Abstract
AIM: To evaluate the efficacy of 6 noninvasive liver fibrosis models and to identify the most valuable model for the prediction of liver fibrosis stage in chronic hepatitis B (CHB) patients.
METHODS: Seventy-eight CHB patients were consecutively enrolled in this study. Liver biopsy was performed and blood serum was obtained at admission. Histological diagnosis was made according to the METAVIR system. Significant fibrosis was defined as stage score ≥ 2, severe fibrosis as stage score ≥ 3. The diagnostic accuracy of 6 noninvasive liver fibrosis models, including serum aspartate aminotransferase (AST) to platelet ratio index (APRI), FIB-4, Forn’s index, Fibrometer, Hepascore, and Shanghai Liver Fibrosis Group’s index (SLFG), was investigated.
RESULTS: The APRI, FIB-4 and Forn’s index under receiver operating characteristic curve (AUROC) for significant fibrosis were 0.71, 0.75 and 0.79, respectively, with a diagnosis accuracy of 67%, 77% and 80%, respectively, and 0.80, 0.87 and 0.86, respectively, under the AUROC for severe fibrosis. The Hepascore, SLFG, and Fibrometer were 0.80, 0.83 and 0.85, respectively under the AUROC for significant fibrosis (P < 0.01). The diagnosis accuracy of Hepascore and SLFG was 86% and 88%, respectively. The Hepascore, SLFG, and Fibrometer were 0.95, 0.93, and 0.94, respectively, under the AUROC for severe fibrosis (P < 0.01).
CONCLUSION: The models containing direct serum markers have a better diagnostic value than those not containing direct serum markers.
Keywords: Chronic hepatitis B, Liver fibrosis, Serum marker, Noninvasive model, Receiver operating curve
INTRODUCTION
Chronic hepatitis B virus (HBV) infection affects 350 million individuals worldwide. At least one million people chronically infected with HBV would die of chronic liver diseases each year[1]. Thus, it is important to prevent the progression of early liver fibrosis to cirrhosis[2]. Although liver biopsy is the gold standard for the assessment of fibrosis, it has several disadvantages, such as poor patient compliance, sampling error, limited usefulness for dynamic surveillance, and poor intra- and inter-observation concordance[3-5]. Considering these limitations, noninvasive histology predictors are urgently needed[6].
Since single fibrosis surrogate cannot measure fibrosis, an alternative approach combined with a number of parameters can generate algorithms capable of evaluating fibrosis. A number of noninvasive models containing serum markers, such as serum aspartate aminotransferase (AST) to platelet ratio index (APRI), FIB-4, Forn’s index, Fibrometer, Hepascore, Shanghai Liver Fibrosis Group’s index (SLFG) have been studied worldwide[7-12]. Additionally, except for SLFG, little has been known about the role of these models in predicting fibrosis stage of chronic hepatitis B (CHB) because most studies were performed in chronic hepatitis C (CHC). China has a high prevalence of CHB, and most hepatocellular carcinomas result from chronic HBV infection. Therefore, we carried out this study to identify the best practical noninvasive model of liver fibrosis in CHB.
MATERIALS AND METHODS
Patients
Seventy-eight consecutive eligible CHB patients who underwent a liver biopsy in March 2006-August 2008 at Zhongshan Hospital, Fudan University, Shanghai, China, were included in this study. Blood serum was collected and stored at -80°C for further test. Chronic HBV infection was diagnosed based on positive surface antigen of HBV (HBsAg) and fluctuated alanine aminotransferase. Exclusion criteria included chronic liver disease due to other causes or co-infection with hepatitis D, clinically overt cirrhosis, previous or concomitant anti-HBV therapy, alcohol consumption exceeding 20 g/d in men and exceeding 10 g/d in women. Data were retrospectively analyzed. The study protocol was approved by the Institutional Review Board in our hospital. Written informed consent was obtained from each patient.
Liver histology and quantification of fibrosis
Liver tissue was obtained by sono-guided percutaneous biopsy (Bard®, Magnum®, 18G, USA) and stained with hematoxylin-eosin-safran and Masson’s trichrome. Fibrosis staging (F) and inflammatory activity (A) were decided according to the METAVIR system. Fibrosis staging was divided into F0-F4 (F0 = no fibrosis, F1 = portal fibrosis without septa, F2 = periportal fibrosis with few septa, F3 = septal fibrosis with many septa, and F4 = cirrhosis). Inflammatory activity was divided into A0-A3 (A0 = no histologic necroinflammatory activity, A1 = minimal activity, A2 = moderate activity, A3 = severe activity). The activity was assessed by integrating the severity and intensity of piecemeal (periportal) and lobular necrosis[13]. According to the American Association for the Study of Liver Disease Practice Guidelines, we defined significant fibrosis as METAVIR fibrosis with a score ≥ 2 (F2, 3, 4) and severe liver fibrosis as METAVIR fibrosis with a score ≥ 3 (F3, 4)[14].
