Abstract
A total of 1,618 ticks [420 individual (adults) and pooled (larvae and nymphs) samples], 369 rodents (Apodemus agrarius, Rattus norvegicus, Tscherskia triton, Mus musculus, and Myodes regulus), and 34 shrews (Crocidura lasiura) that were collected in northern Gyeonggi-do near the Demilitarized Zone (DMZ) of Korea during 2004-2005, were assayed by PCR for selected zoonotic pathogens. From a total of 420 individual and pooled tick DNA samples, Anaplasma (A.) phagocytophilum (16), A. platys (16), Ehrlichia (E.) chaffeensis (63), Borrelia burgdorferi (16), and Rickettsia spp. (198) were detected using species-specific PCR assays. Out of 403 spleens from rodents and shrews, A. phagocytophilum (20), A. platys (34), E. chaffeensis (127), and Bartonella spp. (24) were detected with species-specific PCR assays. These results suggest that fevers of unknown causes in humans and animals in Korea should be evaluated for infections by these vector-borne microbial pathogens.
Keywords: Bartonella, Borrelia, Rickettsia, rodents, Crocidura lasiura, tick-borne pathogens
Introduction
Korea is a northeast Asian peninsular country with four clearly demarked seasons. Seventy percent of the land area is mountainous, with interspersed fertile river valleys. Ticks are commonly collected during the early spring through late autumn, while are few ticks are collected during the cold winter season. Many wild animals inhabit the Demilitarized Zone (DMZ) and the area adjacent to it, and these animals are hosts to ticks and serve as reservoirs for tick-borne pathogens [17]. The Korean and US military have numerous small to large training sites near the DMZ where large populations of small mammals (rodents and insectivores) and occasional deer, wild pigs, and other small mammals are found [32]. Additionally, tourist activity is expected to increase in the area in the near future, which may increase the risk of human exposure to ticks and the pathogens they harbor [5,16].
Ectoparasites (e.g. ticks and fleas) are vectors of a number of pathogens that are important to humans and also veterinary practice. Ticks are harmful ectoparasites that directly or indirectly cause a variety of disease states in their host. Ticks are known vectors of protozoa, rickettsiae, bacteria, and viruses, that may cause serious and life-threatening illnesses in human. Screening ticks for disease-causing pathogens using molecular epidemiological tools provides useful data on the distribution and prevalence of tick-borne pathogens. Moreover, with increases in the mean global annual temperatures of 1℃ since the 1880s [10], it is predicted that the temperate Korean climate may be altered to a subtropical climate. These environmental changes may potentially alter the distribution of wild animals and the arthropod vectors and the pathogens they transmit. Tick-borne encephalitis was previously thought to not exist in Korea, but recent evidence from molecular testing of ticks and rodents suggests that it is present in Korea [19]. Many of the pathogenic agents transmitted by ticks, including Ehrlichia spp., Anaplasma spp., Borrelia spp., Bartonella spp., and Rickettsia spp., are known to be human and animal pathogens worldwide [8,20,29].
Recent seroepidemiological findings documented the presence of human monocytic ehrlichiosis and human granulocytic anaplasmosis in Korea [11,26]. Molecular evidence of Ehrlichia and Anaplasma spp. was identified in ticks collected from animals and grass vegetation in Korea [17,21]. Additionally, a spotted fever group Rickettsia, similar to Rickettsia (R.) japonica, was identified in Haemaphysalis (H.) longicornis ticks by PCR, and antibodies to these organisms were detected in human patients with acute febrile illness [14].
The United States Forces Korea rodent- and tick-borne disease surveillance program was initiated to provide ecological and epidemiological information on potential risks of infection for personal who occupy or train in various environments near the DMZ. This is especially important when considering recent serological evidence that confirmed the presence of Ehrlichia (E.) chaffeensis and Anaplasma (A.) phagocytophilum [11,26].
The purpose for this study was to identify vector-borne pathogens in ticks, rodents and shrews in order to provide more accurate risk assessment of tick-borne pathogens that may affect human and animal health in Korea.
