Abstract
The relevance of the ring-infected erythrocyte surface antigen (RESA) of Plasmodium falciparum as a malaria vaccine candidate has been questioned of late because RESA-deficient parasites have been found to multiply normally in culture or in monkeys. The RESA-2 gene was recently described as a pseudogene highly homologous to RESA. In this report, we demonstrate that RESA-2 is not a pseudogene, because we were able to detect RESA-2 transcripts in asexual blood stages of multiple isolates by using polymerase chain reaction on reverse-transcribed mRNA. Transcription of RESA-2 was observed whether or not the isolates studied expressed the RESA protein.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berzins K., Perlmann H., Wåhlin B., Ekre H. P., Högh B., Petersen E., Wellde B., Schoenbechler M., Williams J., Chulay J. Passive immunization of Aotus monkeys with human antibodies to the Plasmodium falciparum antigen Pf155/RESA. Infect Immun. 1991 Apr;59(4):1500–1506. doi: 10.1128/iai.59.4.1500-1506.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
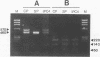
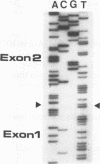
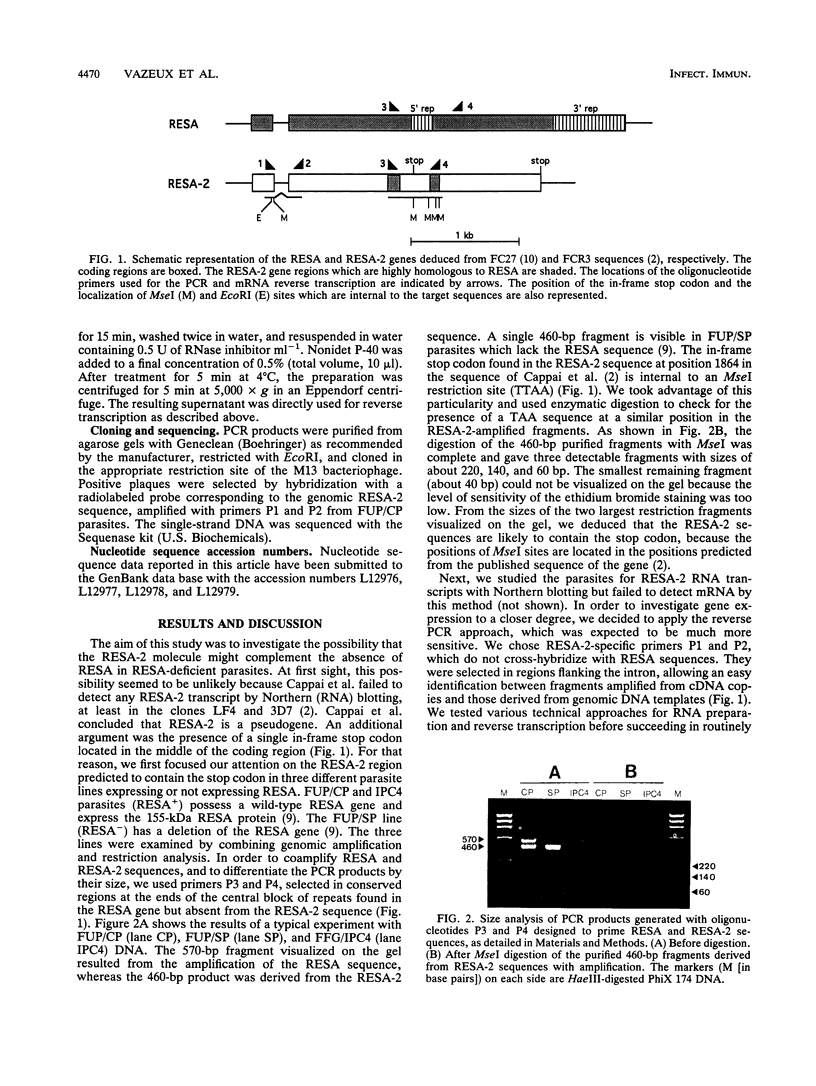
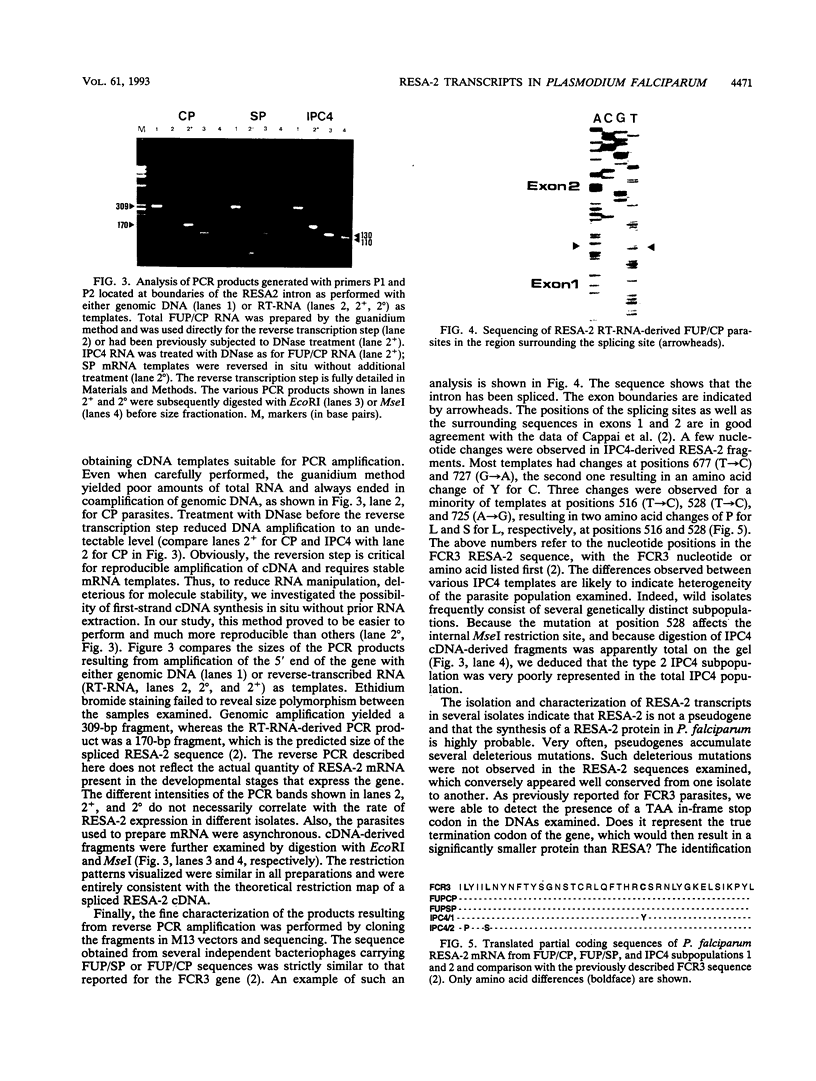
- Cappai R., Kaslow D. C., Peterson M. G., Cowman A. F., Anders R. F., Kemp D. J. Cloning and analysis of the RESA-2 gene: a DNA homologue of the ring-infected erythrocyte surface antigen gene of Plasmodium falciparum. Mol Biochem Parasitol. 1992 Sep;54(2):213–221. doi: 10.1016/0166-6851(92)90113-x. [DOI] [PubMed] [Google Scholar]
- Cappai R., van Schravendijk M. R., Anders R. F., Peterson M. G., Thomas L. M., Cowman A. F., Kemp D. J. Expression of the RESA gene in Plasmodium falciparum isolate FCR3 is prevented by a subtelomeric deletion. Mol Cell Biol. 1989 Aug;9(8):3584–3587. doi: 10.1128/mcb.9.8.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins W. E., Anders R. F., Pappaioanou M., Campbell G. H., Brown G. V., Kemp D. J., Coppel R. L., Skinner J. C., Andrysiak P. M., Favaloro J. M. Immunization of Aotus monkeys with recombinant proteins of an erythrocyte surface antigen of Plasmodium falciparum. Nature. 1986 Sep 18;323(6085):259–262. doi: 10.1038/323259a0. [DOI] [PubMed] [Google Scholar]