Serum parameters
Following parameters, including AST, alanine aminotransferase (ALT), γ-glutamyl transpeptidase (GGT), bilirubin, total cholesterol, urea, prothrombin time (PT), prothrombin index (PI), hemoglobin and platelet count (PLT) were assayed. AST, ALT, GGT, bilirubin, total cholesterol, urea were tested with Hitachi 7600, Japan. PT and PI were tested with Sysmex CA7000, Japan. Hemoglobin and PLT was tested with Sysmex routine blood test pipeline, Japan. The reference value was 0-75 IU/L for ALT and AST. Serum α2-macroglobulin (A2M) (GenWay Biotech, San Diego, USA) and hyaluronic acid (HA) (Shanghai High Medical Biotech, Shanghai, China) concentrations were measured by enzyme linked immunosorbent assay.
APRI, FIB-4, Forn’s index, SLFG, Hepascore, and Fibrometer were detected according to the following formulas: APRI = [AST (/ULN)/PLT (109/L)] × 100, FIB-4 = [age (yr) × AST (U/L)]/{[PLT (109/L)] × (ALT (U/L)]1/2}, Forn’s index = 7.811 - 3.131 × ln [PLT (109/L)] + 0.781 × ln [GGT (U/L)] + 3.467 × ln [age (yr)] - 0.014 × [cholesterol (g/L)], SLFG = 10 × eY/(1+eY), Y = - 13.995 + 3.220 × lg [A2M (g/L)]+ 3.096 × lg [age (yr)] + 2.254 × lg [GGT (U/L)] + 2.437 × lg [HA (ng/mL)], Hepascore = eY/(1+eY), Y = - 4.185818 - [0.0249 × age (yr)] + [0.7464 × sex (male = 1, female = 0)] + [1.0039 × A2M (g/L)] + [0.0302 × HA (ng/mL)] + [0.0691 × TB (μmol/L)] - [0.0012 × GGT (U/L)]; Fibrometer = - 0.007 × PLT (109/L) - 0.049 × PI (%) + 0.012 × AST (U/L) + 0.005 × A2M (g/L) + 0.021 × HA (ng/mL) - 0.270 × urea (mmol/L) + 0.027 × Age (yr) + 3.718.
Statistical analysis
Statistical analysis was performed using Spearman’s two-tail test and univariate analysis. P < 0.05 was considered statistically significant. Sensitivity, specificity, positive and negative predictive values (NPV and PPV) were calculated by using cutoffs according to the original studies. The overall diagnostic performance of scores was evaluated by area under ROC curves (AUROCs). We used DANA method, which was developed by Poynard et al[15,16], to adjust the observed AUROCs in our study. All the AUROCs were adjusted to a standard DANA of 2.5 using the formula: Adjusted AUROC (AdAUC) = Observed AUROC + 0.1056 × (2.5 - Observed DANA). The AUROCs were compared with the method of Hanley-McNeil[17].
RESULTS
Patient characteristics
The mean age of the 78 patients (66 males, 12 females) was 32.6 ± 12.3 years. The mean length of liver biopsies was 18.2 ± 3.4 mm, and the liver specimen length was longer than 15 mm. Significant fibrosis was found in 32 patients (41.0%), severe fibrosis in 19 patients (24.4%), and early cirrhosis in 9 patients (11.5%), respectively. Main features of the patients are summarized in Table 1.
Table 1.
