Abstract
During development, multiple cell types within a tissue often arise from a common pool of progenitor cells (PCs). PCs typically expand in number, while simultaneously producing post-mitotic cells (PMCs). This balance is partly regulated by transcription factors that are expressed within PCs, such as the basic helix–loop–helix (bHLH) gene mouse atonal homolog 7 (Math5), which is expressed in retinal PCs. Here we report that alternative splicing (AS) of Math5 serves as another layer of regulation of Math5 activity. Specifically, Math5, a single exon gene, is alternatively spliced such that the major isoform lacks the entire coding sequence. Similarly, neurogenin 3 (Ngn3), a Math5 paralog expressed in pancreatic PCs, is also alternatively spliced such that the major isoform fails to code for Ngn3 protein. The consequence of reducing the abundance of protein-coding isoforms is likely crucial, as we found that introduction of coding isoforms leads to a reduction in cycling PCs. Thus, AS can limit the number of PCs expressing key regulatory proteins that control PC expansion versus PMC production.
Keywords: Alternative splicing, Math5, Ngn3, noncoding, retina, progenitor cells, post-mitotic cells
The finding that the human genome has ∼23,621 protein-coding genes was quite surprising, as the Drosophila genome has 14,000 protein-coding genes. It is generally accepted that mammals derive their proteome complexity by employing alternative splicing (AS) (Black 2000). Indeed, a recent study showed that 92%–94% of human genes are alternatively spliced (Wang et al. 2008). Moreover, it is becoming evident that AS defects may play a vital role in neurological and other disorders (Licatalosi and Darnell 2006; Cooper et al. 2009). The importance of AS in development is underscored by the disease myotonic dystrophy (DM), where the fetal splice patterns for a core set of genes is retained in adults (Philips et al. 1998; Kanadia et al. 2003). In essence, DM can be seen as a developmental defect (Kanadia et al. 2003, 2006; Cooper et al. 2009).
During development, progenitor cells (PCs) must increase their own numbers while producing post-mitotic cells (PMCs) to create a mature tissue. This aspect of development has been investigated with an emphasis on understanding the transcriptome, which has led to the focus on transcription factors. One such factor is mouse atonal homolog 7 (Math5), which is among the first basic helix–loop–helix (bHLH) transcription factors expressed in a significant subset of multipotent retinal PCs at embryonic day 11 (E11) (Brown et al. 1998; Saul et al. 2008). Math5 function has been extensively investigated, and there exists two independent mouse knockout lines for Math5 (Brown et al. 2001; Wang et al. 2001), as well as a zebrafish loss-of-function mutant (Kay et al. 2001). In these mutants, exit from cell cycle was perturbed along with loss of at least 95% of the retinal ganglion cells (RGCs) (Kay et al. 2001; Le et al. 2006). To better understand how Math5 might be regulated for its multiple roles, we performed a thorough analysis of the structure of Math5 transcripts.
