Abstract
We evaluated the pharmacokinetics of ciprofloxacin in serum (n = 6) and urine (n = 4) in goats following a single intravenous administration of 4 mg/kg body weight. The serum concentration-time curves of ciprofloxacin were best fitted by a two-compartment open model. The drug was detected in goat serum up to 12 h. The elimination rate constant (β) and elimination half-life (t1/2β) were 0.446 ± 0.04 h-1 and 1.630 ± 0.17 h, respectively. The apparent volume of distribution at steady state (Vdss) was 2.012 ± 0.37 l/kg and the total body clearance (ClB) was 16.27 ± 1.87 ml/min/kg. Urinary recovery of ciprofloxacin was 29.70% ± 10.34% of the administered dose within 36 h post administration. In vitro serum protein binding was 41% ± 13.10%. Thus, a single daily intravenous dose of 4 mg/kg is sufficient to maintain effective levels in serum and for 36 h in urine, allowing treatment of systemic, Gram-negative bacterial infections and urinary tract infections by most pathogens.
Keywords: ciprofloxacin, disposition kinetics, goat, urinary excretion
Introduction
Ciprofloxacin is a fluoroquinolone derivative in veterinary medicine with outstanding antibacterial activity primarily against Gram-negative pathogens, but also Gram-positive bacteria and some Chlamydia, Mycoplasma, and many Mycobacterium species [12,23,24]. The pharmacokinetics of ciprofloxacin has been investigated in dogs, rats and monkeys, rabbits, ponies, goats, cow calves, and buffalo calves [4,8,29,30,33,34,37]. Because of the lack of sufficient pharmacokinetic studies in goats and potential species differences, we evaluated the pharmacokinetics of ciprofloxacin after intravenous administration in goats.
Materials and Methods
Experimental animals
Ten clinically healthy goats (15-20 kg) were procured from a local breeding farm in Jammu, India, and housed in a well-ventilated house. All de-wormed animals were housed in hygienic departmental shed for 20 d prior to experiments for acclimatization, and allowed free access to pasture, ad libitum water, and received once daily concentrated feed ration. No treatments were performed for two weeks before study initiation. One day prior to the experiment, the goats underwent thorough physical and clinical examinations.
Experimental design
The study was performed in two phases. In phase I, six goats were used for evaluating the disposition kinetics of ciprofloxacin. A single intravenous dose of 4 mg/kg body weight of ciprofloxacin hydrochloride monohydrate in sterile distilled water (Cadilla Labs Private, India) was administered into the jugular vein of the six goats. Blood samples of 6-7 ml were obtained directly from the jugular vein using disposable needles. The blood samples were collected in un-heparinized test tubes just prior to and at 2.5, 5, 10, 20, 30, 45, 60 and 90 min and 2, 3, 4, 6, 9 and 12 h after drug administration. Blood samples were allowed to clot at ambient temperature for the collection of serum. The separated serum was then centrifuged for 15 min at 3,000 g to obtain clear supernatant fluid and stored at -20℃ until analysis, usually within 2-3 days.
In phase II, the urinary excretion of ciprofloxacin was investigated in four goats. The dose and route of administration were the same as in phase I. The animals were placed into metabolic stalls prior to start of the experiment and total urine was collected. The urine samples were collected at 0-3, 3-6, 6-9, 9-12, 12-24, 24-36, and 36-48 h after drug administration. The whole volume of urine was measured and 10 ml urine samples were taken for analysis.
Ciprofloxacin concentrations in serum and urine were determined using the agar well diffusion assay [5], using E. coli ATCC-25922 as test organisms grown on Mueller Hinton agar. This method correlates well with HPLC studies [16]. Standard concentrations (0.015 to 4 mg/ml) were prepared in pooled untreated goat serum and urine, and showed a mean correlation coefficient (r) > 0.99% for both serum and urine. The serum concentration time profile showed a correlation coefficient (r2) value of 0.982 ± 0.009. The intra-assay and inter-assay precision variability were <10% for standard concentrations in both serum and urine. The lower limit of quantification of the ciprofloxacin assay was 0.015 µg/ml.