Main characteristics of patients studied
| Patients (n = 78) | F0F1 (n = 46) | F2F3F4 (n = 32) | P value (F0F1 vs F2F3F4) | |
| Age (mean ± SD, yr) | 32.6 ± 12.3 | 29.6 ± 12.0 | 36.9 ± 11.4 | 0.009 |
| Men (n, %) | 66 (84.6) | 38 (82.6) | 28 (87.5) | 0.113 |
| CHB family history (n, %) | 29 (37.2) | 18 (39.1) | 11 (34.4) | 0.104 |
| WBC (mean ± SD, 109/L) | 5.3 ± 1.4 | 5.5 ± 1.2 | 4.9 ± 1.6 | 0.060 |
| Hb (mean ± SD, g/L) | 142.6 ± 15.7 | 145.6 ± 13.4 | 138.3 ± 17.8 | 0.044 |
| PLT (mean ± SD, 109/L) | 170.2 ± 51.5 | 185.9 ± 40.7 | 147.6 ± 57.3 | 0.002 |
| TB [median (interquartile range), μmol/L] | 15.4 (11.5-20.6) | 14.7 (10.6-19.1) | 16.7 (12.1-24.1) | 0.087 |
| CB [median (interquartile range), μmol/L] | 5.7 (4.0-7.8) | 5.5 (3.9-7.1) | 6.4 (4.5-10) | 0.057 |
| ALT [interquartile median (range), U/L] | 115 (55-241) | 93.5 (32-240) | 132 (76-263) | 0.165 |
| AST [interquartile median (range), U/L] | 67.5 (38-121) | 56 (30-95) | 86.5 (41-152) | 0.042 |
| GGT [interquartile median (range), U/L] | 52.5 (27-76) | 36.5 (21-59) | 66.5 (46-94) | 0.006 |
| Alb (mean ± SD, g/L) | 42.4 ± 5.1 | 44.0 ± 4.8 | 40.2 ± 4.6 | 0.001 |
| PT (mean ± SD, s) | 12.0 ± 1.1 | 11.5 ± 0.9 | 12.6 ± 0.9 | < 0.001 |
| PI (mean ± SD, s) | 1.00 ± 0.08 | 1.03 ± 0.07 | 0.95 ± 0.06 | < 0.001 |
| TC (mean ± SD, mmol/L) | 3.8 ± 0.8 | 3.8 ± 0.8 | 3.7 ± 0.9 | 0.135 |
| HA [interquartile median (range), ng/mL] | 125 (75-224) | 88 (49-129) | 167 (116-382) | < 0.001 |
| A2M (mean ± SD, g/L) | 2.96 ± 0.58 | 2.73 ± 0.48 | 3.28 ± 0.55 | < 0.001 |
| Lg HBV-DNA (mean ± SD) | 6.0 ± 1.9 | 5.9 ± 2.0 | 6.1 ± 2.1 | 0.314 |
| HBeAg positive (n, %) | 55 (70.5) | 32 (69.6) | 23 (71.9) | 0.223 |
| Liver specimen length (mean ± SD, mm) | 18.2 ± 3.4 | 18.4 ± 3.3 | 17.9 ± 3.6 | 0.254 |
| METAVIR A stage (n, %) | ||||
| A0 | 4 (5.1) | |||
| A1 | 41 (52.5) | |||
| A2 | 32 (41.1) | |||
| A3 | 1 (1.3) | |||
| METAVIR F stage (n, %) | ||||
| F0 | 13 (16.7) | |||
| F1 | 33 (42.3) | |||
| F2 | 13 (16.7) | |||
| F3 | 10 (12.8) | |||
| F4 | 9 (11.5) |
SD: Standard deviation; WBC: Leucocyte; Hb: Hemoglobin; TB: Total bilirubin; CB: Conjugated bilirubin; Alb: Albumin; TC: Total cholesterol.
Correlation between non-invasive model and fibrosis stage
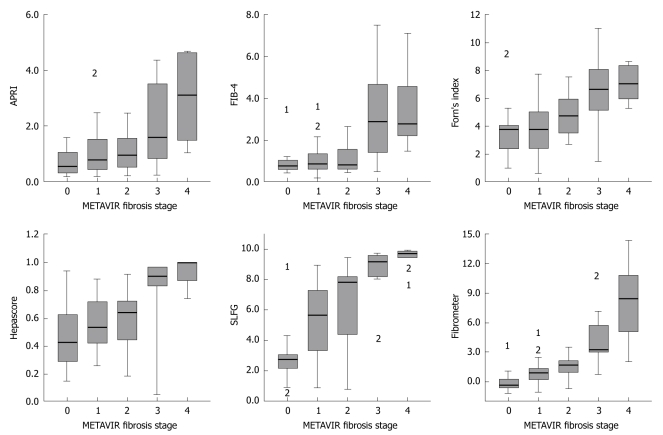
METAVIR fibrosis stages were significantly correlated with APRI, FIB-4, Forn’s index, Fibrometer, Hepascore and SLFG. A better correlation was observed between Fibrometer (r = 0.69), SLFG (r = 0.68) and Hepascore (r = 0.62) (P < 0.001). The box-plots of fibrosis scores are shown in Figure 1. A correlation was also found between scores and histological activity, especially between SLFG (r = 0.55), Fibrometer (r = 0.54) and APRI (r = 0.51) (P < 0.001).