Results and Discussion
Math5, a single exon gene, is alternatively spliced during development
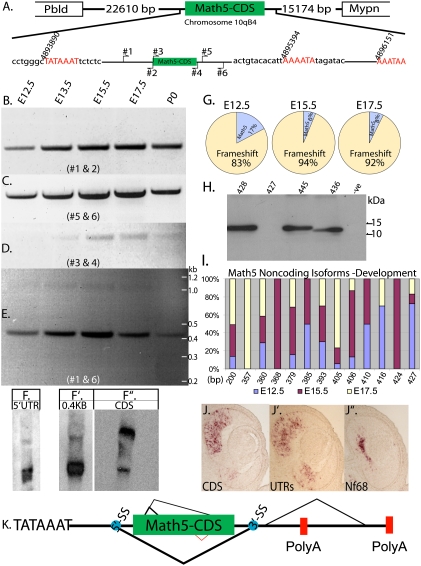
Currently, in the National Center for Bioinformatics Information (NCBI) database, Math5 is represented as a gene with a single exon encoding the entire protein. The 5′ untranslated region (UTR), the 3′UTR, the transcription start signal (TATA), and the polyadenylation signal, remained uncharacterized. Thus, the transcriptional start and polyadenylation sites were determined using 5′RACE and 3′RACE, respectively (Supplemental Fig. 1). A single transcription start site at nucleotide position 4,893,890 (chromosome 10qB4) was found, while there were at least two distinct polyadenylation sites: one at position 4,895,394 and on at 4,896,151 (Fig. 1A). Next, RT–PCR analysis was performed to determine the expression of Math5 across embryonic development from E12.5 to postnatal day 0 (P0). Three sets of primers were used to interrogate the expression of Math5: the 5′UTR (primers 1 and 2), the coding sequence (CDS) (primers 3 and 4), and the 3′UTR (primers 5 and 6) (Fig. 1A). The PCR products of the 5′UTR and 3′UTR were fairly equivalent in their levels, while that of the CDS was lower in comparison (Fig. 1B–D). First, to rule out problems with amplification of the CDS, primers 1 and 6 were used to amplify the predicted protein-coding isoform. Surprisingly, two bands were observed: a prominent band at a molecular weight (MW) of 0.4 kb, and a lower-intensity band at the expected size of 1.0 kb (Fig. 1E). The presence of two isoforms was confirmed by Northern blot analysis. A 5′UTR probe showed two bands: a strong lower-MW band, and a weak higher-MW band (Fig. 1F). This was predicted, as Math5 has a single transcription start site, so that the 5′UTR should be common to all of the RNA species. A similar result was obtained when the 0.4-kb band obtained by RT–PCR (Fig. 1E) was used as a probe (Fig. 1F′). Moreover, the relative intensities of the two bands observed on the Northern analyses (Fig. 1F,F′) were consistent with the results of the RT–PCR analysis (Fig. 1E), thus confirming the existence of two RNA species, as implied by the PCR analysis. Interestingly, the CDS probe bound predominantly to the higher-MW band on the Northern blot (Fig. 1F). This suggests that the higher-MW band detected on the Northern blots (Fig. 1F,F′) is most likely a CDS-containing isoform. It must be noted that the CDS probe also hybridized at a lower level to a lower-MW band, which could be produced by AS of the 3′UTR and/or the usage of an alternate polyadenylation site (Supplemental Fig. 2).
Figure 1.
Math5 is alternatively spliced during development. (A) Schematic representation of the Math5 locus, with the size of the intergenic sequence separating the upstream (Pbld) and downstream (Mypn) genes, respectively. Schematic of the Math5 gene, with the transcription start site (TATA box) and the polyadenylation sites, along with the position of the primers used for RT–PCR analysis. The nucleotide sequence around the transcription start and polyadenylation sites that were identified by RACE are shown along with the nucleotide positions that correspond to the NCBI database. RT–PCR analysis for Math5 across retinal development with primer pairs #1 and #2 (B), #5 and #6 (C), #3 and #4 (D), and #1 and #6 (E). (F) Total RNA blot probed with a 5′UTR probe. (F′) Total RNA blot probed with the 0.4-kb band from E. (F″) Total RNA blot probed with CDS. (G) Quantification of the Math5 CDS fragments amplified from E12.5, E15.5, and E17.5, with primers 3 and 4 showing the percentage of the isoforms in which the CDS frame is maintained and those with a frameshift. (H) Immunoblot analysis of 293T cells transfected with different isoforms of Math5 that have different AS events. The second lane is from cells transfected with the Math5 CDS (427-bp) isoform that could theoretically code for protein if an alternate start codon (GTG) were used. The last lane is untransfected control, which is represented as −ve. (I) Distribution of the Math5 CDS isoforms that result in a frameshift and do not code for protein observed across development. The X-axis shows the size of the isoform and the Y-axis shows the percentage of each isoform. (J–J″) RNA in situ hybridization on serial E12.5 retinal sections probed with DIG-labeled antisense riboprobes made from either the 0.4-kb or 1-kb isoforms of Math5 along with Nf68, which labels differentiating RGCs. (K) An updated schematic of the Math5 gene with all of the AS events, with the thickness of the lines indicating the major to minor splicing events. The blue spots indicate the splice sites that received the highest score for being a spice site by bioinformatic analysis (Supplemental Fig. 3).