The extent of protein binding was determined in vitro using an equilibrium dialyzing technique as described by Kunin [18]. To estimate the protein binding of ciprofloxacin, the drug was dissolved in 0.06 M phosphate buffer (pH 7.0) and antibiotic-free healthy goat serum at 0.5, 0.75, 1.0, 1.5, and 2.0 µg/ml. The differences in the diameters of the inhibition zone between the solution of the drugs in the buffer and serum were calculated.
Pharmacokinetics analysis
The compartment pharmacokinetics of serum concentration-time curves after single intravenous injection were analyzed for each goat by Top-Fit v. 2.0 [13]. The best-fit model was selected based on Akaike's Information Criterion and the Schwartz test [31,40]. Model-dependent pharmacokinetic parameters were obtained as described by Baggot [3] and Gibaldi and Perrier [11]. The two compartment open model was the best fit for intravenous injection of ciprofloxacin. Statistical moments were also used to compute the non-compartmental pharmacokinetic analysis [11,40]. The non-compartment model was used to determine the area under concentration-time curve (AUC), and area under the first moment curve (AUMC), using the linear trapezoidal rule with extrapolation to time infinity. Mean residence time (MRT) and systemic clearance (ClB) were calculated as MRT = AUMC/AUC and ClB = Dose/AUC, respectively. The apparent volumes of distribution at steady state were calculated as VdSS = (Dose × AUMC)/AUC2.
Results
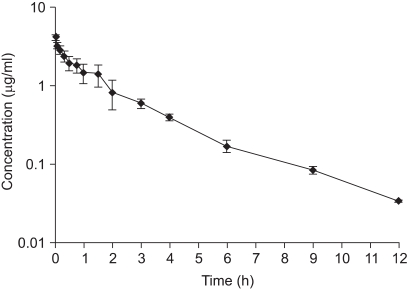
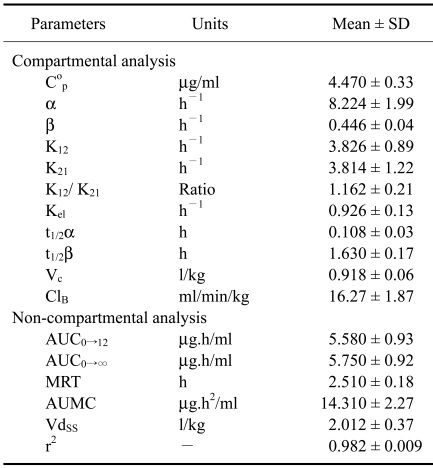
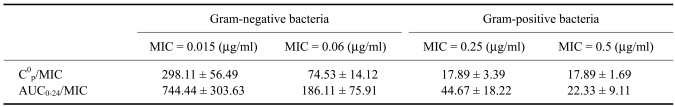
Mean serum ciprofloxacin concentrations following a single intravenous administration of 4 mg/kg body weight are presented in Fig. 1. The drug was detected in goat serum up to 12 h. Following intravenous administration, the elimination half-life (t1/2β), volume of distribution at steady state (Vdss), total body clearance (ClB), and area under curve from 0→∞ (AUC0→∞) were estimated to be 1.63 ± 0.17 h, 2.012 ± 0.37 l/kg, 16.27 ± 1.87 ml/min/kg, and 5.75 ± 0.92 µg.h/ml, respectively (Table 1). The efficacy predictors, C0p/ minimum inhibitory concentration (MIC) and AUC0-24/MIC can predict antimicrobial efficacy of fluoroquinolones and to reduce selection for resistance [36]. Efficacy predictors for Gram-negative bacteria at MIC=0.06 µg/ml and gram-positive bacteria at MIC = 0.5 µg/ml were C0p/MIC = 74.53 ± 14.12, AUC0-24/MIC = 186.11 ± 75.91 and C0p/MIC = 17.89 ± 1.69, AUC0-24/MIC = 22.33 ± 9.11, respectively (Table 3).
Fig. 1.
Semi-logarithmic graph depicting the serum concentration-time profile of ciprofloxacin in goat following single intravenous dose of 4 mg/kg body weight (n = 6).
Table 1.