Figure 1.
Model values according to METAVIR fibrosis stages. The top and bottom of each box are the 25th and 75th centile interval, the line through the box is the median and the error bars are the 5th and 95th centile interval. 1 and 2 indicate the extreme values.
Overall diagnostic performance of serum markers
The mean levels of AST, GGT, PT, PI, A2M and HA were higher in patients with F2-F4 fibrosis than in those with F0-F1 fibrosis (P < 0.01). The mean levels of hemoglobin, PLT and albumin were lower in patients with F2-F4 fibrosis than in those with F0-F1 fibrosis (P < 0.01). Multiple regression analysis showed that A2M and HA were the independent factors for significant fibrosis (A2M, OR = 5.36, 95% CI: 1.58-18.13, P = 0.007; HA, OR = 1.01, 95% CI: 1.00-1.02, P = 0.007). AUROC was used to evaluate the overall diagnostic performance of scores (Table 2).
Table 2.
AUROC for F0F1 vs F2-4 and F0-2 vs F3-4
|
F0-1 vs F2-4 |
F0-2 vs F3-4 |
||||||
| AUROC | SD | 95% CI | AdAUC | AUROC | SD | 95% CI | |
| APRI | 0.71 | 0.06 | 0.59-0.83 | 0.75 | 0.80 | 0.06 | 0.67-0.92 |
| FIB-4 | 0.75 | 0.06 | 0.63-0.87 | 0.79 | 0.87 | 0.06 | 0.76-0.99 |
| Forn’s index | 0.79 | 0.05 | 0.69-0.90 | 0.83 | 0.86 | 0.06 | 0.75-0.96 |
| Hepascore | 0.80 | 0.05 | 0.70-0.91 | 0.84 | 0.95 | 0.02 | 0.90-0.99 |
| SLFG | 0.83 | 0.05 | 0.73-0.93 | 0.86 | 0.93 | 0.03 | 0.87-0.99 |
| Fibrometer | 0.85 | 0.05 | 0.75-0.94 | 0.88 | 0.94 | 0.03 | 0.88-0.99 |
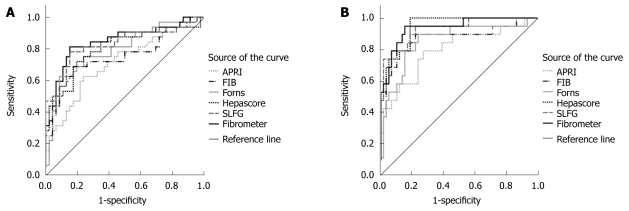
The APRI, FIB-4, Forn’s index, Hepascore, SLFG and Fibrometer were 0.71, 0.75, 0.79, 0.80, 0.83, and 0.85 respectively under the AUROC for F0-F4 (Figure 2A), and 0.75, 0.79, 0.83, 0.84, 0.86, and 0.88 respectively under the adjusted AUROC for F0-F4 with DANA method. The AUROC for Fibrometer, SLFG and Hepascore was better than that for APRI and FIB-4 (P < 0.01).
Figure 2.
ROC curves for the 6 fibrosis models to discriminate METAVIR fibrosis stages F0-1 from F2-4 (A) and F0-2 from F3-4 (B).
The APRI, FIB-4, Forn’s index, Hepascore, SLFG and Fibrometer were 0.80, 0.87, 0.86, 0.95, 0.93, and 0.94 under the AUROC for F0-F4 (Figure 2B). The Hepascore, Fibrometer and SLFG levels were significantly higher than the APRI level under the AUROC for F0-F4 (P < 0.01).
Sensitivity, specificity, positive and negative predictive values
NPV and PPV for the diagnosis of significant fibrosis are presented in Table 3. Cutoffs were chosen for each model as previously described. This analysis could not be performed for Fibrometer since no cutoff was provided in the study by Calès et al[10]. When different cutoffs were used for each model, the percentage of classifiable subjects was 45%-78%, with a diagnostic accuracy of 67%-86% (Table 4). Significant fibrosis (F2-4) was predicted in 13%-32% of patients with their PPV ranged 62%-88%. Since lower cutoffs were originally described to rule out significant fibrosis, attention must be paid to NPV ranging 76%-85%. The best positive predictive value (PPV = 0.88) for significant fibrosis was observed when SLFG > 8.7.
