Abstract
The recent expansion of the seventh cholera pandemic into South America emphasizes the need for a safe, long-lasting, protective, and nonreactogenic vaccine for this disease. Since the predominant Vibrio cholerae O1 strains in the world today are of the El Tor biotype, a bivalent vaccine containing both classical and El Tor biotypes may be desirable. We have constructed a new oral vaccine candidate, V. cholerae CVD110 El Tor, Ogawa, from which all toxin genes so far identified in V. cholerae have been deleted. Three of these genes, those encoding cholera toxin (ctx), zonula occludens toxin (zot), and accessory cholera enterotoxin (ace), are located on a 4.5-kb virulence cassette flanked by repetitive sequences (RS1 elements). Homologous recombination between these RS1 elements resulted in the deletion of this virulence cassette to yield V. cholerae CVD109. Insertion of genes encoding mercury resistance (mer) and the cholera toxin B subunit (ctxB) into the hemolysin locus (hlyA) produced CVD110. This insertion serves three purpose. (i) It genetically tags the vaccine strain so as to distinguish it from wild-type V. cholerae O1. (ii) It produces cholera toxin B subunit in order to elicit antitoxic immunity. (iii) It inactivates the hemolysin gene, rendering the strain nonhemolytic on sheep erythrocyte plates. Supernatants from V. cholerae CVD110 cultures are nonreactogenic when assayed in Ussing chambers.
Full text
PDF

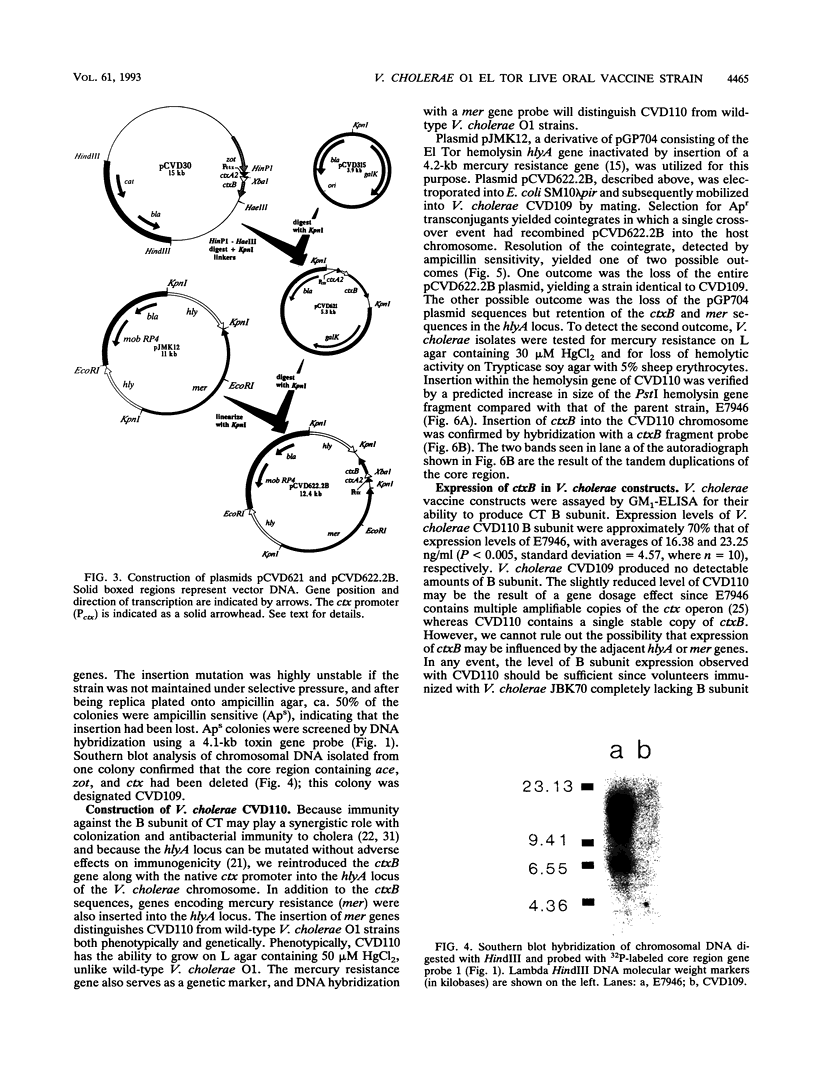
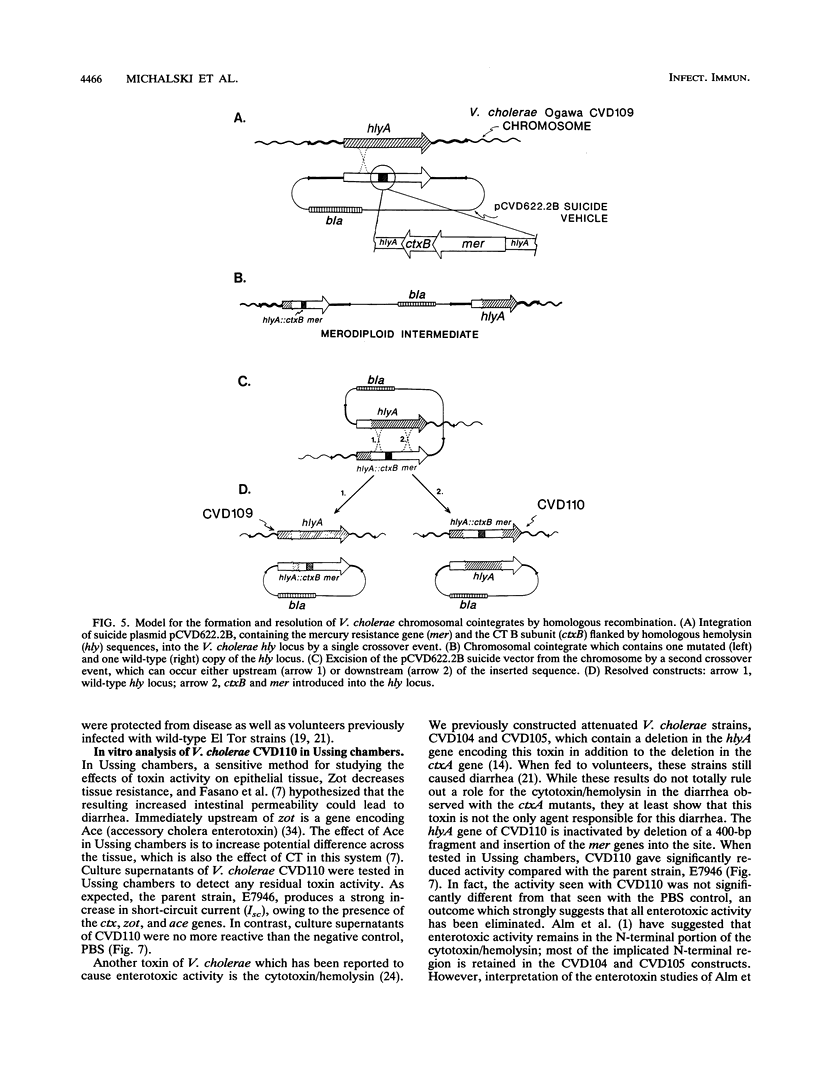


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alm R. A., Mayrhofer G., Kotlarski I., Manning P. A. Amino-terminal domain of the El Tor haemolysin of Vibrio cholerae O1 is expressed in classical strains and is cytotoxic. Vaccine. 1991 Aug;9(8):588–594. doi: 10.1016/0264-410x(91)90247-4. [DOI] [PubMed] [Google Scholar]