Very few Math5 RNA species code for protein
The two isoforms of Math5 discovered by RT–PCR were characterized by sequence analysis. As expected, the 0.4-kb band contained the 5′UTR. However, it was directly spliced to the 3′UTR such that the entire CDS was omitted; thus, this isoform will be referred to as the entire CDS omitted form (ECO form) (Supplemental Fig. 2C). The 1-kb band represented isoforms that included the CDS [InC form(s)] (Supplemental Fig. 2). Interestingly, the InC form(s) contained several isoforms that were alternatively spliced such that the N terminus was perturbed (Supplemental Fig. 2B). Therefore, we sought to determine the fine structure of the InC forms. Primers 3 and 4 (Fig. 1A) were employed for PCR amplification of just the CDS from E12.5, E15.5, and E17.5, and the products were cloned into the pGEMT-T vector and sequenced (Supplemental Table 1). Extensive AS in the N terminus of the CDS was observed such that most of the isoforms had a frameshift. To rule out the possibility that the spliced isoforms within the CDS were a result of PCR artifacts, perhaps due to high GC content, the same primers (3 and 4) were used to amplify the CDS from genomic DNA. Only the single predicted PCR product was observed (Supplemental Fig. 2), suggesting that the multiple isoforms from cDNA were produced in vivo. Thus, we proceeded to examine the spliced variants within the CDS across development. At E12.5, only 17% of the CDS isoforms coded for Math5 protein, while at E15.5 and E17.5, 6% and 8%, respectively, coded for Math5 protein (Fig. 1G). The CDS RNA isoforms that did not encode Math5 protein fell into two categories: one that did not code for protein, and one that could make a protein unrelated to Math5. This latter protein showed similarity to a rat protein called isoform CRA_a (NCBI accession no. EDL97357), when the sequence was BLASTed against the NCBI database. However, no such protein was identified in the mouse database, which led us to assign these isoforms to the non-Math5 protein-coding RNA category. Within the group of transcripts with the CDS, but without a structure that allowed Math5 protein production, there were 12 isoforms from 200 to 427 base pairs (bp) (Fig. 1I). One group of isoforms had a frameshift such that it could theoretically code for Math5 protein if an alternate start codon (GTG) was employed (Supplemental Fig. 3B). To test if the GTG codon was used for translation, expression constructs containing the isoforms that used either ATG or GTG as their potential start codon were transfected into 293T cells (Fig. 1H). Extracts were made and immunoblotted for Math5. No immunoreactive band for Math5 was observed in the lane with the clone that employed GTG as a potential start codon (Fig. 1H, second lane), suggesting that GTG is likely not used as a start codon.
The entire sequence of Math5, from the TATA box (Fig. 1A) to the last polyadenylation signal (Fig. 1A), was used for an analysis of predicted splice sites (SS) using an algorithm available at http://spliceport.cs.umd.edu/SplicingAnalyser2.html. This algorithm predicts the 5′ SS and the 3′ SS and assigns a score to indicate the strength of the sequence to serve as a SS. A single 5′ SS and four 3′ SS were predicted (Supplemental Fig. 4B). The score for the 5′ SS was lower than two of the 3′ SS. Notably, the strongest score for the 3′ SS was for the one that is used to produce the major isoform that lacks the entire CDS; i.e., yielding the ECO form (Fig. 1E). Finally, the 5′ and all of the 3′ SS identified have motifs of the major U2 class spliceosome machinery. The sequence for the 5′ SS is 100% conserved in rats, orangutans, and humans, while that of the fourth 3′ SS has a single nucleotide (G-to-A) change in orangutans and humans (Supplemental Fig. 4C). Moreover, some of the AS events observed in the N terminus of the CDS shown here were predicted independently by the Swiss Bioinformatics Institute (SBI) (as shown on the University of California at Santa Cruz Genome Browser). However, there is one AS event in the C terminus that was predicted by the SBI (Fig. 1K, red) that was not observed in the developing retina. Interestingly, the EST that was used by the SBI to predict this AS event was from the cerebellum, perhaps suggesting differential regulation of Math5 in different tissues.