Pharmacokinetic parameters of ciprofloxacin in goat (n = 6) following a single intravenous administration with 4 mg/kg body weight
Cop-Serum drug concentration at t=0; α and β-hybrid rate constants represent the slopes of distribution and elimination phases, respectively; t1/2α-distribution half-lives; t1/2β-elimination half-lives; kel-first order elimination rate constant, Vc-volume of distribution from central compartment; VdSS-volume of distribution at steady-state; K12-rate constant of transfer of drug from central compartment into the tissue compartment; K21-rate constant of transfer of drug from tissue compartment into the central compartment; AUC0→12-Area under the serum concentration vs. time curve from 0 to 12 h; AUC0→∞-Area under the serum concentration vs. time curve from 0 to ∞; AUMC area under the first moment curve; MRT-mean residence time; ClB-total body clearance; r2-Correlation coefficient fit curve of serum concentration vs time profile.
Table 3.
Efficacy predictors (C0p/MIC and AUC0-24/MIC) estimated for ciprofloxacin against Gram-negative and Gram-positive bacteria
For calculations the applied values were: C0p = 4.47 µg/ml. AUC0-24 = 11.16 µg.h/ml. This value is obtained after doubling the value of AUC0-12.
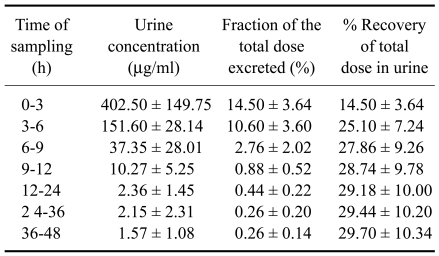
Ciprofloxacin concentrations in urine were much higher than in serum (Table 2, Fig. 1). Urinary recovery of ciprofloxacin was 29.70 ± 10.34% of the administered dose within 36 h post administration (Table 2). The in vitro protein percentage of ciprofloxacin at different concentration (0.5, 0.75, 1. 1.5, 2 µg/ml) was 20.5, 35.2, 49.3, 51.0, and 49.0, respectively with the mean value 41.0 ± 13.1%. The in vitro protein binding of ciprofloxacin to serum protein was 41.0 ± 13.10%, and increased with increasing doses.
Table 2.
Urinary excretion of ciprofloxacin and fraction of the dose (%) excreted in aliquots in goats following a single intravenous injection of 4 mg/kg body weight (n = 4)
All data are mean ± SD.
Discussion
Following intravenous administration of ciprofloxacin (4 mg/kg), no adverse effect or toxic manifestation was observed. Ciprofloxacin concentration versus time data is best described by a biphasic curve and was similar to the disposition in pre-ruminant calves and piglets, goats, dogs, ponies, sheep, buffalo calves and cats [1,2,8,22,25,29,30]. The distribution of the ciprofloxacin appeared to be quite rapid, as indicated by short distribution half-life (0.108 ± 0.03 h) and 2.11 fold reduction in the serum drug concentration within 30 min of its administration. The distribution half-life (t1/2α) in goats was similar to murrah buffalo calves [29]. However, the values were lower than the observed distribution half-life in pre-ruminant calves [25], cats [2], and cow calves [17].
The elimination half-life was similar to that in rabbits [4] and goats [28]. Higher values were reported in cats [2], horses [26], cow calves [34], buffalo calves [30], and men [6]. Ciprofloxacin showed a Vdss (2.012 ± 0.37 l/kg) that indicates the drug is well distributed to extra-vascular tissue, as a volume of distribution of one indicates good extra-vascular tissue distribution [3]. This is supported by the high K12/K21 ratio, which indicates that the drug moves freely between the body compartments, as reported in cats [2]. The Vdss values were similar in rabbits [9] and horses [26], but lower than in cats [2] and ponies [8]. Ciprofloxacin clearance in goats was similar to dogs [1], calves [25] and pigs [10]. This value in goats was higher than reported in men [6], buffalo calves [30], and cat [2] but lower than in lactating cow [15], and cow calves [34].
Ciprofloxacin was detected in urine following intravenous administration, with a mean of 29.70% ± 10.34% of the total administered dose of ciprofloxacin recovered in urine within 36 h. Urine concentrations up to 36 h were above MIC90 for both Gram-positive and Gram-negative pathogens responsible for urinary tract infections, suggesting that ciprofloxacin could be used to treat goats with urinary tract infections caused by bacterial strains resistant to other commonly used antimicrobials.
Ciprofloxacin (0.5 to 2.0 µg/ml) protein binding averaged 41.0%, comparable with Joos et al. [16] and Hoffken et al. [14] who reported serum binding between 21.9% and 39.6% in human.