- Barrineau P., Gilbert P., Jackson W. J., Jones C. S., Summers A. O., Wisdom S. The DNA sequence of the mercury resistance operon of the IncFII plasmid NR1. J Mol Appl Genet. 1984;2(6):601–619. [PubMed] [Google Scholar]
- Baudry B., Fasano A., Ketley J., Kaper J. B. Cloning of a gene (zot) encoding a new toxin produced by Vibrio cholerae. Infect Immun. 1992 Feb;60(2):428–434. doi: 10.1128/iai.60.2.428-434.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash R. A., Music S. I., Libonati J. P., Craig J. P., Pierce N. F., Hornick R. B. Response of man to infection with Vibrio cholerae. II. Protection from illness afforded by previous disease and vaccine. J Infect Dis. 1974 Oct;130(4):325–333. doi: 10.1093/infdis/130.4.325. [DOI] [PubMed] [Google Scholar]
- Clemens J. D., van Loon F., Sack D. A., Rao M. R., Ahmed F., ChakrabortY J., Kay B. A., Khan M. R., Yunus M. D., Harris J. R. Biotype as determinant of natural immunising effect of cholera. Lancet. 1991 Apr 13;337(8746):883–884. doi: 10.1016/0140-6736(91)90207-6. [DOI] [PubMed] [Google Scholar]
- Fasano A., Baudry B., Pumplin D. W., Wasserman S. S., Tall B. D., Ketley J. M., Kaper J. B. Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5242–5246. doi: 10.1073/pnas.88.12.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feachem R. G. Environmental aspects of cholera epidemiology. I. A review of selected reports of endemic and epidemic situations during 1961-1980. Trop Dis Bull. 1981 Aug;78(8):675–698. [PubMed] [Google Scholar]
- Glass R. I., Libel M., Brandling-Bennett A. D. Epidemic cholera in the Americas. Science. 1992 Jun 12;256(5063):1524–1525. doi: 10.1126/science.1598586. [DOI] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Iwanaga M., Yamamoto K., Higa N., Ichinose Y., Nakasone N., Tanabe M. Culture conditions for stimulating cholera toxin production by Vibrio cholerae O1 El Tor. Microbiol Immunol. 1986;30(11):1075–1083. doi: 10.1111/j.1348-0421.1986.tb03037.x. [DOI] [PubMed] [Google Scholar]