As an additional test for the different isoforms, we performed RNA in situ hybridization (ISH) on serial E12.5 retinal sections, using probes for either the CDS or the UTRs. As expected, Math5 ISH signal was observed in the area containing PCs. Moreover, the region containing the PCs enriched for the InC form(s) was the dorsal central retina. This is the same region where one can observe the RGCs in the inner neuroblastic region, as shown by the ISH for the RGC marker Nf68 (Fig. 1J). As retinal development proceeds in a central-to-peripheral manner, it was interesting to note that the leading edge of Math5 expression was peripheral to the region of Nf68 expression; i.e., where there was not yet RGC production (Fig. 1J). This peripheral domain showed greater expression of the ECO form relative to the InC form(s) (Fig. 1J′). This suggests that, when transcription of Math5 is initiated in PCs prior to RGC production, the PCs are more likely to have the ECO form. To further investigate this possibility, we performed dissociated ISH (DISH) with CDS and UTR probes. In order to mark PCs, E12.5 retinas were pulsed for 1 h with 5-ethyl-2′-deoxyuridine (EdU). EdU, a thymidine analog, incorporates into the genome during DNA synthesis, thereby marking all PCs in the S phase of the cell cycle. ISH with CDS and UTR probes for Math5 showed that only 17% of the EdU+ cells were positive for the CDS probe, while 34% of the EdU+ cells were positive for the UTR probe (Supplemental Fig. 5). Taken together, the data suggest that Math5 undergoes extensive AS such that there are very few (less than ∼5%) InC forms. Within the pool of InC forms only a small fraction (∼6%–17%) could code for Math5 protein. Thus, it is likely that very few (less than ∼1%) Math5 mRNA+ cells express significant levels of the protein.
The major isoform of neurogenin 3 (Ngn3) does not code for Ngn3 protein
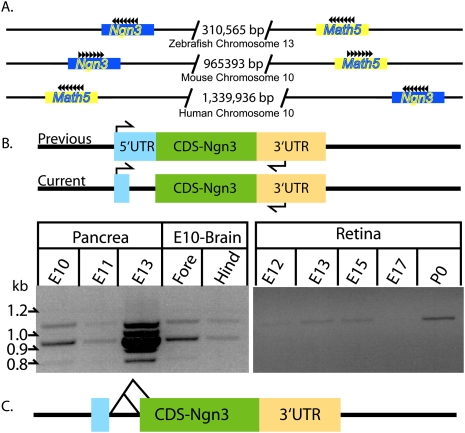
Next, we investigated whether the findings regarding AS of Math5 were unique to Math5. We focused on Math4b (also known as Ngn3), which, like Math5, is expressed in early PCs in a different tissue: the pancreas (Apelqvist et al. 1999; Gradwohl et al. 2000; Schwitzgebel et al. 2000; van Eyll et al. 2006; Burlison et al. 2008). Additionally, Ngn3 was designated as a single exon gene located on chromosome 10qB4 (Sommer et al. 1996), the same chromosome as Math5 (Fig. 2A). These two genes are on the same chromosome in zebrafish, mice, and humans (Fig. 2A), suggesting that Math5 and Ngn3 shared a recent common ancestor. Importantly, a recent study that employed ISH and immunohistochemistry (IHC) on serial sections of the developing pancreas showed that Ngn3 RNA is expressed in more cells than Ngn3 protein (Villasenor et al. 2008). Moreover, analogous to the phenotype of Math5 loss of function, loss of Ngn3 reduces the production of a subset of cells: the endocrine cells (Gradwohl et al. 2000; Grapin-Botton et al. 2001). These data suggested that Ngn3 might be alternatively spliced such that the majority of the isoforms of Ngn3 would not code for protein. Therefore, the AS status of Ngn3 was examined during pancreatic development by RT–PCR analysis (Fig. 2B). Again, primers in the 5′UTR and 3′UTR yielded two bands, with the lower-MW band more abundant than the higher-MW band. Sequence analysis revealed that the lower-MW isoform was alternatively spliced, such that a frameshift introduced a premature stop codon, rendering it unable to code for Ngn3 protein (Fig. 2C). Given the proximity of Ngn3 to Math5 on chromosome 10qB4, we also investigated the expression and AS status of Ngn3 in the retina and the forebrain and hindbrain. In the retina, expression of Ngn3 across development (E12.5–E17.5) was very low and was not subjected to AS. In the brain at E10.5, Ngn3 also was expressed and was alternatively spliced as in the pancreas (Fig. 2B). In all, these data suggest that the discrepancy in the amount of Ngn3 mRNA and protein is at least partly explained by AS. Like Math5, the 5′ and both of the 3′ SS were of the U2 class and are conserved 100% in rats, orangutans, and humans (Supplemental Fig. 4D).