Materials and Methods
Study sites
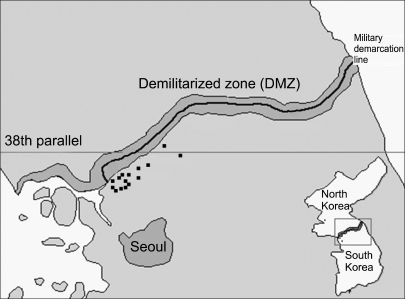
Ticks were collected in the field by dragging and flagging grass vegetation and forested ground cover (fallen leaves, clumps of grasses and scattered shrubs). Ticks also were removed from various wild rodents (Apodemus agrarius, Rattus norvegicus, Tscherskia triton, Mus musculus, and Myodes regulus) and a shrew (Crocidura lasiura) that were live-trapped at US military installations and training sites in northern Gyeonggi-do near the DMZ (Fig. 1).
Fig. 1.
Collection sites were conducted in northern Gyeonggi-do near the Demilitarized Zone of Korea. The small black squares indicate sample collection sites.
Tick collections
During March through September 2004, a total of 1,618 ticks were collected from grass vegetation and forest leaf litter (933 ticks) and wild rodents (685 ticks) at 17 sites (Fig. 1). Based on microscopic examination, ticks were identified to species and developmental stage characterized. Adult ticks were stored and assayed individually, while the nymphs and larvae were pooled (1-6 and 1-30 ticks per pool, respectively) into 420 sample pools (62 from wild rodents and 358 from grass vegetation and forest leaf litter) and stored at -70℃ until they were assayed.
Tissue samples
A total of 403 small mammals (369 wild rodents and 34 shrews) belonging to six species, six different genera, and two families were live captured at US military installations and training sites in northern Gyeonggi-do near the DMZ in Korea from August 2004 through June 2005 using Sherman traps (3" × 5" × 9" folding traps; H.B. Sherman Traps, USA). The live-caught rodents and shrews were transported to Korea University where they were euthanized in accordance with the Korea University animal use protocol, their abdominal cavities opened aseptically, and spleen samples collected and stored individually at -70℃ until assayed.
DNA extraction
DNA was extracted from pools of larvae, nymphs and individual adult ticks. A total of 747 and 174 nymphs were collected by tick drag/flag and from rodents and a shrew, respectively, and these were placed in 215 pools according to collection site, while DNA was extracted from 186 individual adult ticks (76 males and 110 females) and 19 pools of larvae with using DNeasy tissue kits (Qiagen, Germany) (Table 1). Individual ticks and pools of ticks were mechanically homogenized using sterile scissors and a manual homogenizer (General Biosystem, Korea). DNA extraction was performed using DNeasy tissue kits (Qiagen, Germany) in accordance with instructions provided by the manufacturer.
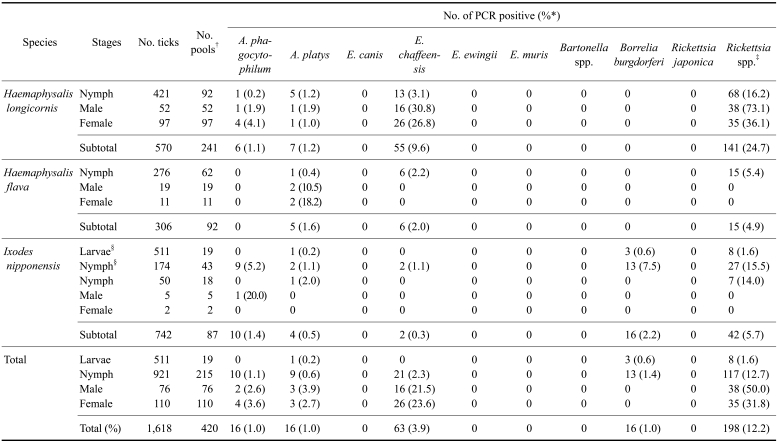
Table 1.