- Ketley J. M., Michalski J., Galen J., Levine M. M., Kaper J. B. Construction of genetically marked Vibrio cholerae O1 vaccine strains. FEMS Microbiol Lett. 1993 Jul 15;111(1):15–21. doi: 10.1111/j.1574-6968.1993.tb06355.x. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Black R. E., Clements M. L., Cisneros L., Nalin D. R., Young C. R. Duration of infection-derived immunity to cholera. J Infect Dis. 1981 Jun;143(6):818–820. doi: 10.1093/infdis/143.6.818. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Black R. E., Clements M. L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983 Dec;47(4):510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Herrington D., Ketley J., Losonsky G., Tacket C. O., Tall B., Cryz S. Safety, immunogenicity, and efficacy of recombinant live oral cholera vaccines, CVD 103 and CVD 103-HgR. Lancet. 1988 Aug 27;2(8609):467–470. doi: 10.1016/s0140-6736(88)90120-1. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Herrington D., Losonsky G., Morris J. G., Clements M. L., Black R. E., Tall B., Hall R. Volunteer studies of deletion mutants of Vibrio cholerae O1 prepared by recombinant techniques. Infect Immun. 1988 Jan;56(1):161–167. doi: 10.1128/iai.56.1.161-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus H., Ketley J. M., Kaper J. B., Holmes R. K. Effects of DNase production, plasmid size, and restriction barriers on transformation of Vibrio cholerae by electroporation and osmotic shock. FEMS Microbiol Lett. 1990 Mar 1;56(1-2):149–154. doi: 10.1111/j.1574-6968.1990.tb04139.x. [DOI] [PubMed] [Google Scholar]