Figure 2.
Ngn3 is alternatively spliced during development. (A) Schematic representation of the chromosomes on which the Math5 and Ngn3 are found in zebrafish, mice, and humans along with the size of the intergenic sequences. (B) A schematic showing the previous and the current gene structure of Ngn3 and the position of the primers used for RT–PCR analysis. RT–PCR analysis across three stages of pancreatic development, along with forebrain and hindbrain at E10.5 and the retina across retinal development. (C) A schematic of the Ngn3 gene with the two AS events.
Math5 and Ngn3 drive cells to exit the cell cycle
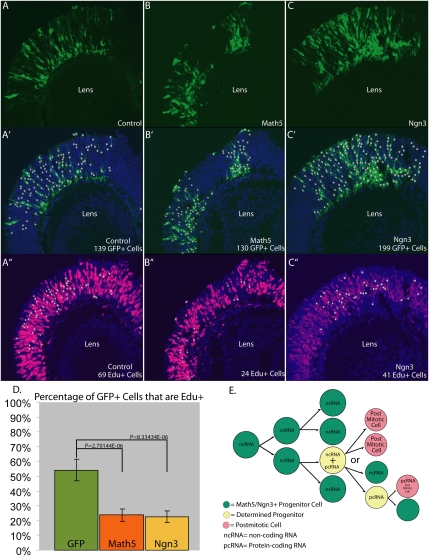
The observation that the majority of the isoforms of both Ngn3 and Math5 do not code for protein suggests that AS is employed to restrict the number of PCs expressing protein, where they might promote exit from cell cycle, as bHLH proteins have been shown to do in other systems; i.e., muscle (Crescenzi et al. 1990; Sorrentino et al. 1990; Kitzmann et al. 1998). To test whether expression of a coding isoform in PCs might indeed lead to exit from cell cycle, a Math5 or Ngn3 cDNA encoding functional protein was electroporated into E12.5 retinal explants, which are comprised almost entirely of PCs (Fig. 3A–C). The electroporated explants were cultured for 24 h and then pulsed with EdU for 1 h prior to fixation. Any GFP+ cell that is in S phase (thus, a cycling PC) should be EdU+. Confocal microscopy and quantification of all of the GFP+ cells that were EdU+ revealed that, in control explants electroporated with only GFP, at least 54% of the GFP+ cells were in S phase (Fig. 3D). However, following electroporation of Math5 or Ngn3, the number of GFP+ cells that were EdU+ was reduced to 24% and 23%, respectively (Fig. 3D). Thus, the expression of Math5 or Ngn3 protein can lead to a significant reduction in the number of PCs in S phase, which would lead to a decrease in the progenitor pool. Indeed, these data are in agreement with previous studies in which misexpression of Math5 or Xath5 (Xenopus homolog of Math5) protein in different stages of the developing Xenopus embryo produced more neurons (Brown et al. 1998). A previous experiment in which Ngn3 was expressed more broadly in early pancreatic PCs using the Ipf1/Pdx1 promoter (Apelqvist et al. 1997, 1999) showed that Ngn3 drove precocious endocrine cell production, leading to depletion of the progenitor pool. Consequently, there were no PCs left to produce cells that are born later, such as the exocrine cells (Apelqvist et al. 1999).
