Abstract
The cJun N-terminal kinase 1 (JNK1) is implicated in diet-induced obesity. Indeed, germline ablation of the murine Jnk1 gene prevents diet-induced obesity. Here we demonstrate that selective deficiency of JNK1 in the murine nervous system is sufficient to suppress diet-induced obesity. The failure to increase body mass is mediated, in part, by increased energy expenditure that is associated with activation of the hypothalamic–pituitary–thyroid axis. Disruption of thyroid hormone function prevents the effects of nervous system JNK1 deficiency on body mass. These data demonstrate that the hypothalamic–pituitary–thyroid axis represents an important target of metabolic signaling by JNK1.
Keywords: JNK1, obesity, insulin resistance, thyroid hormone
Human obesity represents a serious world-wide health problem. One consequence of obesity is the development of insulin resistance, hyperglycemia, and metabolic syndrome that can lead to β-cell dysfunction and type 2 diabetes (Kahn et al. 2006). It is therefore important that we gain an understanding of the physiology and pathophysiology of the development of obesity, because this knowledge represents a basis for the design of potential therapeutic interventions.
The cJun N-terminal kinase 1 (JNK1) represents one signaling pathway that has been implicated in diet-induced obesity (Weston and Davis 2007). JNK1 is activated when mice are fed a high-fat diet (HFD) (Hirosumi et al. 2002). Moreover, Jnk1−/− mice are protected against HFD-induced weight gain (Hirosumi et al. 2002). The mechanism that accounts for the effect of germline JNK1 deficiency to control body weight is unclear. Tissue-specific deficiency of JNK1 in fat, muscle, liver, and myeloid cells does not affect HFD-induced weight gain (Sabio et al. 2008, 2009, 2010). A different organ must therefore play a major role in the diet-induced regulation of body weight by JNK1. The brain represents a possible site of JNK1 function because the hypothalamus and pituitary gland are known to regulate metabolism, including feeding behavior, physical activity, and energy expenditure (Lenard and Berthoud 2008).
The purpose of this study was to investigate the role of JNK1 in the brain. Our approach was to examine the effect of selective ablation of the Jnk1 gene in the mouse nervous system. We found that HFD-fed control (NWT) mice gained substantially greater body weight than JNK1-deficient (NKO) mice. The decreased weight gain by NKO mice was accounted for by decreased food intake, increased physical activity, and increased energy expenditure. These changes were associated with increased amounts of thyroid hormone in the blood and increased expression of thyroid hormone-responsive genes in target tissues. Importantly, pharmacological inhibition of thyroid hormone markedly attenuated NKO phenotypes. These data demonstrate that the hypothalamic–pituitary–thyroid axis is a major target of the JNK1 signaling pathway that regulates metabolism.
Results
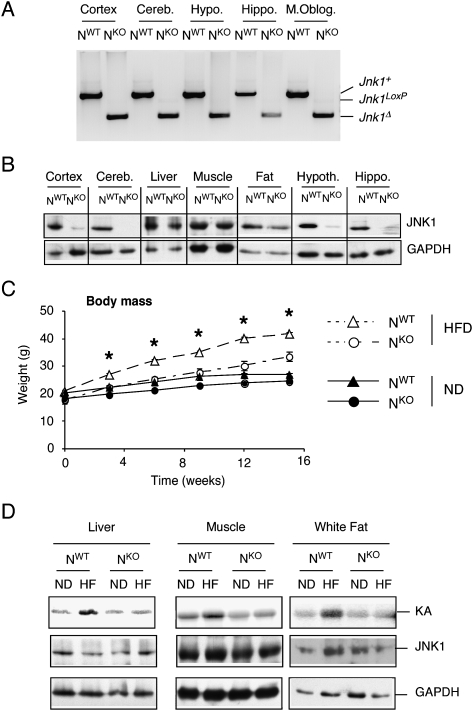
To investigate the role of JNK1 in the nervous system, we created compound mutant mice (Nestin-cre Jnk1LoxP/LoxP) with a selective defect in the expression of JNK1. Genotype analysis of NWT and NKO mice demonstrated that the Jnk1LoxP allele was efficiently deleted in the nervous system of NKO mice (Fig. 1A). Thus, the Jnk1 gene was ablated in all regions of the CNS of NKO mice that we examined, including the cortex, cerebellum, hypothalamus, hippocampus, and medulla oblongata (Fig. 1A). Immunoblot analysis demonstrated markedly reduced JNK1 protein in these subregions of the brain and normal amounts of JNK1 in liver, muscle, and adipose tissue (Fig. 1B). Control studies demonstrated that the Jnk1 gene was not deleted in β cells of the Islets of Langerhans in NKO mice (Supplemental Fig. S1). These data indicate that NKO mice exhibit a tissue-specific defect in JNK1 expression. NKO mice therefore represent a model for the analysis of nervous system-specific JNK1 deficiency.
Figure 1.