Table 3.
Sensitivity, specificity, predictive values and likelihood ratios of scores according to different cutoffs for the diagnosis of significant fibrosis
| Score | Cutoff | % |
Significant fibrosis (F2-4) |
|||||
| Sen | Spe | PPV | NPV | +LR | -LR | |||
| APRI | < 0.50 | 27 | 0.84 | 0.35 | 0.47 | 0.76 | 1.29 | 0.46 |
| > 1.50 | 32 | 0.47 | 0.80 | 0.62 | 0.69 | 2.35 | 0.66 | |
| FIB-4 | < 1.45 | 65 | 0.63 | 0.85 | 0.74 | 0.76 | 4.20 | 0.44 |
| > 3.25 | 13 | 0.25 | 0.96 | 0.80 | 0.65 | 6.25 | 0.78 | |
| Forn’s index | < 4.20 | 46 | 0.75 | 0.63 | 0.59 | 0.78 | 2.03 | 0.40 |
| > 6.90 | 18 | 0.34 | 0.96 | 0.85 | 0.68 | 8.50 | 0.69 | |
| Hepascore | < 0.50 | 23 | 0.88 | 0.50 | 0.55 | 0.85 | 1.74 | 0.26 |
| > 0.84 | 27 | 0.50 | 0.91 | 0.80 | 0.72 | 5.56 | 0.55 | |
| SLFG | < 3.00 | 23 | 0.91 | 0.33 | 0.48 | 0.83 | 1.36 | 0.27 |
| > 8.70 | 22 | 0.47 | 0.96 | 0.88 | 0.72 | 11.75 | 0.55 | |
Sen: Sensitivity; Spe: Specificity; PPV: Positive predictive value; NPV: Negative predictive value; +LR: Positive likelihood ratio; -LR: Negative likelihood ratio.
Table 4.
Percentage of classifiable subjects, correct prediction, diagnostic accuracy and biopsies that could be avoided n (%)
| Models | Cut-offs | Classifiable subjects | Correct prediction | Diagnostic accuracy | Biopsy avoided |
| APRI | < 0.50 | 21 (27) | 16 (76) | 67% | 31 (40) |
| > 1.50 | 25 (32) | 15 (62) | |||
| FIB-4 | < 1.45 | 51 (65) | 39 (76) | 77% | 47 (60) |
| > 3.25 | 10 (13) | 8 (80) | |||
| Forn’s index | < 4.20 | 36 (46) | 28 (78) | 80% | 40 (51) |
| > 6.90 | 14 (18) | 12 (85) | |||
| Hepascore | < 0.50 | 18 (23) | 15 (85) | 82% | 32 (41) |
| > 0.84 | 21 (27) | 17 (80) | |||
| SLFG | < 3.00 | 18 (23) | 15 (83) | 86% | 30 (38) |
| > 8.70 | 17 (22) | 15 (88) |
DISCUSSION
Noninvasive models have been proposed for the assessment of liver fibrosis. The diagnostic performance of APRI, FIB-4, Forn’s index, Fibrometer, Hepascore and SLFG was evaluated for the assessment of liver fibrosis in CHB patients. These models are mainly based on two kinds of serum markers, direct and indirect. Direct serum markers are directly linked to the modifications in extracellular matrix (ECM) metabolism. Indirect serum markers have no direct link with liver fibrosis but reflect liver dysfunction or other phenomena caused by fibrosis. We focused on the serum markers of fibrosis. The main end-point of our study was to evaluate the global diagnostic performance of models by comparing their AUROCs. Our study indicated that models containing direct serum markers (Fibrometer, SLFG, Hepascore) performed much more accurately than models containing only indirect serum markers (APRI, FIB-4, Forn’s index).
Direct serum markers are useful for assessing the speed of liver fibrogenesis. HA, a component of ECM, is a glycosaminoglycan synthesized by hepatic stellate cells and degraded by liver sinusoidal cells[18]. A2M is a protease inhibitor with its concentration increased due to stellate cell activation and liver fibrosis[19]. Studies have demonstrated that HA and A2M levels are correlated with hepatic fibrosis in patients with CHB or CHC[11,20-22], which is consistent with the findings in our study. Multiple regression analysis in our study showed that HA and A2M were the independent factor for significant fibrosis and had a better diagnostic accuracy.