The total number of ticks and the number of individuals (adults) and pools (larvae and nymphs) assayed, and the number of pools PCR positive by stage and gender (adults) for selected rickettsial pathogens
*Percent = No. of PCR positive/No. of ticks ×100. †Ticks were pooled in groups of 1-5 ticks (nymphs) and 2-30 ticks (larvae), and the adults were individually assayed. ‡Spotted fever group of Rickettsia. §Ticks collected from small mammals. All other ticks assayed were collected by tick drags.
Detection of tick-borne pathogens by PCR
Purified DNA was used for the detection of tick-borne pathogens using conventional and nested PCR [16]. PCR assays using genomic DNAs and species-specific primers, as previously described, were used to identify selected zoonotic pathogens [18].
Nested PCR: The nested PCR technique was used for the detection of A. phagocytophilum by amplifying a 926 bp fragment of A. phagocytophilum-specific 16S rRNA gene in a total volume of 25 µl as previously described [4]. Species-specific primers for A. platys, E. chaffeensis, E. ewingii, and E. canis were used in the nested PCR assays [23,24]. The primers ECC and ECB were used to amplify all Ehrlichia spp. [6,7]. The primers EPLAT5 and EPLAT3 were used for A. platys-specific amplification [22], the primers HE1 and HE3 were used for E. chaffeensis-specific amplification [3], the primers EE52 and HE3 were used for E. ewingii-specific amplification [23], and the primers ECAN5 and HE3 were used for E. canis-specific amplification [23].
Conventional PCR: Identification of Bartonella spp., E. muris, Borrelia (B.) burgdorferi, R. rickettsii, and R. japonica was performed using conventional PCR with the species-specific primers [9,30,33]. The citrate synthase gene (gltA) was selected for the identification of E. muris [14]. The primers BhCS (781p) and BhCS (1137n) were used for Bartonella spp. amplification [24]. The gltA gene was used for the identification of Bartonella spp. The ospC gene was selected for the identification of B. burgdorferi. A pair of synthesized oligonucleotide primers derived from the gene sequence encoding the 190 kDa antigen of R. rickettsii, Rr190.70p and Rr190.602n, as described by Regnery et al. [30], was used for the PCR amplification of R. rickettsii DNA. Species-specific primers, Rj10 and Rj5, were used for the R. japonica 17 kDa antigen gene fragment [9]. PCR reactions were performed using 50-100 ng of template DNA, a species-specific primer set, and the PCR mixture. The PCR products were electrophoresed in 1% agarose gel; they were then stained with ethidium bromide and photographed using a still video documentation system (Gel Doc 2000; BioRad, USA).
Isolation of Bartonella sp.
Small mammal spleens were collected in 2 ml tubes and maintained on dry ice for transportation and subsequently used for culture isolation. The spleens were homogenized and then plated on fresh chocolate agar and allowed to incubate in 5% CO2 at 35℃ for up to 4 weeks. The single colonies that grew were scraped for identification of Bartonella spp. The isolates were then confirmed as Bartonella spp. by PCR and DNA sequencing. Culture isolates were stored at -70℃ in frozen medium [a total of 100 ml; M199 tissue culture medium with glutamine and Earle's salts (GIBCO, USA), 1 ml of ×100 glutamine (GIBCO, USA), 1 ml of ×100 sodium pyruvate (GIBCO, USA), 20% bovine fetal calf serum (heat inactivated), and 3 ml sodium bicarbonate (7.5% solution) (GIBCO, USA), 10% DMSO, pH: 7.1-7.4] for later use.
Results
A total of 1,618 ticks from two genera and three species [570 H. longicornis, 306 H. flava and 742 Ixodes (I.) nipponensis] was collected from grass vegetation and forest leaf litter (933 ticks) and small mammals (685 ticks) from 2004 to 2005 near or at US military installations and training sites in northern Gyeonggi-do near the DMZ, Korea (Fig. 1, Table 1). H. longicornis ticks were the most frequently collected species from the grass fields. Except for one H. flava, all ticks taken from captured small mammals were I. nipponensis larvae and nymphs (Table 1).