An optimum dosage is derived by correlating the important pharmacodynamic variables like in vitro MIC data with pharmacokinetic variables. The antibacterial activity of the fluoroquinolones is dependent on the drug concentration and the MIC of the micro-organisms [38]. Antimicrobial drugs that act predominantly by concentration-dependent mechanisms generally exert significant post-antibiotic, sub-minimum inhibitory concentration effects. Such drugs continue to inhibit bacterial growth for a period of hours after they have been completely removed from the system. Optimal outcomes with this type of bactericide require high concentrations, with therapeutic success correlating with the AUC/MIC ratio, while prevention of the development of resistance correlating with the Cop/MIC ratio [32]. Accordingly, the Cop/MIC90 and AUC24/MIC90 are the best parameters for predicting the antimicrobial effects of fluoroquinolones [21]. For fluoroquinolones, a Cop/MIC90 higher than 3 produced 99% reduction in bacterial count, and a Cop/MIC90 of 8 or higher prevented the emergence of resistant organisms [7]. In addition, an AUC24/MIC90 higher than 100 should give maximum clinical and bacteriology efficacy [36]. The numerical values of Cop/MIC90 and AUC24/MIC90 are substitute markers for predicting optimal dosage [35]. The AUC/MIC for cattle, sheep, goats, and camels were lower than 100-125 [19].
Lower and upper MIC90 values were used for the calculation of dosage regimen [39]. MIC90 values of 0.015 and 0.06 µg/ml were used for Gram-negative bacteria (E. coli, Pasteurella spp., Salmonella spp., Klebsiella pneumonia, Proteus mirabilis, Bordetella bronchiseptica, and Haemophilus spp.). However, values for Gram-positive bacteria (Staphylococcus aureus, Staphylococcus intermedius, Staphylococcus spp.) were 0.25 and 0.5 µg/ml [2]. MIC90 values of 0.007 and 1 µg/ml were used for selected veterinary bacterial pathogens [27].
The efficacy predictors, AUC0-24/MIC and C0p/MIC ratios (Table 3) are lower for Gram-negative than Gram-positive bacteria. However, for treatment of gram-positive bacteria with greater MIC, the predicted efficacy is lower. The minimum therapeutic concentration of fluoroquinolones is 0.02-0.5 µg/ml [20]. Here, ciprofloxacin concentration in serum was MIC < 1 µg/ml [28] up to 12 h, a level suitable for Gram-negative bacteria, and levels in urine up to 36 h were sufficient for treating both Gram-negative and Gram-positive microorganisms. Ciprofloxacin also shows post-antibiotic effects (PAE) that persist for 4 to 8 h. We therefore recommend once daily, intravenous ciprofloxacin at 4 mg/kg to maintain effective levels in serum or 36 h intervals for treating urinary tract infections in goats.
In conclusion, ciprofloxacin showed high efficacy predictors against Gram-negative bacteria with PAE. In addition, the high concentration of ciprofloxacin excreted in urine up to 36 h makes it a better therapeutic option for treating systemic infections, especially of Gram-negative bacterial origin, as well as acute urinary tract infections of resistant strains.