It has been reported that APRI under the AUROC for significant fibrosis is 0.76 (95% CI: 0.74-0.79)[23]. It has been shown that the accuracy of APRI under the AUROC for significant fibrosis is 0.63 and 0.72 in CHB patients[24,25]. Zhang et al[26] showed that APRI has low diagnostic accuracy of liver fibrosis and APRI combined HA can achieve a better diagnostic accuracy of liver fibrosis. FIB-4 has a high diagnostic accuracy of severe fibrosis[27]. Mallet et al[28] reported that FIB-4 is 0.81 under the AUROC for severe fibrosis. In our study, the FIB-4 was 0.87 under the AUROC for severe fibrosis. The reported Forn’s index is 0.76 under the AUROC for significant fibrosis[29]. In our study, the Forn’s index was 0.79 under the AUROC for significant fibrosis. The diagnostic value of APRI, FIB-4 and Forn’s index was much lower in CHB patients than in CHC patients.
Calès et al[10] have developed Fibrometer for the diagnosis of significant fibrosis and found that its diagnostic performance is stable in patients with different chronic liver diseases[30,31]. Hepascore has been used in diagnosis of significant and severe fibrosis[12]. In our study, the Hepascore was 0.80 and 0.95 under the AUROC for significant and severe fibrosis. SLFG is the first developed model in CHB patients. In our study, the NPV and PPV of SLFG under AUROC are similar to the reported data[11]. These results indicat that Fibrometer, SLFG and Hepascore can be used in diagnosis of liver fibrosis. However, these noninvasive models should be validated in a larger number of CHB patients.
Broadly speaking, no true noninvasive model could exactly reflect liver fibrosis. Transient elastography (fibroScan) is another noninvasive method to detect the mean liver stiffness for diagnosing fibrosis. However, it is expensive and may be limited in those with narrow intercostal spaces, morbid obesity or significant ascites[32,33]. These noninvasive models can be used in clinical management of CHB by offering an attractive alternative to liver biopsy.
In our study, since the sample size was small, further study is needed before these models are used in clinical practice. Validating against not only histological stage scores but also digital image analysis and clinical outcomes may also be a better choice.
In conclusion, serologic models containing direct serum markers of Hepascore, SLFG, and Fibrometer have better diagnostic values in CHB patients than those containing only indirect serum markers of APRI, FIB-4, Forn’s index.
COMMENTS
Background
Clinically, developing noninvasive methods for diagnosing liver fibrosis is important. Noninvasive models have been established most in hepatitis C patients.
Research frontiers
Noninvasive models have been proposed for the assessment of liver fibrosis. These models have been evaluated at many medical centers in chronic hepatitis C (CHC), but few in chronic hepatitis B (CHB). In this study, the efficacy of 6 noninvasive models was evaluated and the more valuable models were identified for predicting liver fibrosis in CHB.
Innovations and breakthroughs
The efficacy of 6 noninvasive models was evaluated in a cohort of Chinese patients with CHB. The results indicate that their efficacy is not influenced by ethnic and virus factors. This study also showed that the serologic models containing direct serum markers, such as hyaluronic acid (HA) and α2-macroglobulin (A2M) have better diagnostic values in CHB patients than in those not containing direct serum markers.
Applications
Noninvasive models can be used in diagnosis of liver fibrosis in patients with CHB in China or in Asia.
Terminology
DANA method: A method used to adjust the differences caused by the prevalence of fibrosis stages. Standard prevalence is defined as a prevalence of 0.20 for each of the five stages.
Peer review
The study adds information that helps establishment of strategies against liver fibrosis diagnosis using noninvasive methods. The study was scientifically designed. The manuscript is logical and readable.
