.1007/s10147-008-0798-7. [DOI] [PubMed] [Google Scholar]
- 15.Brand B, Oesterhelweg L, Binmoeller KF, Sriram PV, Bohnacker S, Seewald S, De Weerth A, Soehendra N. Impact of endoscopic ultrasound for evaluation of submucosal lesions in gastrointestinal tract. Dig Liver Dis. 2002;34:290–297. doi: 10.1016/s1590-8658(02)80150-5. [DOI] [PubMed] [Google Scholar]
- 16.Hwang JH, Kimmey MB. The incidental upper gastrointestinal subepithelial mass. Gastroenterology. 2004;126:301–307. doi: 10.1053/j.gastro.2003.11.040. [DOI] [PubMed] [Google Scholar]