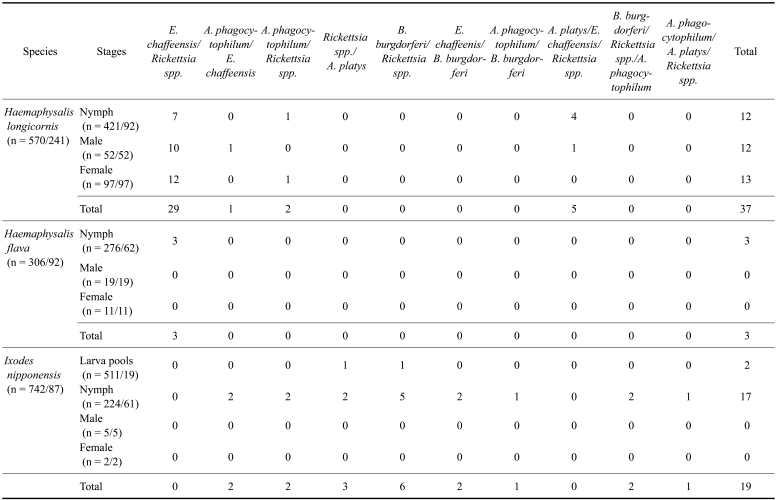
Species-specific PCR assays were performed using DNA samples from 420 individuals and pools of ticks, and DNA samples from spleens of 403 small mammals. Five of the ten tick-borne pathogens examined in this study were detected in ticks [A. phagocytophilum (16, 1.0%), A. platys (16, 1.0%), E. chaffeensis (63, 3.9%), B. burgdorferi (16, 1.0%), and Rickettsia spp. (198, 12.2%)] (Table 1). At least fifty-one ticks had a mixed infection with two pathogens: E. chaffeensis and Rickettsia spp. (32 samples), A. phagocytophilum and E. chaffeensis (3 samples), A. phagocytophilum and Rickettsia spp. (4 samples), Rickettsia spp. and A. platys (3 samples), B. burgdorferi and Rickettsia spp. (6 samples), E. chaffeensis and B. burgdorferi (2 samples), and A. phagocytophilum and B. burgdorferi (1 sample) (Table 2). At least eight ticks had mixed infections with three pathogens: A. platys, E. chaffeensis and Rickettsia spp. (5 samples), B. burgdorferi, Rickettsia spp. and A. phagocytophilum (2 samples), and A. phagocytophilum, A. platys and Rickettsia spp. (1 sample) (Table 2).
Table 2.
The number of mixed infections observed in ticks collected from grass vegetation and forest leaf litter and small mammals
The numbers in parenthesis are the number of ticks/the number of pooled DNAs and/or single DNAs.
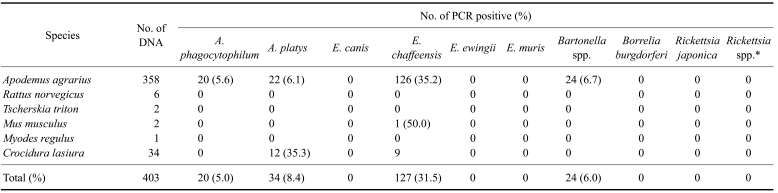
A total of 403 small mammals were collected from US military installations and training sites in northern Gyeonggi-do near the DMZ, and these included five rodents, Apodemus agrarius (358), Rattus norvegicus (6), Tscherskia triton (2), Mus musculus (2), Myodes regulus (1) and a shrew, Crocidura lasiura (34) (Table 3). Four of the ten tick-borne pathogens examined in this study were detected by PCR in the small mammals [A. phagocytophilum (20, 5.0%), A. platys (34, 8.4%), E. chaffeensis (127, 31.5%) and Bartonella spp. (24, 6.0%)] (Table 3). Apodemus agrarius was PCR positive for A. phagocytophilum, A. platys, E. chaffeensis and Bartonella spp., while Mus musculus was only positive for E. chaffeensis. Crocidura lasiura was positive only for A. platys and E. chaffeensis (Table 3).
Table 3.
Tick-borne pathogens identified by PCR from the spleens of small mammals
*Spotted fever group of Rickettsia.