Figure 3.
Math5 and Ngn3 overexpression lead to reduction in PC proliferation (A–C). GFP+ cells in retinal sections of E12.5 retinal explants that were electroporated with plasmids expressing GFP (A), Math5 plus GFP (B), and Ngn3 plus GFP (C). (A′–C′) Quantification of GFP+ cells shown in A–C using the automated spot-detection (white circles) algorithm (IMARIS, Bitplane) with a diameter threshold of 5 μm and pixel intensity of 21. (A″–C″) Quantification of the number of GFP+ cells identified in A′–C′ that are also EdU+. (D) Bar graph of data in A′–C″ representing the number of GFP+ cells that were EdU+ in control (green), Math5 plus GFP (red), and Ngn3 (yellow). The P-values shown here were obtained by performing the Student's t-test. (E) A model showing how AS can regulate the number of PCs by regulating the production of noncoding RNA versus protein-coding RNA.
In conclusion, we propose a model in which PCs that express genes such as Math5 or Ngn3 use AS to reduce the level of protein found in PCs. A relatively large number of cells may transcriptionally activate such genes, but AS may have evolved as another layer of regulation to limit protein production. We predict that the regulation via AS is such that only a small subset of cells make enough protein to promote cell cycle exit. Thus, AS would regulate the balance of PC proliferation and the production of PMCs. In addition, within the cells that exit cell cycle, these transcription factors may be required for induction of a specific cell fate(s), and here again the levels might be important for this activity. Here we showed that AS is employed in early PCs of two different tissues: the retina and the pancreas. Thus, it would not be surprising if similar events were observed elsewhere in the developing embryo. Finally, if bHLH transcription factors, such as Math5 and Ngn3, with a relatively simple gene structure can employ AS, then a re-examination of the AS of other single exon genes in the mammalian genome will shed light on the depth of this mode of post-transcriptional regulation.
Materials and methods
5′RACE and 3′RACE
E15.5 retinae were harvested and used for total RNA preparation, using TriZol (Invitrogen). According to the manufacturer's protocol, 10 μg of total RNA were subjected to the 5′RACE and 3′RACE using FirstChoice RLM-RACE Kit (Ambion).
RT–PCR analysis
Retinae from E12.5 -to P0 embryos and pancreas from E10.5, E11.5, and E13.5 embryos, along with forebrain and hindbrain from E10.5 embryos, were harvested and lysed in TriZol (Invitrogen) followed by total RNA preparation. Five micrograms of total RNA were converted to cDNA by using oligo-dT (300 ng), random hexamers (300 ng) (Invitrogen), and Transcriptor (Roche) according to the manufacturers' protocols. Primers and PCR conditions used for amplifying Math5 5′UTR, 3′UTR, CDS and full length are shown in Supplemental Tables 2 and 3.
Northern blot
Total RNA (8 μg) from E15.5 retinae were run on a 1% formaldehyde-agarose gel and transferred to a nylon membrane (Amersham), and each lane was cut so that each strip could be probed with different probes. The probe sequence for the ECO form is shown in Supplemental Figure 2, and that for the CDS clone #E12-P-2 is listed in Supplemental Table 1. The blot for 5′UTR was done separately, and the probe sequence is that which is amplified by the two primers in Supplemental Table 2.
RNA ISH
Probes and protocols for section ISH using Math5 ECO form and CDS RNA, along with Nf68 RNA, were described previously (Trimarchi et al. 2007; Kanadia et al. 2008).
DISH
Retinae from E12.5 embryos without the lens were harvested and cultured for 1 h with 10 μM EdU, followed by dissociation of the retinae and fluorescence ISH as described previously (Trimarchi et al. 2007; Kanadia et al. 2008).
Immunblot analysis
Transfection of 293T cells with Math5 CDS expression constructs was performed using Lipofectamine-2000 (Invitrogen), followed by immunoblot analysis using rabbit anti-Math5 polyclonal antibody (Abcam) as described previously (Kanadia et al. 2008).