Footnotes
Supported by Grant for Master Degree Students of Fudan University
Peer reviewers: Damiao Carlos Moraes Santos, DCM, PhD, Bio-Manguinhos, Fundación Oswaldo Cruz, Avenida Brasil 4365, Manguinhos, Rio de Janeiro, 21040360, Brazil; Roberto J Carvalho-Filho, MD, PhD, Hepatitis Section, Division of Gastroenterology, Federal University of Sao Paulo, Rua Botucatu, 740, 2.o andar, Vila Clementino, State of Sao Paulo, 04023-060, Brazil
S- Editor Wang JL L- Editor Wang XL E- Editor Ma WH
References
- 1.Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733–1745. doi: 10.1056/NEJM199712113372406. [DOI] [PubMed] [Google Scholar]
- 2.Afdhal NH, Nunes D. Evaluation of liver fibrosis: a concise review. Am J Gastroenterol. 2004;99:1160–1174. doi: 10.1111/j.1572-0241.2004.30110.x. [DOI] [PubMed] [Google Scholar]
- 3.Little AF, Ferris JV, Dodd GD 3rd, Baron RL. Image-guided percutaneous hepatic biopsy: effect of ascites on the complication rate. Radiology. 1996;199:79–83. doi: 10.1148/radiology.199.1.8633176. [DOI] [PubMed] [Google Scholar]
- 4.Lindor KD, Bru C, Jorgensen RA, Rakela J, Bordas JM, Gross JB, Rodes J, McGill DB, Reading CC, James EM, et al. The role of ultrasonography and automatic-needle biopsy in outpatient percutaneous liver biopsy. Hepatology. 1996;23:1079–1083. doi: 10.1002/hep.510230522. [DOI] [PubMed] [Google Scholar]
- 5.Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF) Hepatology. 2000;32:477–481. doi: 10.1053/jhep.2000.16602. [DOI] [PubMed] [Google Scholar]
- 6.Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38 Suppl 1:S38–S53. doi: 10.1016/s0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 7.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 8.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 9.Forns X, Ampurdanès S, Llovet JM, Aponte J, Quintó L, Martínez-Bauer E, Bruguera M, Sánchez-Tapias JM, Rodés J. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986–992. doi: 10.1053/jhep.2002.36128. [DOI] [PubMed] [Google Scholar]
- 10.Calès P, Oberti F, Michalak S, Hubert-Fouchard I, Rousselet MC, Konaté A, Gallois Y, Ternisien C, Chevailler A, Lunel F. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology. 2005;42:1373–1381. doi: 10.1002/hep.20935. [DOI] [PubMed] [Google Scholar]
- 11.Zeng MD, Lu LG, Mao YM, Qiu DK, Li JQ, Wan MB, Chen CW, Wang JY, Cai X, Gao CF, et al. Prediction of significant fibrosis in HBeAg-positive patients with chronic hepatitis B by a noninvasive model. Hepatology. 2005;42:1437–1445. doi: 10.1002/hep.20960. [DOI] [PubMed] [Google Scholar]
- 12.Adams LA, Bulsara M, Rossi E, DeBoer B, Speers D, George J, Kench J, Farrell G, McCaughan GW, Jeffrey GP. Hepascore: an accurate validated predictor of liver fibrosis in chronic hepatitis C infection. Clin Chem. 2005;51:1867–1873. doi: 10.1373/clinchem.2005.048389. [DOI] [PubMed] [Google Scholar]
- 13.Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20:15–20. [PubMed] [Google Scholar]
- 14.Strader DB, Wright T, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147–1171. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- 15.Poynard T, Halfon P, Castera L, Munteanu M, Imbert-Bismut F, Ratziu V, Benhamou Y, Bourlière M, de Ledinghen V. Standardization of ROC curve areas for diagnostic evaluation of liver fibrosis markers based on prevalences of fibrosis stages. Clin Chem. 2007;53:1615–1622. doi: 10.1373/clinchem.2007.085795. [DOI] [PubMed] [Google Scholar]
- 16.Poynard T, Muntenau M, Morra R, Ngo Y, Imbert-Bismut F, Thabut D, Messous D, Massard J, Lebray P, Moussalli J, et al. Methodological aspects of the interpretation of non-invasive biomarkers of liver fibrosis: a 2008 update. Gastroenterol Clin Biol. 2008;32:8–21. doi: 10.1016/S0399-8320(08)73990-3. [DOI] [PubMed] [Google Scholar]
- 17.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 18.McGary CT, Raja RH, Weigel PH. Endocytosis of hyaluronic acid by rat liver endothelial cells. Evidence for receptor recycling. Biochem J. 1989;257:875–884. doi: 10.1042/bj2570875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawser CA, Iredale JP, Winwood PJ, Arthur MJ. Rat hepatic stellate cell expression of alpha2-macroglobulin is a feature of cellular activation: implications for matrix remodelling in hepatic fibrosis. Clin Sci (Lond) 1998;95:179–186. [PubMed] [Google Scholar]