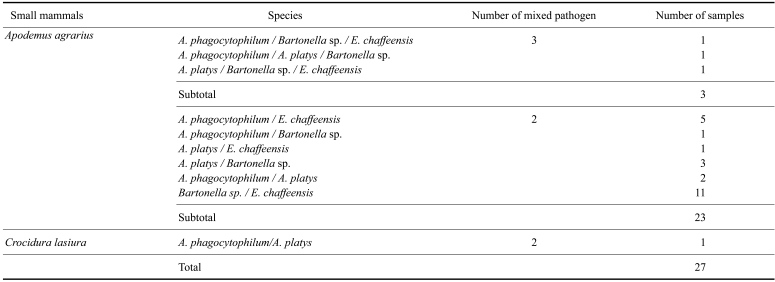
A total of 376 small mammals had single infections with rickettsial pathogens, while 26 Apodemus agrarius had mixed infections of two (23 samples), or three (3 samples) pathogens and a single Crocidura lasiura was positive for two pathogens (Table 4).
Table 4.
The number of mixed infections observed in small mammals
The frozen and homogenized samples of spleens of Apodemus agrarius were cultured and grew as a non-hemolytic gram-negative organism after 14 days, with only a few small white colonies. PCR amplification from the 10 isolates using gltA primers produced a 356 bp fragment and sequencing results were strongly suggestive of Bartonella elizabethae by phylogenetic analysis [17].
Discussion
An analysis of ticks and small mammal tissues demonstrated a high rate of infection of tick-borne pathogens in northern Gyeonggi-do near the DMZ. Most Ehrlichia and Anaplasma spp. tick-borne infections occur in Ixodes spp. in the US and Europe [1,31]. In Asia, Ehrlichia spp. was previously identified from Haemaphysalis spp. as well as Ixodes spp. [13,17,18]. H. longicornis are widespread throughout Korea, and especially around the pastures for grazing cattle or where deer congregate.
I. nipponensis are two-host ticks with larvae and nymphs found on rodents and a shrew. Infection rates of Rickettsia spp. (56.5%) and B. burgdorferi (25.8%) were relatively high among the selected rodents and a shrew tested. Ticks collected from grass vegetation and forest leaf litter were negative for B. burgdorferi, which may be a result of the small sample size of I. nipponensis from the "collected vegetation". In experimentally infected mice, B. burgdorferi DNA can be detected from the foot and lymph nodes by PCR until 55 days post-inoculation [25]. In that study, B. burgdorferi DNA was detected from the spleen tissues 15 days post inoculation, but not at 55 days post inoculation. Persistent infections have also been reported in the skin, blood, CSF and synovial fluid of human patients [2,25]. In the present study, B. burgdorferi DNA was not detected from the spleens of rodents and a shrew or the ticks, but was identified from the I. nipponensis removed from the small mammals. This suggests that wild rodents are a natural reservoir of B. burgdorferi in Korea, with I. nipponensis as an important vector for the larger animal hosts.
In this study, there was a very high prevalence of Rickettsia spp. in H. longicornis, H. flava and I. nipponensis ticks, but not in rodents and a shrew. Our previous studies during 2001 through 2003 detected Rickettsia spp. only from H. longicornis and Apodemus agrarius [18]. The PCR primer set in the previous studies targeted the R. rickettsii rOmpA gene [30], and we were able to sequence the amplicons. The resultant phylogenetic tree showed that Korean rickettsias were closely related to the Rickettsia spp. strain FUJ98 in China [18].
Additionally, these results showed that only one Ixodes spp. tick collected from vegetation was found infected with A. phagocytophilum (0.1%) [18]. In the present study, the A. phagocytophilum infection rate observed in rodents and a shrew tissues (5.6%) was similar to the rate of infection for I. nipponensis ticks collected from rodents and a shrew (5.2%), while only 1.8% of I. nipponensis collected from vegetation were positive for A. phagocytophilum.
Specific DNA of E. canis, E. ewingii, E. muris and R. japonica was not amplified in this study. There have been previous reports of the spotted fever group rickettsiosis, including R. japonicus, in Korean patients and ticks [15,28].