Creation of mice with nervous system-specific JNK1 deficiency. (A) Genotype analysis of genomic DNA isolated from the cortex, cerebellum (Cereb.), hippocampus (Hippo.), hypothalamus (Hypoth.), and medulla oblongata (M. Oblog.) of Nes-Cre+ Jnk1+/+ (NWT) mice and Nes-Cre+ Jnk1LoxP/LoxP (NKO) mice was performed to detect the presence of Jnk1+, Jnk1LoxP, and Jnk1Δ alleles. (B) Extracts prepared from the cortex, cerebellum, liver, muscle (quadriceps), fat (epididymal adipose tissue), hypothalamus, and hippocampus of NWT and NKO mice were examined using immunoblot analysis by probing with antibodies to JNK1 and GAPDH. (C) NWT and NKO male mice (8–10 wk old) were fed either a chow diet (ND) or a HFD (16 wk). The weight of the mice was measured (mean ± SD; n = 10). The HFD-induced weight gain of NWT was significantly greater than NKO mice (P < 0.05). (D) NWT and NKO mice were fed a chow diet (ND) or a HFD for 16 wk. JNK activity in the liver, quadriceps muscle, and epididymal adipose tissue was measured in a protein kinase assay (KA) assay using cJun and [γ-32P]ATP as substrates. The cell extracts used for the protein kinase assay were also examined by immunoblot analysis by probing with antibodies to JNK1 and GAPDH.
Nervous system JNK1 is required for HFD-induced weight gain
It has been established that HFD-fed Jnk1−/− mice exhibit a severe defect in the development of diet-induced obesity (Hirosumi et al. 2002). However, selective JNK1 deficiency in adipose tissue, liver, muscle, or myeloid cells caused no defect in HFD-induced obesity (Sabio et al. 2008, 2009, 2010). These findings indicate that JNK1 function in another organ accounts for the effects of whole-body JNK1 deficiency to suppress HFD-induced weight gain. We therefore tested whether nervous system-specific JNK1 deficiency might prevent HFD-induced weight gain. Comparison of chow-fed and HFD-fed NWT and NKO mice demonstrated that nervous system-specific JNK1 deficiency markedly reduced weight gain caused by a HFD (Fig. 1C).
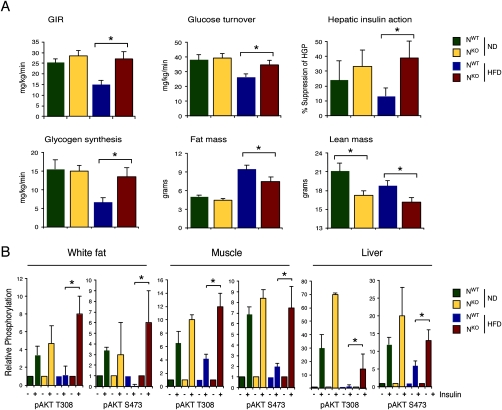
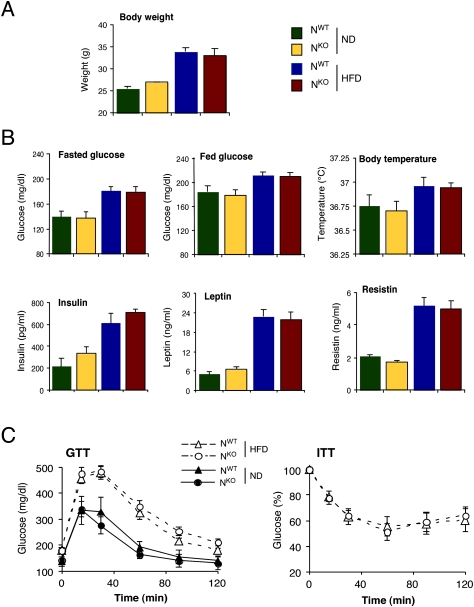
Examination of organ mass at necropsy indicated a significant reduction in the weight of epididymal white fat, intrascapular brown fat, quadriceps muscle, and liver in HFD-fed NKO mice compared with NWT mice (Supplemental Fig. S2). In contrast, no significant difference in heart mass was detected between NKO and NWT mice (Supplemental Fig. S2). Measurement of lean and fat mass using 1H-MRS indicated that, while reduced fat accumulation by NKO mice was detected, the NKO mice also exhibited reduced lean mass compared with NWT mice (Fig. 2A). These data suggest that the defect in HFD-induced weight gain observed in NKO mice was due to a reduction in both fat and lean body mass.
Figure 2.
Effect of nervous system-specific JNK1 deficiency on insulin sensitivity. (A) Insulin sensitivity was measured using a hyperinsulinemic–euglycemic clamp in conscious NKO and NWT mice. The steady-state glucose infusion rate (GIR), whole-body glucose turnover, hepatic insulin action, and whole-body glycogen plus lipid synthesis are presented. Fat and lean body mass were measured by 1H-MRS. The data presented are the mean ± SE for six to approximately eight experiments. Statistically significant differences between NKO mice and NWT mice are indicated ([*] P < 0.05). (B) Chow-fed (ND) and HFD-fed NWT and NKO mice were treated by intraperitoneal injection of insulin (1.5 U/kg body mass). Extracts prepared from epididymal adipose tissue, quadriceps muscle, and liver at 15 min post-injection were examined by immunoblot analysis with antibodies to phospho-AKT (Thr-308 and Ser-473) and AKT. Quantitation was performed using the Odyssey infrared imaging system (LI-COR Biosciences). The data presented are the mean ± SD (n = 3). Statistically significant differences between NKO mice and NWT mice are indicated ([*] P < 0.05).
The resistance to weight gain in HFD-fed NKO mice may account for the finding that HFD-induced JNK activation in adipose tissue, muscle, and liver of NWT mice was not detected in NKO mice (Fig. 1D).