- 20.McHutchison JG, Blatt LM, de Medina M, Craig JR, Conrad A, Schiff ER, Tong MJ. Measurement of serum hyaluronic acid in patients with chronic hepatitis C and its relationship to liver histology. Consensus Interferon Study Group. J Gastroenterol Hepatol. 2000;15:945–951. doi: 10.1046/j.1440-1746.2000.02233.x. [DOI] [PubMed] [Google Scholar]
- 21.Myers RP, Ratziu V, Imbert-Bismut F, Charlotte F, Poynard T. Biochemical markers of liver fibrosis: a comparison with historical features in patients with chronic hepatitis C. Am J Gastroenterol. 2002;97:2419–2425. doi: 10.1111/j.1572-0241.2002.05997.x. [DOI] [PubMed] [Google Scholar]
- 22.Myers RP, Tainturier MH, Ratziu V, Piton A, Thibault V, Imbert-Bismut F, Messous D, Charlotte F, Di Martino V, Benhamou Y, et al. Prediction of liver histological lesions with biochemical markers in patients with chronic hepatitis B. J Hepatol. 2003;39:222–230. doi: 10.1016/s0168-8278(03)00171-5. [DOI] [PubMed] [Google Scholar]
- 23.Shaheen AA, Myers RP. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis C-related fibrosis: a systematic review. Hepatology. 2007;46:912–921. doi: 10.1002/hep.21835. [DOI] [PubMed] [Google Scholar]
- 24.Wai CT, Cheng CL, Wee A, Dan YY, Chan E, Chua W, Mak B, Oo AM, Lim SG. Non-invasive models for predicting histology in patients with chronic hepatitis B. Liver Int. 2006;26:666–672. doi: 10.1111/j.1478-3231.2006.01287.x. [DOI] [PubMed] [Google Scholar]
- 25.Sebastiani G, Vario A, Guido M, Alberti A. Sequential algorithms combining non-invasive markers and biopsy for the assessment of liver fibrosis in chronic hepatitis B. World J Gastroenterol. 2007;13:525–531. doi: 10.3748/wjg.v13.i4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang YX, Wu WJ, Zhang YZ, Feng YL, Zhou XX, Pan Q. Noninvasive assessment of liver fibrosis with combined serum aminotransferase/platelet ratio index and hyaluronic acid in patients with chronic hepatitis B. World J Gastroenterol. 2008;14:7117–7121. doi: 10.3748/wjg.14.7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, Pol S. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 28.Mallet V, Dhalluin-Venier V, Roussin C, Bourliere M, Pettinelli ME, Giry C, Vallet-Pichard A, Fontaine H, Pol S. The accuracy of the FIB-4 index for the diagnosis of mild fibrosis in chronic hepatitis B. Aliment Pharmacol Ther. 2009;29:409–415. doi: 10.1111/j.1365-2036.2008.03895.x. [DOI] [PubMed] [Google Scholar]
- 29.Adler M, Gulbis B, Moreno C, Evrard S, Verset G, Golstein P, Frotscher B, Nagy N, Thiry P. The predictive value of FIB-4 versus FibroTest, APRI, FibroIndex and Forns index to noninvasively estimate fibrosis in hepatitis C and nonhepatitis C liver diseases. Hepatology. 2008;47:762–763; author reply 763. doi: 10.1002/hep.22085. [DOI] [PubMed] [Google Scholar]
- 30.Calès P, Boursier J, Oberti F, Hubert I, Gallois Y, Rousselet MC, Dib N, Moal V, Macchi L, Chevailler A, et al. FibroMeters: a family of blood tests for liver fibrosis. Gastroenterol Clin Biol. 2008;32:40–51. doi: 10.1016/S0399-8320(08)73992-7. [DOI] [PubMed] [Google Scholar]
- 31.Leroy V, Halfon P, Bacq Y, Boursier J, Rousselet MC, Bourlière M, de Muret A, Sturm N, Hunault G, Penaranda G, et al. Diagnostic accuracy, reproducibility and robustness of fibrosis blood tests in chronic hepatitis C: a meta-analysis with individual data. Clin Biochem. 2008;41:1368–1376. doi: 10.1016/j.clinbiochem.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 32.Coco B, Oliveri F, Maina AM, Ciccorossi P, Sacco R, Colombatto P, Bonino F, Brunetto MR. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat. 2007;14:360–369. doi: 10.1111/j.1365-2893.2006.00811.x. [DOI] [PubMed] [Google Scholar]
- 33.Cobbold JF, Morin S, Taylor-Robinson SD. Transient elastography for the assessment of chronic liver disease: ready for the clinic? World J Gastroenterol. 2007;13:4791–4797. doi: 10.3748/wjg.v13.i36.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]