Our results demonstrate that ticks and rodents and a shrew captured near the DMZ of Korea were infected with Anaplasma, Ehrlichia, Bartonella, Borrelia, and Rickettsia spp. Although infections with Ehrlichia and Anaplasma spp. have generally been considered to be observed only in a defined range of hosts, including rodents and some large mammals, our studies suggest that several Ehrlichia and Anaplasma spp. can be transmitted to a variety of hosts in nature. Therefore, additional efforts to define the spectrum of host susceptibility in domestic and wild animals are needed.
H. longicornis, H. flava and I. nipponensis should be considered as potential vectors of A. phagocytophilum, A. platys, E. chaffeensis and Rickettsia spp., while Apodemus agrarius, Crocidura lasiura and Mus musculus may be reservoir hosts of selected tick-borne pathogens in Korea.
Until now, there have not been reports of clinical cases for A. phagocytophilum, E. chaffeensis and B. elizabethae in humans and animals in the Korea, as compared with the numerous reports throughout the world. For some diseases, such as rabies and malaria, there have been reported outbreaks along the DMZ [12,27]. Therefore, in the future, it will become important to perform surveillance for pathogens, including Anaplasma, Ehrlichia, Bartonella, Borrelia, and Rickettsia spp., in vectors and wild animals, as well as in civilian and military populations that reside or train near the DMZ. It is imperative to continue the efforts to identify additional tick-borne pathogens to further disclose the extent and possible public health significance of these agents.
Acknowledgments
Funding for portions of this work was provided by the US Department of Defense Global Emerging Infections Surveillance and Response System, Silver Spring, MD, the Armed Forces Medical Intelligence Center, Ft Detrick, MD. Dr. Joon-seok Chae received funding from the LG Yeonam Foundation.
References
- 1.Adelson ME, Rao RVS, Tilton RC, Cabets K, Eskow E, Fein L, Occi JL, Mordechai E. Prevalence of Borrelia burgdorferi, Bartonella spp. Babesia microti, and Anaplasma phagocytophila in Ixodes scapularis ticks collected in northern New Jersey. J Clin Microbiol. 2004;42:2799–2801. doi: 10.1128/JCM.42.6.2799-2801.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. Diagnosis of Lyme borreliosis. Clin Microbiol Rev. 2005;18:484–509. doi: 10.1128/CMR.18.3.484-509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson BE, Sumner JW, Dawson JE, Tzianabos T, Greene CR, Olson JG, Fishbein DB, Olsen-Rasmussen M, Holloway BP, George EH, Azad AF. Detection of the etiologic agent of human ehrlichiosis by polymerase chain reaction. J Clin Microbiol. 1992;30:775–780. doi: 10.1128/jcm.30.4.775-780.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barlough JE, Madigan JE, Derock E, Bigornia L. Nested polymerase chain reaction for detection of Ehrlichia equi genomic DNA in horses and ticks (Ixodes pacificus) Vet Parasitol. 1996;63:319–329. doi: 10.1016/0304-4017(95)00904-3. [DOI] [PubMed] [Google Scholar]
- 5.Chae JS, Kim CM, Kim EH, Hur EJ, Klein TA, Kang TK, Lee HC, Song JW. Molecular epidemiological study for tick-borne disease (Ehrlichia and Anaplasma spp.) surveillance at selected U.S. military training sites/installations in Korea. Ann N Y Acad Sci. 2003;990:118–125. doi: 10.1111/j.1749-6632.2003.tb07349.x. [DOI] [PubMed] [Google Scholar]
- 6.Dawson JE, Biggie KL, Warner CK, Cookson K, Jenkins S, Levine JF, Olson JG. Polymerase chain reaction evidence of Ehrlichia chaffeensis, an etiologic agent of human ehrlichiosis, in dogs from southeast Virginia. Am J Vet Res. 1996;57:1175–1179. [PubMed] [Google Scholar]