JNK1 deficiency increases insulin sensitivity
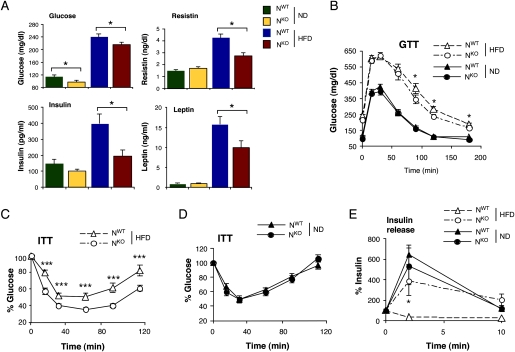
The hyperglycemia and hyperinsulinemia caused by feeding a HFD to NWT mice was significantly reduced in HFD-fed NKO mice (Fig. 3A). Similarly, the HFD-induced increase in the blood concentration of adipokines (leptin, resistin, and IL6) was markedly attenuated in HFD-fed NKO mice (Fig. 3A; Supplemental Fig. S3). Consistent with these observations, HFD-fed NKO mice were more glucose-tolerant (Fig. 3B), more responsive in an insulin tolerance test (Fig. 3C), and exhibited increased glucose-induced insulin release (Fig. 3E) compared with HFD-fed NWT mice. These data indicate that HFD-fed NKO mice show increased insulin sensitivity and improved β-cell function compared with HFD-fed NWT mice. To confirm this conclusion, we conducted a hyperinsulinemic–euglycemic clamp study in conscious mice. This analysis demonstrated significant increases in steady-state glucose infusion rate, insulin-stimulated whole-body glucose turnover, glycogen plus lipid synthesis, and hepatic insulin action in HFD-fed NKO mice compared with HFD-fed NWT mice (Fig. 2A). These data confirmed that HFD-fed NKO mice are more insulin-sensitive than HFD-fed NWT mice.
Figure 3.
JNK1 deficiency in the nervous system partially protects mice against the metabolic effects of feeding a HFD. (A) Blood glucose concentration in chow-fed (ND) and HFD-fed NWT and NKO mice fasted overnight. The blood concentration of insulin, resistin, and leptin in mice fasted overnight is also presented. The data represent the mean ± SD (n = 10). Statistically significant differences between NKO and NWT are indicated ([*] P < 0.05). (B) Glucose tolerance tests (GTT) on chow-fed (ND) and HFD-fed NWT and NKO mice were performed by measurement of blood glucose concentration in animals following intraperitoneal injection of glucose (1 g/kg). The data presented represent the mean ± SD (n = 10∼15). Statistically significant differences between NKO and NWT are indicated ([*] P < 0.05). (C,D) Insulin tolerance tests (ITT) on NWT and NKO mice fed either a chow diet (ND) or a HFD were performed by intraperitoneal injection of insulin (1.5 U/kg body mass). The concentration of blood glucose was measured (mean ± SD; n = 10). Statistically significant differences between NKO and NWT are indicated ([***] P < 0.001). (E) Glucose-induced insulin release. The effect of administration of glucose (2 g/kg body mass) by intraperitoneal injection on blood insulin concentration was examined (mean ± SD; n = 13∼15). Statistically significant differences between NWT and NKO mice are indicated ([*] P < 0.05).
To obtain biochemical evidence for insulin sensitivity, we examined insulin-stimulated AKT activation in adipose tissue, muscle, and liver of NKO and NWT mice (Fig. 2B). Insulin treatment increased AKT activation in chow-fed NKO and NWT mice. Insulin-stimulated AKT activation was suppressed in adipose tissue, muscle, and liver of HFD-fed NWT mice, demonstrating insulin resistance in these organs (Fig. 2B). In contrast, studies of NKO mice demonstrated that the HFD did not inhibit insulin-stimulated AKT activation in adipose tissue and muscle, and only partially suppressed AKT activation in liver (Fig. 2B). These data strongly support the conclusion that NKO mice exhibit protection against HFD-induced insulin resistance. This finding is consistent with the observation that NKO mice did not gain weight in response to feeding a HFD (Fig. 1C).