References
- 1.Abadía AR, Aramayona JJ, Muñoz MJ, Pla Delfina JM, Saez MP, Bregante MA. Disposition of ciprofloxacin following intravenous administration in dogs. J Vet Pharmacol Ther. 1994;17:384–388. doi: 10.1111/j.1365-2885.1994.tb00264.x. [DOI] [PubMed] [Google Scholar]
- 2.Albarellos GA, Kreil VE, Landoni MF. Pharmacokinetics of ciprofloxacin after single intravenous and repeat oral administration to cats. J Vet Pharmacol Ther. 2004;27:155–162. doi: 10.1111/j.1365-2885.2004.00573.x. [DOI] [PubMed] [Google Scholar]
- 3.Baggot JD. Principles of Drug Distribution in Domestic Animals: The Basis of Veterinary Clinical Pharmacology. Philadelphia: Saunders; 1977. pp. 144–189. [Google Scholar]
- 4.Barriere SL, Kaatz GW, Schaberg DR, Fekety R. Altered pharmacokinetic disposition of ciprofloxacin and vancomycin after single and multiple doses in rabbits. Antimicrob Agents Chemother. 1987;31:1075–1078. doi: 10.1128/aac.31.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett JV, Brodie JL, Benner EJ, Kirby WM. Simplified, accurate method for antibiotic assay of clinical Specimens. Appl Microbiol. 1966;14:170–177. doi: 10.1128/am.14.2.170-177.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campoli-Richards DM, Monk JP, Price A, Benfield P, Todd PA, Ward A. Ciprofloxacin: A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1988;35:373–447. doi: 10.2165/00003495-198835040-00003. [DOI] [PubMed] [Google Scholar]
- 7.Craig WA. Pharmacokinetic/Pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1–10. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 8.Dowling PM, Wilson RC, Tyler JW, Duran SH. Pharmacokinetics of ciprofloxacin in ponies. J Vet Pharmacol Ther. 1995;18:7–12. doi: 10.1111/j.1365-2885.1995.tb00543.x. [DOI] [PubMed] [Google Scholar]
- 9.El-Seidi IA. Effect of pregnancy and lactation on pharmacokinetic properties of ciprofloxacin in rabbits. Wiener Tierarztliche Monatsschrift. 2000;87:147–152. [Google Scholar]
- 10.Fang BH, Feng QH, Chen ZL, Wang ZQ. Bioavailability and Pharmacokinetics of ciprofloxacin in pigs. Chinese J Vet Sc. 1999;19:588–590. [Google Scholar]
- 11.Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. New York: Dekker; 1982. pp. 1–494. [Google Scholar]
- 12.Hackbarth CJ, Chamers HF, Stella F, Shibl AM, Sande MA. Ciprofloxacin in experimental Pseudomonas aeruginosa meningitis in rabbits. J Antimicrob Chemother. 1986;18(Suppl D):65–69. doi: 10.1093/jac/18.supplement_d.65. [DOI] [PubMed] [Google Scholar]
- 13.Heinzel G, Woloszczak R, Thomann P. Topfit Version 2.0: Pharmacokinetic and Pharmacodynamic Data Analysis System for PC. Jena: Gustav Fischer Verlag GmbH; 1993. [Google Scholar]
- 14.Höffken G, Lode H, Prinzing C, Borner K, Koeppe P. Pharmacokinetics of ciprofloxacin after oral and parenteral administration. Antimicrob Agents Chemother. 1985;27:375–379. doi: 10.1128/aac.27.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jayakumar K, Honnegowda, Narayana K. Pharmacokinetics of ciprofloxacin in lactating cows. Indian Vet J. 2000;77:765–767. [Google Scholar]
- 16.Joos B, Ledergerber B, Flepp M, Bettex JD, Lüthy R, Siegenthaler W. Comparison of high-pressure liquid chromatography and bioassay for determination of ciprofloxacin in serum and urine. Antimicrob Agents Chemother. 1985;27:353–356. doi: 10.1128/aac.27.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar R, Kumar V, Verma SP, Uppal RP. Pharmacokinetics of ciprofloxacin in cow calves. Indian J Anim Sci. 1997;67:505–506. [Google Scholar]
- 18.Kunin CM. Clinical pharmacology of the new penicillins. 1. The importance of serum protein binding in determining antimicrobial activity and concentration in serum. Clin Pharmacol Ther. 1966;7:166–179. doi: 10.1002/cpt196672166. [DOI] [PubMed] [Google Scholar]
- 19.Lees P, Shojaee Aliabadi F. Rational dosing of antimicrobial drugs: animals versus humans. Int J Antimicrob Agents. 2002;19:269–284. doi: 10.1016/s0924-8579(02)00025-0. [DOI] [PubMed] [Google Scholar]