- Coppel R. L., Cowman A. F., Anders R. F., Bianco A. E., Saint R. B., Lingelbach K. R., Kemp D. J., Brown G. V. Immune sera recognize on erythrocytes Plasmodium falciparum antigen composed of repeated amino acid sequences. 1984 Aug 30-Sep 5Nature. 310(5980):789–792. doi: 10.1038/310789a0. [DOI] [PubMed] [Google Scholar]
- Cowman A. F., Coppel R. L., Saint R. B., Favaloro J., Crewther P. E., Stahl H. D., Bianco A. E., Brown G. V., Anders R. F., Kemp D. J. The ring-infected erythrocyte surface antigen (RESA) polypeptide of Plasmodium falciparum contains two separate blocks of tandem repeats encoding antigenic epitopes that are naturally immunogenic in man. Mol Biol Med. 1984 Jun;2(3):207–221. [PubMed] [Google Scholar]
- Fandeur T., Bonnefoy S., Mercereau-Puijalon O. In vivo and in vitro derived Palo Alto lines of Plasmodium falciparum are genetically unrelated. Mol Biochem Parasitol. 1991 Aug;47(2):167–178. doi: 10.1016/0166-6851(91)90176-7. [DOI] [PubMed] [Google Scholar]
- Fandeur T., Gysin J., Mercereau-Puijalon O. Protection of squirrel monkeys against virulent Plasmodium falciparum infections by use of attenuated parasites. Infect Immun. 1992 Apr;60(4):1390–1396. doi: 10.1128/iai.60.4.1390-1396.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro J. M., Coppel R. L., Corcoran L. M., Foote S. J., Brown G. V., Anders R. F., Kemp D. J. Structure of the RESA gene of Plasmodium falciparum. Nucleic Acids Res. 1986 Nov 11;14(21):8265–8277. doi: 10.1093/nar/14.21.8265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley M., Tilley L., Sawyer W. H., Anders R. F. The ring-infected erythrocyte surface antigen of Plasmodium falciparum associates with spectrin in the erythrocyte membrane. Mol Biochem Parasitol. 