JNK1 deficiency reduces food intake
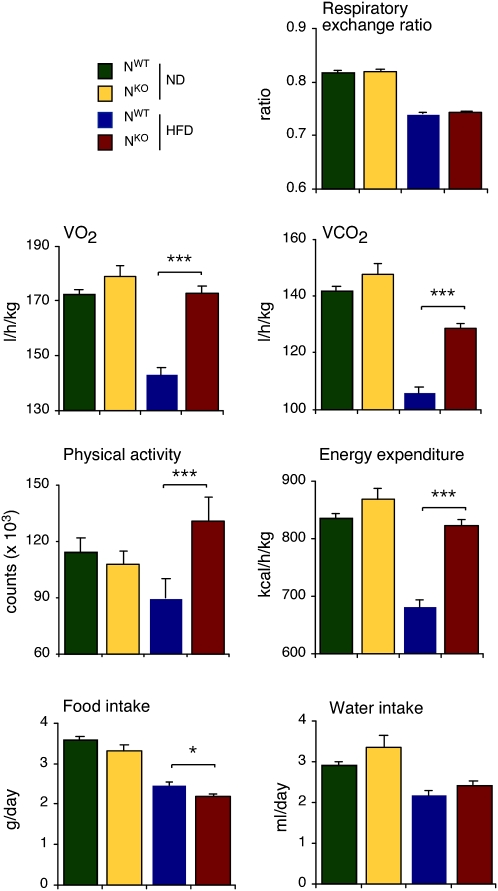
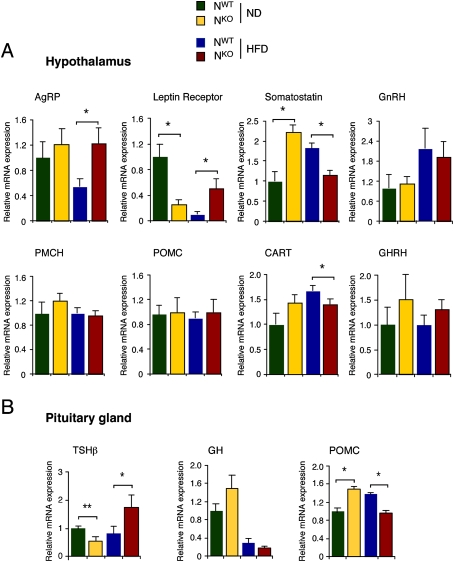
Metabolic cage analysis demonstrated that HFD-fed NKO mice consumed slightly less food than HFD-fed NWT mice (Fig. 4). This decrease in food intake may contribute to the failure of HFD-fed NKO mice to gain weight. We did not detect changes in the expression of hypothalamic neuropeptides that regulate satiety (agouti-related protein [AgRP], cocaine and amphetamine-regulated transcript [CART], promelanin-concentrating hormone [PMCH], and pro-opiomelanocortin [POMC]) that might account for decreased feeding behavior by HFD-fed NKO mice (Fig. 5A). However, increased leptin signaling might contribute to the observed reduction in food intake. The adipokine leptin acts on multiple subsets of neurons in the CNS to suppress feeding behavior (Myers et al. 2009). The blood leptin concentration was reduced in HFD-fed NKO mice compared with HFD-fed NWT mice (Fig. 3A). Nevertheless, increased hypothalamic leptin signaling in HFD-fed NKO mice could mediate increased leptin sensitivity. It has been established that leptin receptor expression is regulated by blood leptin concentration and obesity (Townsend et al. 2008). Indeed, leptin receptor expression is down-regulated in HFD-fed mice (Townsend et al. 2008). However, feeding a HFD did not down-regulate leptin receptor expression in the hypothalamus of HFD-fed NKO mice (Fig. 5A). This failure of leptin receptor down-regulation may contribute to decreased feeding by HFD-fed NKO mice compared with HFD-fed NWT mice.
Figure 4.
Comparison of energy balance of NWT and NKO mice using metabolic cages. Mice were examined during a 3-d period to measure the food and water consumption, gas exchange (VO2 and VCO2), respiratory exchange quotient [VCO2]/[VO2 ], energy expenditure, and physical activity (mean ± SE, n = 6). Statistically significant differences between NKO mice and NWT mice are indicated ([*] P < 0.05; [**] P < 0.01; [***] P < 0.001).
Figure 5.
Gene expression in the hypothalamus and pituitary gland of NWT and NKO mice. (A) NWT and NKO mice were fed a chow diet (ND) or a HFD (16 wk) and then fasted overnight. The expression of AgRP, leptin receptor, somatostatin, gonadotrophin-releasing hormone (GnRH), PMCH, POMC, CART, and GH-releasing hormone (GHRH) mRNA in the hypothalamus was measured by quantitative RT–PCR. The data were normalized to the expression of Gapdh mRNA in each sample. The data are presented as the mean ± SD (n = 6). Statistically significant differences between NWT and NKO mice are indicated ([*] P < 0.05). (B) The expression of TSHβ, GH, and POMC mRNA in the pituitary gland was measured by quantitative RT–PCR. The data were normalized to the expression of Gapdh mRNA in each sample. The data are presented as the mean ± SD (n = 6). Statistically significant differences between NWT and NKO mice are indicated ([*] P < 0.05; [**] P < 0.01).
JNK1 deficiency increases energy expenditure
We performed metabolic cage analysis of NKO and NWT mice to determine the effects of nervous system-specific JNK1 deficiency on energy balance (Fig. 4). No difference in the respiratory exchange quotient ([VCO2]/[VO2]) between NKO and NWT mice was detected. However, the HFD-fed NKO mice exhibited a large increase in physical activity and energy expenditure compared with HFD-fed NWT mice (Fig. 4). This effect of JNK1 deficiency to cause increased energy expenditure and physical activity is likely to be a major determinant of JNK1-regulated obesity, and may largely account for the failure of HFD-fed NKO mice to gain weight.