- 7.Dawson JE, Stallknecht DE, Howerth EW, Warner C, Biggie K, Davidson WR, Lockhart JM, Nettles VF, Olson JG, Childs JE. Susceptibility of white-tailed deer (Odocoileus virginianus) to infection with Ehrlichia chaffeensis, the etiologic agent of human ehrlichiosis. J Clin Microbiol. 1994;32:2725–2728. doi: 10.1128/jcm.32.11.2725-2728.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fritz CL, Kjemtrup AM. Lyme borreliosis. J Am Vet Med Assoc. 2003;223:1261–1270. doi: 10.2460/javma.2003.223.1261. [DOI] [PubMed] [Google Scholar]
- 9.Furuya Y, Katayama T, Yoshida Y, Kaiho I. Specific amplification of Rickettsia japonica DNA from clinical specimens by PCR. J Clin Microbiol. 1995;33:487–489. doi: 10.1128/jcm.33.2.487-489.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen J, Ruedy R, Glascoe J, Sato N. GISS analysis of surface temperature change. J Geophys Res. 1999;104:30997–31022. [Google Scholar]
- 11.Heo EJ, Park JH, Koo JR, Park MS, Park MY, Dumler JS, Chae JS. Serologic and molecular detection of Ehrlichia chaffeensis and Anaplasma phagocytophila (Human granulocytic ehrlichiosis agent) in Korean patients. J Clin Microbiol. 2002;40:3082–3085. doi: 10.1128/JCM.40.8.3082-3085.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyun BH, Lee KK, Kim IJ, Lee KW, Park HJ, Lee OS, An SH, Lee JB. Molecular epidemiology of rabies virus isolates from South Korea. Virus Res. 2005;114:113–125. doi: 10.1016/j.virusres.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Inokuma H, Beppu T, Okuda M, Shimada Y, Sakata Y. Detection of ehrlichial DNA in Haemaphysalis ticks recovered from dogs in Japan that is closely related to a novel Ehrlichia sp. found in cattle ticks from Tibet, Thailand, and Africa. J Clin Microbiol. 2004;42:1353–1355. doi: 10.1128/JCM.42.3.1353-1355.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inokuma H, Brouqui P, Drancourt M, Raoult D. Citrate synthase gene sequence: a new tool for phylogenetic analysis and identification of Ehrlichia. J Clin Microbiol. 2001;39:3031–3039. doi: 10.1128/JCM.39.9.3031-3039.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang WJ, Kim JH, Choi YJ, Jung KD, Kim YG, Lee SH, Choi MS, Kim IS, Walker DH, Park KH. First serologic evidence of human spotted fever group rickettsiosis in Korea. J Clin Microbiol. 2004;42:2310–2313. doi: 10.1128/JCM.42.5.2310-2313.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim CM, Kim JY, Yi YH, Lee MJ, Cho MR, Shah DH, Klein TA, Kim HC, Song JW, Chong ST, O'Guinn ML, Lee JS, Lee IY, Park JH, Chae JS. Detection of Bartonella species from ticks, mites and small mammals in Korea. J Vet Sci. 2005;6:327–334. [PubMed] [Google Scholar]
- 17.Kim CM, Kim MS, Park MS, Park JH, Chae JS. Identification of Ehrlichia chaffeensis, Anaplasma phagocytophilum, and A. bovis in Haemaphysalis longicornis and Ixodes persulcatus ticks from Korea. Vecter Borne Zoonotic Dis. 2003;3:17–26. doi: 10.1089/153036603765627424. [DOI] [PubMed] [Google Scholar]
- 18.Kim CM, Yi YH, Yu DH, Lee MJ, Cho MR, Desai AR, Shringi S, Klein TA, Kim HC, Song JW, Baek LJ, Chong ST, O'Guinn ML, Lee JS, Lee IY, Park JH, Foley J, Chae JS. Tick-borne rickettsial pathogens in ticks and small mammals in Korea. Appl Environ Microbiol. 2006;72:5766–5776. doi: 10.1128/AEM.00431-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SY, Yun SM, Han MG, Lee IY, Lee NY, Jeong YE, Lee BC, Ju YR. Isolation of tick-borne encephalitis viruses from wild rodents, South Korea. Vector Borne Zoonotic Dis. 2008;8:7–13. doi: 10.1089/vbz.2006.0634. [DOI] [PubMed] [Google Scholar]