Electroporation and explant cultures of E12.5 retinae
Retinae with lenses attached were harvested at E12.5, followed by electroporation of either pCAG-GFP (1 μg/μL), pCAG-GFP (1 μg/μL) plus pCAG-Math5 (1 μg/μL), or pCAG-GFP (1 μg/μL) plus pCAG-Ngn3 (1 μg/μL) in a final volume of 60 μL of PBS (pH 7.4). Electroporation and explant culture was conducted as described previously (Matsuda and Cepko 2004). Twenty-four hours post-culture, EdU at a final concentration of 10 μM was added to the culture media. The retinae were left in EdU for 1 h, then fixed in 4% paraformaldehyde for 20 min at room temperature. The retinae were mounted for cryosections, which was followed by confocal microscopy (Leica, DMIRE2). All of the figures were processed by using IMARIS-bitplane, Adobe Photoshop, and Adobe Illustrator.
Acknowledgment
This work was funded by the Howard Hughes Medical Institute.
Footnotes
Article published online ahead of print. Article and publication date are online at http://www.genesdev.org/cgi/doi/10.1101/gad.1847110.
Supplemental material is available at http://www.genesdev.org.
References
- Apelqvist A, Ahlgren U, Edlund H. Sonic hedgehog directs specialised mesoderm differentiation in the intestine and pancreas. Curr Biol. 1997;7:801–804. doi: 10.1016/s0960-9822(06)00340-x. [DOI] [PubMed] [Google Scholar]
- Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- Black DL. Protein diversity from alternative splicing: A challenge for bioinformatics and post-genome biology. Cell. 2000;103:367–370. doi: 10.1016/s0092-8674(00)00128-8. [DOI] [PubMed] [Google Scholar]
- Brown NL, Kanekar S, Vetter ML, Tucker PK, Gemza DL, Glaser T. Math5 encodes a murine basic helix-loop-helix transcription factor expressed during early stages of retinal neurogenesis. Development. 1998;125:4821–4833. doi: 10.1242/dev.125.23.4821. [DOI] [PubMed] [Google Scholar]
- Brown NL, Patel S, Brzezinski J, Glaser T. Math5 is required for retinal ganglion cell and optic nerve formation. Development. 2001;128:2497–2508. doi: 10.1242/dev.128.13.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlison JS, Long Q, Fujitani Y, Wright CV, Magnuson MA. Pdx-1 and Ptf1a concurrently determine fate specification of pancreatic multipotent progenitor cells. Dev Biol. 2008;316:74–86. doi: 10.1016/j.ydbio.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper TA, Wan L, Dreyfuss G. RNA and disease. Cell. 2009;136:777–793. doi: 10.1016/j.cell.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crescenzi M, Fleming TP, Lassar AB, Weintraub H, Aaronson SA. MyoD induces growth arrest independent of differentiation in normal and transformed cells. Proc Natl Acad Sci. 1990;87:8442–8446. doi: 10.1073/pnas.87.21.8442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grapin-Botton A, Majithia AR, Melton DA. Key events of pancreas formation are triggered in gut endoderm by ectopic expression of pancreatic regulatory genes. Genes & Dev. 2001;15:444–454. doi: 10.1101/gad.846001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanadia RN, Johnstone KA, Mankodi A, Lungu C, Thornton CA, Esson D, Timmers AM, Hauswirth WW, Swanson MS. A muscleblind knockout model for myotonic dystrophy. Science. 2003;302:1978–1980. doi: 10.1126/science.1088583. [DOI] [PubMed] [Google Scholar]
- Kanadia RN, Shin J, Yuan Y, Beattie SG, Wheeler TM, Thornton CA, Swanson MS. Reversal of RNA missplicing and myotonia after muscleblind overexpression in a mouse poly(CUG) model for myotonic dystrophy. Proc Natl Acad Sci. 2006;103:11748–11753. doi: 10.1073/pnas.0604970103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanadia RN, Clark VE, Punzo C, Trimarchi JM, Cepko CL. Temporal requirement of the alternative-splicing factor Sfrs1 for the survival of retinal neurons. Development. 2008;135:3923–3933. doi: 10.1242/dev.024620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay JN, Finger-Baier KC, Roeser T, Staub W, Baier H. Retinal ganglion cell genesis requires lakritz, a zebrafish atonal homolog. Neuron. 2001;30:725–736. doi: 10.1016/s0896-6273(01)00312-9. [DOI] [PubMed] [Google Scholar]