JNK1 deficiency engages the hypothalamic–pituitary–thyroid axis
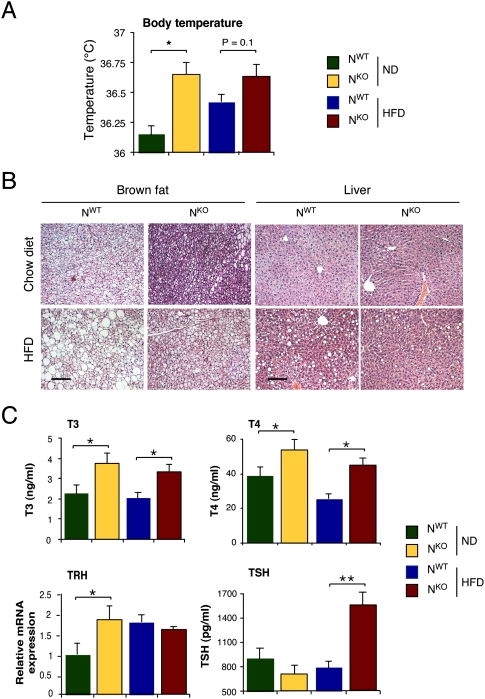
We found that NKO mice exhibited elevated body temperature (Fig. 6A) that was associated with a reduction in lipid accumulation by brown fat and liver in HFD-fed NKO mice compared with HFD-fed NWT mice (Fig. 6B). Gene expression analysis demonstrated that NKO mice expressed larger amounts of mRNA derived from thyroid hormone target genes (Supplemental Fig. S5; Obregon 2008). These data indicate that the thyroid hormone pathway is activated in NKO mice. Indeed, increased levels of T4 and T3 were detected in the blood of NKO mice compared with NWT mice (Fig. 6C). This change was associated with increased expression of thyrotropin-releasing hormone (Trh) mRNA in the hypothalamus of chow-fed NKO mice (Fig. 6C), increased expression of thyroid-stimulating hormone (Tsh) mRNA in the pituitary gland (Fig. 5B), and increased TSH protein in the blood of HFD-fed NKO mice (Fig. 6C). TSH and TRH expression are subject to acute negative feedback regulation by thyroid hormone (Björkman and Ekholm 2000). The presence of high levels of T4 and T3 in the blood of NKO mice under conditions where TSH and TRH expression are elevated suggests that brain JNK1 deficiency disrupts the normal negative feedback regulation of the hypothalamic–pituitary axis.
Figure 6.
Activation of the hypothalamic–pituitary–thyroid hormone axis in NKO mice. (A) The body temperature of chow-fed (ND) and HFD-fed NKO and NWT mice are presented (mean ± SD; n = 8). Statistically significant differences between NKO and NWT mice are indicated ([*] P < 0.05). (B) Sections prepared from intrascapular brown fat and liver of chow-fed (ND) and HFD-fed NKO and NWT mice were stained with hematoxylin and eosin. Bar, 100 μm. (C) The concentration of T3, T4, and TSH in the blood of chow-fed (ND) and HFD-fed NKO and NWT mice was measured by ELISA (mean ± SD, n = 10). The amount of Trh mRNA in the hypothalamus was measured by quantitative RT–PCR analysis and was normalized to the amount of Gapdh mRNA measured in each sample (mean ± SD; n = 6∼7). Statistically significant differences between NKO and NWT mice are indicated ([*] P < 0.05; [**] P < 0.01).
The effect of JNK1 deficiency on TSH expression suggests that brain-specific JNK1 knockout mice may have altered pituitary function. Indeed, HFD-fed NKO mice were found to have decreased amounts of adrenocorticotropic hormone (ACTH) and increased amounts of growth hormone (GH) in the blood compared with HFD-fed NWT mice (Supplemental Fig. S4). These data support the conclusion that JNK1 is required for normal pituitary function.
The hypothalamic–pituitary–thyroid axis contributes to metabolic regulation by JNK1
To test whether increased thyroid hormone signaling was causally related to the defect in HFD-induced weight gain in NKO mice, we examined the effect of treatment of mice with propylthiouracil (PTU), a drug that inhibits thyroperoxidase and prevents T4 production by the thyroid gland (Björkman and Ekholm 2000). We treated NKO and NWT mice with PTU in the drinking water and examined the effect of feeding a chow diet or a HFD. Analysis of intrascapular brown fat demonstrated that PTU treatment suppressed the increased expression of thyroid hormone-responsive genes in NKO mice (Supplemental Fig. S5). These data demonstrate that PTU treatment represents an effective model to study the role of thyroid hormone signaling in NKO and NWT mice. We found that the PTU-treated NKO and NWT mice showed similar increases in body weight when fed a HFD (Fig. 7A; Supplemental Fig. S6). No significant differences in glucose, insulin, and adipokine (leptin and resistin) concentrations in the blood or body temperature between PTU-treated NKO and NWT mice were detected (Fig. 7B). Similarly, no significant differences between PTU-treated NKO and NWT mice were detected in glucose and insulin tolerance tests (Fig. 7C). Together, these data demonstrate that inhibition of thyroid hormone by PTU treatment markedly suppressed the metabolic phenotypes of NKO mice. This analysis supports the conclusion that increased thyroid hormone contributes to the metabolic phenotype of NKO mice.
Figure 7.
Disruption of thyroid hormone signaling prevents the effects of nervous system-specific JNK1 deficiency on HFD-induced weight gain. (A) NKO and NWT mice were treated with PTU in the drinking water. The mice were divided into chow-fed (ND) and HFD-fed groups after 2 wk, and then maintained for an additional 10 wk. The body weight of the mice is presented. No statistically significant differences between NKO and NWT mice were detected (P > 0.05). (B) Chow-fed (ND) and HFD-fed NKO and NWT mice were examined after 12 wk of treatment with PTU. The blood glucose concentration in fed and overnight fasted mice, body temperature, and the blood hormone (insulin, leptin, and resistin) concentrations are presented. No significant differences between NKO and NWT mice were detected (P > 0.05). (C) Glucose tolerance tests (GTT) on PTU-treated chow-fed (ND) and HFD-fed NKO and NWT mice are presented. No significant differences between NKO and NWT mice were detected (P > 0.05). Insulin tolerance tests (ITT) on PTU-treated HFD-fed NKO and NWT mice are presented. No significant differences between NKO and NWT mice were detected (P > 0.05).