1991 May;46(1):137–147. doi: 10.1016/0166-6851(91)90207-m. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Cowman A. F., Walliker D. Genetic diversity in Plasmodium falciparum. Adv Parasitol. 1990;29:75–149. doi: 10.1016/s0065-308x(08)60105-0. [DOI] [PubMed] [Google Scholar]
- Knapp B., Nau U., Hundt E., Küpper H. A. A new blood stage antigen of Plasmodium falciparum highly homologous to the serine-stretch protein SERP. Mol Biochem Parasitol. 1991 Jan;44(1):1–13. doi: 10.1016/0166-6851(91)90215-r. [DOI] [PubMed] [Google Scholar]
- Miller L. H., Howard R. J., Carter R., Good M. F., Nussenzweig V., Nussenzweig R. S. Research toward malaria vaccines. Science. 1986 Dec 12;234(4782):1349–1356. doi: 10.1126/science.2431481. [DOI] [PubMed] [Google Scholar]
- Nolte D., Hundt E., Langsley G., Knapp B. A Plasmodium falciparum blood stage antigen highly homologous to the glycophorin binding protein GBP. Mol Biochem Parasitol. 1991 Dec;49(2):253–264. doi: 10.1016/0166-6851(91)90069-i. [DOI] [PubMed] [Google Scholar]
- Pologe L. G., de Bruin D., Ravetch J. V. A and T homopolymeric stretches mediate a DNA inversion in Plasmodium falciparum which results in loss of gene expression. Mol Cell Biol. 1990 Jun;10(6):3243–3246. doi: 10.1128/mcb.10.6.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruangjirachuporn W., Wåhlin B., Perlmann H., Carlsson J., Berzins K., Wahlgren M., Udomsangpetch R., Wigzell H., Perlmann P. Monoclonal antibodies to a synthetic peptide corresponding to a repeated sequence in the Plasmodium falciparum antigen Pf155. Mol Biochem Parasitol. 1988 May;29(1):19–28. doi: 10.1016/0166-6851(88)90115-6. [DOI] [PubMed] [Google Scholar]
- Wellems T. E., Howard R. J. Homologous genes encode two distinct histidine-rich proteins in a cloned isolate of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6065–6069. doi: 10.1073/pnas.83.16.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]