- McCardell B. A., Madden J. M., Shah D. B. Isolation and characterization of a cytolysin produced by Vibrio cholerae serogroup non-O1. Can J Microbiol. 1985 Aug;31(8):711–720. doi: 10.1139/m85-135. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J. Duplication and amplification of toxin genes in Vibrio cholerae. Cell. 1983 Nov;35(1):253–263. doi: 10.1016/0092-8674(83)90228-3. [DOI] [PubMed] [Google Scholar]
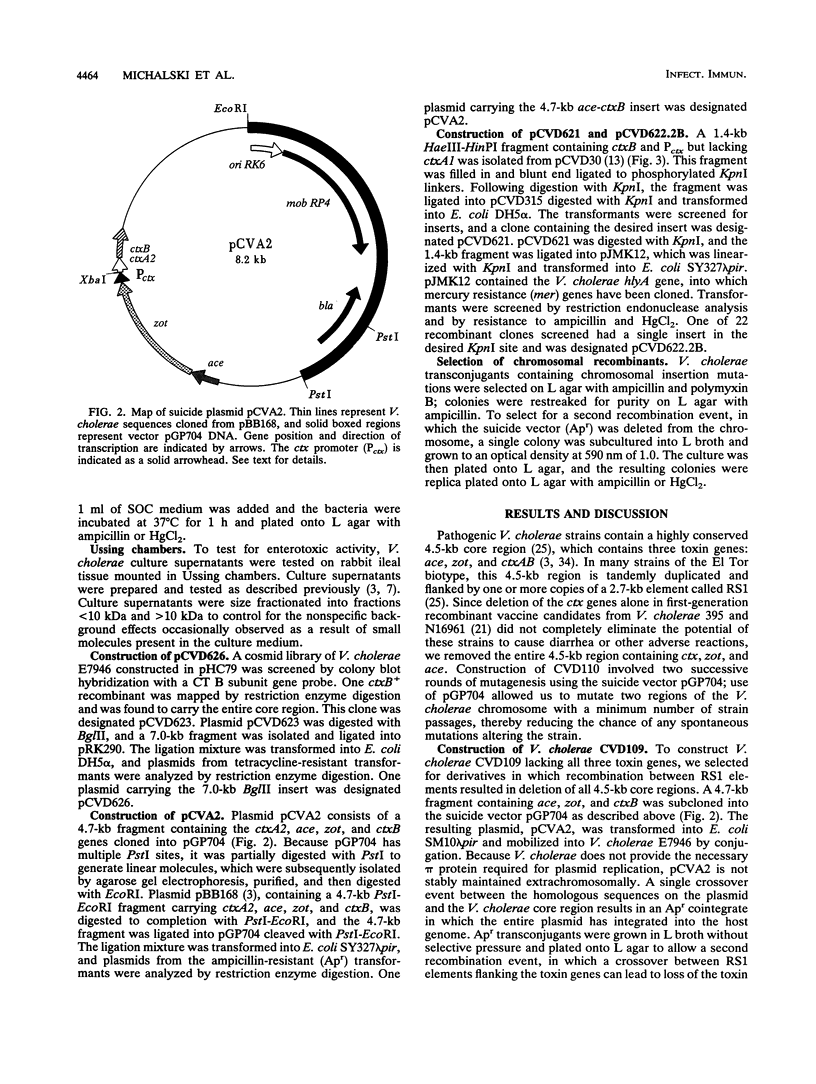
- Miller V. L., Mekalanos J. J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988 Jun;170(6):2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristaino P. A., Levine M. M., Young C. R. Improved GM1-enzyme-linked immunosorbent assay for detection of Escherichia coli heat-labile enterotoxin. J Clin Microbiol. 1983 Oct;18(4):808–815. doi: 10.1128/jcm.18.4.808-815.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. A general method for site-directed mutagenesis in prokaryotes. Nature. 1981 Jan 1;289(5793):85–88. doi: 10.1038/289085a0. [DOI] [PubMed] [Google Scholar]
- Svennerholm A. M., Holmgren J. Synergistic protective effect in rabbits of immunization with Vibrio cholerae lipopolysaccharide and toxin/toxoid. Infect Immun. 1976 Mar;13(3):735–740. doi: 10.1128/iai.13.3.735-740.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacket C. O., Losonsky G., Nataro J. P., Cryz S. J., Edelman R., Kaper J. B., Levine M. M. Onset and duration of protective immunity in challenged volunteers after vaccination with live oral cholera vaccine CVD 103-HgR. J Infect Dis. 1992 Oct;166(4):837–841. doi: 10.1093/infdis/166.4.837. [DOI] [PubMed] [Google Scholar]
- Tauxe R. V., Blake P. A. Epidemic cholera in Latin America. JAMA. 1992 Mar 11;267(10):1388–1390. [PubMed] [Google Scholar]
- Trucksis M., Galen J. E., Michalski J., Fasano A., Kaper J. B. Accessory cholera enterotoxin (Ace), the third toxin of a Vibrio cholerae virulence cassette. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5267–5271. doi: 10.1073/pnas.90.11.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]