To test the contribution of thyroid hormone to the phenotype of whole-body JNK1 knockout mice, we examined the effect of PTU treatment on Jnk1−/− mice. We found that PTU treatment significantly suppressed the effect of whole-body JNK1 deficiency on HFD-induced weight gain, hyperglycemia, glucose intolerance, insulin sensitivity, and glucose-induced insulin release (Supplemental Figs. S7, S8). However, the PTU treatment caused greater suppression of the metabolic phenotype of NKO mice than Jnk1−/− mice, consistent with metabolic roles of JNK1 in both neuronal and nonneuronal tissues.
Discussion
The mechanism of obesity-induced insulin resistance, metabolic syndrome, and type 2 diabetes may involve the JNK1 signaling pathway. Thus, mouse studies have demonstrated that treatment with JNK inhibitors can reduce hyperglycemia and improve insulin sensitivity (Bennett et al. 2003; Kaneto et al. 2004; Stebbins et al. 2008). Moreover, Jnk1−/− mice are protected against the development of HFD-induced obesity and insulin resistance (Hirosumi et al. 2002). Analysis of tissue-specific JNK1 knockout mice demonstrates that JNK1 deficiency in adipose tissue, muscle, liver, or myeloid cells does not prevent HFD-induced weight gain (Sabio et al. 2008, 2009, 2010). Nevertheless, adipose tissue-specific NKO mice do exhibit defects in HFD-induced insulin resistance in adipose tissue and liver (Sabio et al. 2008). Moreover, muscle-specific NKO mice are protected against HFD-induced muscle insulin resistance (Sabio et al. 2010). These observations demonstrate that JNK1 can regulate insulin resistance independently of the effects of JNK1 on HFD-induced weight gain. In addition, this analysis established that JNK1-regulated insulin resistance involves more than one mechanism, including JNK1-regulated expression of inflammatory cytokines (Sabio et al. 2008), JNK1-regulated expression of lipoprotein lipase (Sabio et al. 2010), and JNK1-mediated negative regulatory phosphorylation of the insulin receptor adapter protein IRS1 (Aguirre et al. 2000; Hirosumi et al. 2002; Lee et al. 2003; Sabio et al. 2008, 2010).
JNK1 deficiency in the nervous system is sufficient to protect mice against weight gain caused by feeding a HFD. The weight gain of NWT mice is mediated, in part, by reduced physical activity and energy expenditure. In contrast, feeding a HFD to mice with JNK1 deficiency in the nervous system (NKO mice) does not cause decreased physical activity and energy expenditure. This maintenance of physical activity and energy expenditure in HFD-fed NKO mice contributes to the failure of these mice to gain weight when fed a HFD.
The increased energy expenditure in NKO mice is mediated, in part, by activation of the hypothalamic–pituitary–thyroid axis. This conclusion is based on several lines of evidence, including increased body temperature, increased expression of thyroid hormone-induced genes, and increased amounts of T4 and T3 in the blood of NKO mice compared with NWT mice. Moreover, pharmacological inhibition of thyroid hormone production abolished the metabolic phenotypes of NKO mice, including marked suppression of HFD-induced weight gain. These data identify the hypothalamic–pituitary–thyroid axis as an important target of the metabolic actions of JNK1.
The thyroid hormone pathway is negatively regulated by JNK1. The increased amount of T4 and T3 in the blood of NKO mice compared with NWT mice correlates with increased expression of hypothalamic TRH and pituitary gland TSH. These changes in TRH and TSH expression were unexpected because thyroid hormone exerts powerful negative feedback control of TRH and TSH expression (Björkman and Ekholm 2000). The association of increased T4 and T3 in the blood with increased expression of TRH and TSH in NKO mice indicates that JNK1 deficiency in the brain disrupts the normal negative feedback control of the hypothalamic–pituitary–thyroid axis. An important goal for future studies will be to determine the molecular mechanism of JNK1 regulation of the hypothalamic–pituitary–thyroid axis.
In conclusion, this study demonstrates that JNK1 deficiency in the nervous system is sufficient to account for the role of JNK1 in the regulation of HFD-induced weight gain. This knowledge has important implications for the design of novel therapeutic interventions in the treatment of diet-induced obesity.
Materials and methods
Mice
We previously described Jnk1−/− mice (Dong et al. 1998) and Jnk1LoxP/LoxP mice (Das et al. 2007). Nes-Cre mice (Tronche et al. 1999) were obtained from The Jackson Laboratories, and Rip-CreESR mice (Dor et al. 2004) were obtained from D. Melton (Harvard University). The mice were backcrossed to the C57BL/6J strain (The Jackson Laboratories) and were housed in facilities accredited by the American Association for Laboratory Animal Care (AALAC). The mice were genotyped by PCR analysis of genomic DNA (Das et al. 2007). All studies were performed using male mice (8–24 wk old). The mice were treated with PTU in the drinking water (cherry-flavored Kool-Aid supplemented without or with 1.2 mM PTU [Sigma]). Body temperature was measured using a Microtherma 2 Type “T” Thermometer (Braintree Scientific, Inc.). Rip-CreESR mice were treated with 1 mg of 4-hydroxytamoxifen (Sigma) by intraperitoneal injection once each day for five consecutive days. The animal studies were approved by the Institutional Animal Care and Use Committees of the University of Massachusetts Medical School and the Pennsylvania State University College of Medicine.