- Kitzmann M, Carnac G, Vandromme M, Primig M, Lamb NJ, Fernandez A. The muscle regulatory factors MyoD and myf-5 undergo distinct cell cycle-specific expression in muscle cells. J Cell Biol. 1998;142:1447–1459. doi: 10.1083/jcb.142.6.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TT, Wroblewski E, Patel S, Riesenberg AN, Brown NL. Math5 is required for both early retinal neuron differentiation and cell cycle progression. Dev Biol. 2006;295:764–778. doi: 10.1016/j.ydbio.2006.03.055. [DOI] [PubMed] [Google Scholar]
- Licatalosi DD, Darnell RB. Splicing regulation in neurologic disease. Neuron. 2006;52:93–101. doi: 10.1016/j.neuron.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Cepko CL. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc Natl Acad Sci. 2004;101:16–22. doi: 10.1073/pnas.2235688100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips AV, Timchenko LT, Cooper TA. Disruption of splicing regulated by a CUG-binding protein in myotonic dystrophy. Science. 1998;280:737–741. doi: 10.1126/science.280.5364.737. [DOI] [PubMed] [Google Scholar]
- Saul SM, Brzezinski J, IV, Altschuler RA, Shore SE, Rudolph DD, Kabara LL, Halsey KE, Hufnagel RB, Zhou J, Dolan DF, et al. Math5 expression and function in the central auditory system. Mol Cell Neurosci. 2008;37:153–169. doi: 10.1016/j.mcn.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwitzgebel VM, Scheel DW, Conners JR, Kalamaras J, Lee JE, Anderson DJ, Sussel L, Johnson JD, German MS. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000;127:3533–3542. doi: 10.1242/dev.127.16.3533. [DOI] [PubMed] [Google Scholar]
- Sommer L, Ma Q, Anderson DJ. neurogenins, a novel family of atonal-related bHLH transcription factors, are putative mammalian neuronal determination genes that reveal progenitor cell heterogeneity in the developing CNS and PNS. Mol Cell Neurosci. 1996;8:221–241. doi: 10.1006/mcne.1996.0060. [DOI] [PubMed] [Google Scholar]
- Sorrentino V, Pepperkok R, Davis RL, Ansorge W, Philipson L. Cell proliferation inhibited by MyoD1 independently of myogenic differentiation. Nature. 1990;345:813–815. doi: 10.1038/345813a0. [DOI] [PubMed] [Google Scholar]
- Trimarchi JM, Stadler MB, Roska B, Billings N, Sun B, Bartch B, Cepko CL. Molecular heterogeneity of developing retinal ganglion and amacrine cells revealed through single cell gene expression profiling. J Comp Neurol. 2007;502:1047–1065. doi: 10.1002/cne.21368. [DOI] [PubMed] [Google Scholar]
- van Eyll JM, Passante L, Pierreux CE, Lemaigre FP, Vanderhaeghen P, Rousseau GG. Eph receptors and their ephrin ligands are expressed in developing mouse pancreas. Gene Expr Patterns. 2006;6:353–359. doi: 10.1016/j.modgep.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Villasenor A, Chong DC, Cleaver O. Biphasic Ngn3 expression in the developing pancreas. Dev Dyn. 2008;237:3270–3279. doi: 10.1002/dvdy.21740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SW, Kim BS, Ding K, Wang H, Sun D, Johnson RL, Klein WH, Gan L. Requirement for math5 in the development of retinal ganglion cells. Genes & Dev. 2001;15:24–29. doi: 10.1101/gad.855301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP, Burge CB. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456:470–476. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]