- 20.La Scola B, Liang Z, Zeaiter Z, Houpikian P, Grimont PA, Raoult D. Genotypic characteristics of two serotypes of Bartonella henselae. J Clin Microbiol. 2002;40:2002–2008. doi: 10.1128/JCM.40.6.2002-2008.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SO, Na DK, Kim CM, Li YH, Cho YH, Park JH, Lee JH, Eo SK, Klein TA, Chae JS. Identification and prevalence of Ehrlichia chaffeensis infection in Haemaphysalis longicornis ticks from Korea by PCR, sequencing and phylogenetic analysis based on 16S rRNA gene. J Vet Sci. 2005;6:151–155. [PubMed] [Google Scholar]
- 22.Mathew JS, Ewing SA, Murphy GL, Kocan KM, Corstvet RE, Fox JC. Characterization of a new isolate of Ehrlichia platys (order rickettsiales) using electron microscopy and polymerase chain reaction. Vet Parasitol. 1997;68:1–10. doi: 10.1016/s0304-4017(96)01052-7. [DOI] [PubMed] [Google Scholar]
- 23.Murphy GL, Ewing SA, Whitworth LC, Fox JC, Kocan AA. A molecular and serologic survey of Ehrlichia canis, E. chaffeensis, and E. ewingii in dogs and ticks from Oklahoma. Vet Parasitol. 1998;79:325–339. doi: 10.1016/s0304-4017(98)00179-4. [DOI] [PubMed] [Google Scholar]
- 24.Norman AF, Regnery R, Jameson P, Greene C, Krause DC. Differentiation of Bartonella-like isolates at the species level by PCR-restriction fragment length polymorphism in the citrate synthase gene. J Clin Microbiol. 1995;33:1797–1803. doi: 10.1128/jcm.33.7.1797-1803.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pahl A, Kühlbrandt U, Brune K, Röllinghoff M, Gessner A. Quantitative detection of Borrelia burgdorferi by real-time PCR. J Clin Microbiol. 1999;37:1958–1963. doi: 10.1128/jcm.37.6.1958-1963.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park JH, Heo EJ, Choi KS, Dumler JS, Chae JS. Detection of antibodies to Anaplasma phagocytophilum and Ehrlichia chaffeensis antigens in sera of Korean patients by western immunoblotting andindirect immunofluorescence assays. Clin Diagn Lab Immunol. 2003;10:1059–1064. doi: 10.1128/CDLI.10.6.1059-1064.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park JW, Klein TA, Lee HC, Pacha LA, Ryu SH, Yeom JS, Moon SH, Kim TS, Chai JY, Oh MD, Choe KW. Vivax malaria: a continuing health threat to the Republic of Korea. Am J Trop Med Hyg. 2003;69:159–167. [PubMed] [Google Scholar]
- 28.Park KH, Chang WH, Schwan TG. Identification and characterization of Lyme disease spirochetes, Borrelia burgdorferi sensu lato, isolated in Korea. J Clin Microbiol. 1993;31:1831–1837. doi: 10.1128/jcm.31.7.1831-1837.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parola P, Davoust B, Raoult D. Tick- and flea-borne rickettsial emerging zoonoses. Vet Res. 2005;36:469–492. doi: 10.1051/vetres:2005004. [DOI] [PubMed] [Google Scholar]
- 30.Regnery RL, Spruill CL, Plikaytis BD. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol. 1991;173:1576–1589. doi: 10.1128/jb.173.5.1576-1589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schouls LM, Van De Pol I, Rijpkema SGT, Schot CS. Detection and identification of Ehrlichia, Borrelia burgdorferi sensu lato, and Bartonella species in Dutch Ixodes ricinus ticks. J Clin Microbiol. 1999;37:2215–2222. doi: 10.1128/jcm.37.7.2215-2222.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoon SY, Gye MC, Lee HS. Mammalian fauna in DMZ area. Korean J Environ Biol. 2007;25:215–222. [Google Scholar]
- 33.Wang IN, Dykhuizen DE, Qiu W, Dunn JJ, Bosler EM, Luft BJ. Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics. 1999;151:15–30. doi: 10.1093/genetics/151.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]