RNA analysis
The expression of mRNA was examined by quantitative PCR analysis using a 7500 Fast Real-Time PCR machine. TaqMan assays were used to quantitate Accβ (Mm01204683_m1), Agrp (Mm00475829_g1), Cart (Mm00489086_m1), Ghrh (Mm01250745_m1), Gnrh (Mm01315605_m1), Gh (Mm00433590-g1), Glut4 (Mm00436615-m1), Ldhβ (Mm00493146_m1), Leptin receptor (Mm00434759_m1), Pck1 (Mm00440636_m1), Pmch (Mm242886_g1), Pomc (Mm00599949_m), Somatostatin (Mm 00436671_m1), Spot14 (Mm01273967_m1), Trh (Mm01963590_s1), Tshβ (Mm00437190_m1), and Ucp1 (Mm01244861-m1). The relative mRNA expression was normalized by measurement of the amount of Gapdh mRNA (#4352339E) in each sample using TaqMan assays (Applied Biosystems).
Isolation of pancreatic islets
Murine pancreatic islets were isolated using methods described previously (Mangada et al. 2009).
Immunoblot analysis
Tissue extracts were prepared using Triton lysis buffer (20 mM Tris at pH 7.4, 1% Triton X-100, 10% glycerol, 137 mM NaCl, 2 mM EDTA, 25 mM β-glycerophosphate, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 10 μg/mL of aprotinin and leupeptin). Extracts (20–50 μg of protein) were examined by protein immunoblot analysis by probing with antibodies to AKT, phospho-Thr308 AKT, and phospho-Ser473 AKT (Cell Signaling); and JNK1 and GAPDH (Santa Cruz Biotechnologies). Immunocomplexes were detected by enhanced chemiluminescence (NEN). Quantitation of immunoblots was performed using the Odyssey infrared imaging system (LI-COR Biosciences).
Measurement of blood glucose, adipokine, cytokine, and insulin concentration
Blood glucose was measured with an Ascensia Breeze 2 glucometer (Bayer). Adipokines, cytokines, and insulin in plasma were measured by ELISA using a Luminex 200 machine (Millipore).
Glucose and insulin tolerance tests
The mice were fed a standard chow diet or a HFD (Iso Pro 3000, Purina; and F3282, Bioserve, Inc.) for 16 wk. Glucose and insulin tolerance tests were performed using methods described previously (Mora et al. 2005).
Protein kinase assays
JNK activity was measured using an in vitro protein kinase assay with the substrates cJun and [γ-32P]ATP as substrates (Whitmarsh and Davis 2001).
Hyperinsulinemic–euglycemic clamp studies
The clamp studies were performed at the University of Massachusetts Mouse Phenotyping Center. Briefly, mice were fed a HFD diet (55% fat by calories; Harlan Teklad) or chow diet for 3 wk, and whole-body fat and lean mass were noninvasively measured using 1H-MRS (Echo Medical Systems). Following an overnight fast, a 2-h hyperinsulinemic–euglycemic clamp was conducted in conscious mice with a primed and continuous infusion of human insulin (150 mU/kg body weight priming followed by 2.5 mU/kg/min; Humulin; Eli Lilly), and 20% glucose was infused at variable rates to maintain euglycemia (Kim et al. 2004). Whole-body glucose turnover was assessed with a continuous infusion of [3-3H]glucose, and 2-deoxy-D-[1-14C]glucose (PerkinElmer) was administered as a bolus (10 μCi) at 75 min after the start of the clamps to measure insulin-stimulated glucose uptake in individual organs. At the end of the clamps, mice were anesthetized, and tissues were taken for biochemical analysis (Kim et al. 2004).
Metabolic cages
Mice were housed under controlled temperature and lighting with free access to food and water. The food/water intake, energy expenditure, respiratory exchange ratio, and physical activity were performed (3 d) using metabolic cages (TSE Systems).
Analysis of tissue sections
Histology was performed using tissue fixed in 10% formalin for 24 h, dehydrated, and embedded in paraffin. Sections (7 μm) were cut and stained using hematoxylin and eosin (American Master Tech Scientific).
Statistical analysis
Differences between groups were examined for statistical significance using the Student's test or analysis of variance (ANOVA) with the Fisher's test.
Acknowledgments
We thank D. Greiner for assistance with the isolation of pancreatic islets, J. Leonard for expert advice, D. Melton for providing Rip-CreESR mice, M. Das for providing Jnk1LoxP mice, V. Benoit and J-H. Liu for technical assistance, and K. Gemme for administrative assistance. These studies were supported by grants from the National Institutes of Health (CA65861 to R.J.D., and DK80756 to J.K.K.) and the American Diabetes Association (7-07-RA-80 to J.K.K.). This study was supported by the University of Massachusetts Mouse Phenotyping Center (National Institute of Diabetes and Digestive and Kidney Diseases, Diabetes and Endocrinology Research Center, grant DK52530) and the Penn State Diabetes and Obesity Mouse Phenotyping Center (Pennsylvania State Department of Health Tobacco Settlement Award to J.K.K.). R.J.D. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Article published online ahead of print. Article and publication date are online at http://www.genesdev.org/cgi/doi/10.1101/gad.1878510.