- 20.Leysen DC, Haemers A, Pattyn SR. Mycobacteria and the new quinolones. Antimicrob Agents Chemother. 1989;33:1–5. doi: 10.1128/aac.33.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lode H, Borner K, Koeppe P. Pharmacodynamics of fluoroquinolones. Clin Infect Dis. 1998;27:33–39. doi: 10.1086/514623. [DOI] [PubMed] [Google Scholar]
- 22.Munoz MJ, Llovería P, Santos MP, Abadía AR, Aramayona JJ, Bregante MA. Pharmacokinetics of ciprofloxacin in sheep after single intravenous or intramuscular administration. Vet Q. 1996;18:45–48. doi: 10.1080/01652176.1996.9694613. [DOI] [PubMed] [Google Scholar]
- 23.Neer TM. Clinical pharmacologic features of fluoroquinolone antimicrobial drugs. J Am Vet Med Assoc. 1988;193:577–580. [PubMed] [Google Scholar]
- 24.Neu HC. Ciprofloxacin: a major advance in quinolone chemotherapy. Am J Med. 1987;82(Suppl 4A):1–404. [PubMed] [Google Scholar]
- 25.Nouws JFM, Mevius DJ, Vree TB, Baars AM, Laurensen J. Pharmacokinetics, renal clearance and metabolism of ciprofloxacin following intravenous and oral administration to calves and pigs. Vet Q. 1988;10:156–163. doi: 10.1080/01652176.1988.9694165. [DOI] [PubMed] [Google Scholar]
- 26.Park SC. Pharmacokinetics of ciprofloxacin after intravenous and intramuscular administration in healthy horses. Indian Vet J. 2002;79:904–908. [Google Scholar]
- 27.Prescott JF, Yielding KM. In vitro susceptibility of selected veterinary bacterial pathogens to ciprofloxacin, enrofloxacin and norfloxacin. Can J Vet Res. 1990;54:195–197. [PMC free article] [PubMed] [Google Scholar]
- 28.Pu SJ, Feng QH. Pharmacokinetics of ciprofloxacin and its concentrations in milk and udder tissues in goats with endotoxin induced mastitis. Chin J Vet Sci. 2000;20:271–274. [Google Scholar]
- 29.Raina R, Garg BD, Uppal RP, Jain SK, Rana RD. Pharmacokinetics and urinary excretion of ciprofloxacin in goats. Indian J Pharmacol. 1994;26:83–87. [Google Scholar]
- 30.Raina R, Uppal RP, Kumar V, Garg BD. Pharmacokinetics and dosage of ciprofloxacin in buffalo calves. Indian J Anim Sci. 2000;70:475–477. [Google Scholar]
- 31.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–464. [Google Scholar]
- 32.Shojaee AliAbadi F, Lees P. Antibiotic treatment for animals: effect on bacterial population and dosage regimen optimisation. Int J Antimicrob Agents. 2000;14:307–313. doi: 10.1016/s0924-8579(00)00142-4. [DOI] [PubMed] [Google Scholar]
- 33.Siefert HM, Maruhn D, Maul W, Förster D, Ritter W. Absorption, concentrations in plasma, metabolism and excretion after a single administration of [14C] ciprofloxacin in albino rats and rhesus monkeys. Drug Res. 1986;36:1496–1502. [PubMed] [Google Scholar]
- 34.Singh K, Srivastava AK. Pharmacokinetics and urinary excretion of ciprofloxacin in crossbred cow calves. Indian J Anim Sci. 2000;70:1021–1024. [Google Scholar]
- 35.Toutain PL, Lees P. Integration and modelling of pharmacokinetic and pharmacodynamic data to optimize dosage regimens in veterinary medicine. J Vet Pharmacol Ther. 2004;27:467–477. doi: 10.1111/j.1365-2885.2004.00613.x. [DOI] [PubMed] [Google Scholar]
- 36.Turnidge J. Pharmacokinetics and Pharmacodynamics of fluoroquinolones. Drugs. 1999;58(Suppl 2):29–36. doi: 10.2165/00003495-199958002-00006. [DOI] [PubMed] [Google Scholar]
- 37.Walker RD, Stein GE, Hauptman JG, MacDonald KH, Budsberg SC, Rosser EJ., Jr Serum and tissue cage fluid concentrations of ciprofloxacin after oral administration of the drug to healthy dogs. Am J Vet Res. 1990;51:896–900. [PubMed] [Google Scholar]
- 38.Walker RD. The use of fluoroquinolones for companion animal antimicrobial therapy. Aust Vet J. 2000;78:84–90. doi: 10.1111/j.1751-0813.2000.tb10528.x. [DOI] [PubMed] [Google Scholar]
- 39.Watts JL, Salmon SA, Sanchez MS, Yancey RJ., Jr In vitro activity of premafloxacin, a new extended-spectrum fluoroquinolone, against pathogens of veterinary importance. Antimicrob Agents Chemother. 1997;41:1190–1192. doi: 10.1128/aac.41.5.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamaoka K, Nakagawa T, Uno T. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978;6:165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]