Supplemental material is available at http://www.genesdev.org.
References
- Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH2-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307) J Biol Chem. 2000;275:9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- Bennett BL, Satoh Y, Lewis AJ. JNK: A new therapeutic target for diabetes. Curr Opin Pharmacol. 2003;3:420–425. doi: 10.1016/s1471-4892(03)00068-7. [DOI] [PubMed] [Google Scholar]
- Björkman U, Ekholm R. Biochemistry of thyroid hormone formation and secretion. In: Greer MA, editor. The thyroid gland. Raven Press; New York: 2000. pp. 83–125. [Google Scholar]
- Das M, Jiang F, Sluss HK, Zhang C, Shokat KM, Flavell RA, Davis RJ. Suppression of p53-dependent senescence by the JNK signal transduction pathway. Proc Natl Acad Sci. 2007;104:15759–15764. doi: 10.1073/pnas.0707782104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Yang DD, Wysk M, Whitmarsh AJ, Davis RJ, Flavell RA. Defective T cell differentiation in the absence of Jnk1. Science. 1998;282:2092–2095. doi: 10.1126/science.282.5396.2092. [DOI] [PubMed] [Google Scholar]
- Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- Kaneto H, Nakatani Y, Miyatsuka T, Kawamori D, Matsuoka TA, Matsuhisa M, Kajimoto Y, Ichijo H, Yamasaki Y, Hori M. Possible novel therapy for diabetes with cell-permeable JNK-inhibitory peptide. Nat Med. 2004;10:1128–1132. doi: 10.1038/nm1111. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Higashimori T, Park SY, Choi H, Dong J, Kim YJ, Noh HL, Cho YR, Cline G, Kim YB, et al. Differential effects of interleukin-6 and -10 on skeletal muscle and liver insulin action in vivo. Diabetes. 2004;53:1060–1067. doi: 10.2337/diabetes.53.4.1060. [DOI] [PubMed] [Google Scholar]
- Lee YH, Giraud J, Davis RJ, White MF. c-Jun N-terminal kinase (JNK) mediates feedback inhibition of the insulin signaling cascade. J Biol Chem. 2003;278:2896–2902. doi: 10.1074/jbc.M208359200. [DOI] [PubMed] [Google Scholar]
- Lenard NR, Berthoud HR. Central and peripheral regulation of food intake and physical activity: Pathways and genes. Obesity (Silver Spring) 2008;16:S11–S22. doi: 10.1038/oby.2008.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangada J, Pearson T, Brehm MA, Wicker LS, Peterson LB, Shultz LD, Serreze DV, Rossini AA, Greiner DL. Idd loci synergize to prolong islet allograft survival induced by costimulation blockade in NOD mice. Diabetes. 2009;58:165–173. doi: 10.2337/db08-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora A, Sakamoto K, McManus EJ, Alessi DR. Role of the PDK1–PKB–GSK3 pathway in regulating glycogen synthase and glucose uptake in the heart. FEBS Lett. 2005;579:3632–3638. doi: 10.1016/j.febslet.2005.05.040. [DOI] [PubMed] [Google Scholar]
- Myers MG, Jr, Munzberg H, Leinninger GM, Leshan RL. The geometry of leptin action in the brain: More complicated than a simple ARC. Cell Metab. 2009;9:117–123. doi: 10.1016/j.cmet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obregon MJ. Thyroid hormone and adipocyte differentiation. Thyroid. 2008;18:185–195. doi: 10.1089/thy.2007.0254. [DOI] [PubMed] [Google Scholar]
- Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, Barrett T, Kim JK, Davis RJ. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabio G, Cavanagh-Kyros J, Ko HJ, Jung DY, Gray S, Jun JY, Barrett T, Mora A, Kim JK, Davis RJ. Prevention of steatosis by hepatic JNK1. Cell Metab. 2009;10:491–498. doi: 10.1016/j.cmet.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabio G, Cavanagh-Kyros J, Ko HJ, Jung DY, Gray S, Jun JY, Barrett T, Kim JK, Davis RJ. Role of muscle JNK1 in obesity-induced insulin resistance. Mol Cell Biol. 2010;30:106–115. doi: 10.1128/MCB.01162-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins JL, De SK, Machleidt T, Becattini B, Vazquez J, Kuntzen C, Chen LH, Cellitti JF, Riel-Mehan M, Emdadi A, et al. Identification of a new JNK inhibitor targeting the JNK–JIP interaction site. Proc Natl Acad Sci. 2008;105:16809–16813. doi: 10.1073/pnas.0805677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend KL, Lorenzi MM, Widmaier EP. High-fat diet-induced changes in body mass and hypothalamic gene expression in wild-type and leptin-deficient mice. Endocrine. 2008;33:176–188. doi: 10.1007/s12020-008-9070-1. [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Whitmarsh AJ, Davis RJ. Analyzing JNK and p38 mitogen-activated protein kinase activity. Methods Enzymol. 2001;332:319–336. doi: 10.1016/s0076-6879(01)32212-7. [DOI] [PubMed] [Google Scholar]