Summary
Light has profound behavioral effects on almost all animals, and nocturnal animals show sensitivity to extremely low light levels [1–4]. Crepuscular, i.e., dawn/dusk-active animals such as Drosophila melanogaster are thought to show far less sensitivity to light [5–8]. Here we report that Drosophila respond to extremely low levels of monochromatic blue light. Light levels 3–4 orders of magnitude lower than previously believed impact circadian entrainment and the light-induced stimulation of locomotion known as positive behavioral masking. We use GAL4; UAS- mediated rescue of tyrosine hydroxylase (DTH) mutant (ple) flies to study the roles of dopamine in these processes. We present evidence for two roles of dopamine in circadian behaviors. First, rescue with either a wild type DTH or a DTH mutant lacking neural expression leads to weak circadian rhythmicity, indicating a role for strictly regulated DTH and dopamine in robust circadian rhythmicity. Second, the DTH rescue strain deficient in neural dopamine selectively shows a defect in circadian entrainment to low light, whereas another response to light, positive masking, has normal light sensitivity. These findings imply separable pathways from light input to the behavioral outputs of masking versus circadian entrainment, with only the latter dependent on dopamine.
Results
We developed two assays to study the low light behavioral responses of Drosophila melanogaster. In the first, we examine circadian entrainment of flies to 12:12 LD cycles, followed by 6 hr phase delays concurrent with a 10-fold reduction in light intensity. We use planar diffuse sources of monochromatic blue light (470 ± 20 nm), generated from LEDs (see methods). Blue light is not the standard in the circadian field, but is a wavelength to which flies show maximal circadian photosensitivity [9–11], and is easier to describe than white light sources which are undefined in wavelength composition.
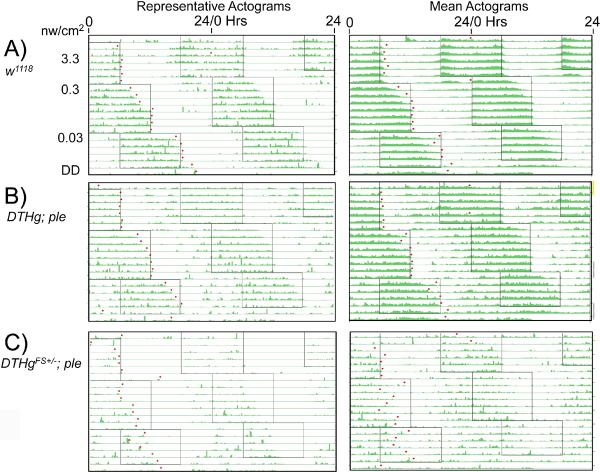
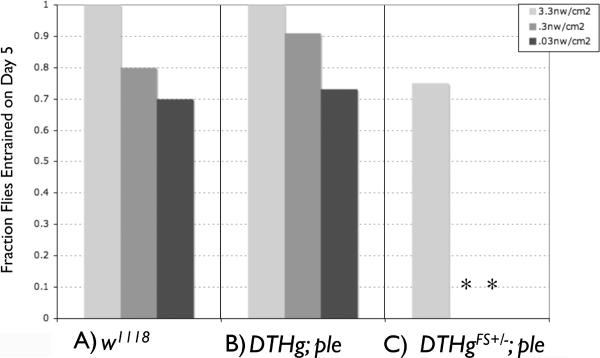
With this assay, we find that wild type flies robustly entrain activity rhythms to light levels as low as 0.03 nW/cm2 (Figure 1A), as determined by activity-off points from representative individual or mean actograms. Summing data from all 12 flies, all flies are entrained by day 5 at 3.3 nW/cm2, and 70% are entrained by day 5 even at 0.03 nW/cm2 (Figure 2A). We have not found the low light threshold for entrainment, since wild type flies still entrain even at 0.001 nW/cm2, though entrainment takes ~10 days at this light level (data not shown).
Figure 1.
Dim Light Actograms of ple Rescue Flies Representative (left) and mean (right) double plotted actograms from low light phase delays. Boxed areas represent light periods, with blue light intensity as shown to the left of the actogram. Left panels: Representative actograms. Right panels: Mean actograms. A) w1118; n= 10; B) DTHg; ple; n=11 C) DTHgFS±; ple; n=8. Flies were allowed to entrain to a given light intensity as indicated, then subjected to simultaneous 6 hr phase delays with a reduction of light intensity. Dots are the computer-called activity offsets used to determine entrainment. The initial LD schedule started at 6 pm, ~12 hours away from their normal LD phase.
Figure 2.
Neural dopamine is required for low light entrainment. Fraction of flies entrained by day 5 at the given light intensity: A) w1118; B) DTHg; ple; C) DTHgFS±; ple. Flies were considered to be entrained if activity offset was within 1 hr of the time of lights off. Asterisks indicate significant differences from the w1118 control at the same light intensity, Chi Squared, P<001.
We examined the role of dopamine in circadian entrainment by utilizing two lines that show complete or partial rescue of the Drosophila pale (ple) locus, encoding tyrosine hydroxylase, the rate limiting enzyme for dopamine biosynthesis (Riemensperger et al, in preparation). In these lines, full rescue of the ple lethality is achieved by driving expression of a genomic UAS-ple transgene, DTHg, with a combination of Ddc- and TH-GAL4 transgenes. These rescued flies show normal lifespan and normal brain dopamine levels (Riemensperger et al. in preparation), and their low light intensity entrainment is indistinguishable from wild type (Figures 1B, 2B).
To study the roles of neural dopamine, flies were constructed by an analogous strategy, but using a UAS-ple transgene, DTHgFS±, containing compensating +1 and −1 reading frame-shifts in a hypoderm-specific and adjacent common exon (Riemensperger et al, in preparation). By this strategy, the hypodermal TH isoform, which provides dopamine that is vital for survival, contains 15 missense amino acids in a non-critical region of the TH protein, such that survival to adult and adult lifespan is normal, whereas the CNS splice isoform is terminated by a nonsense mutation. These flies lack dopamine in the central brain and optic lobes, as determined both by dopamine and TH immunocytochemistry, and by HPLC, with a detection limit of ~2% normal levels (Riemensperger et al, in preparation).
Both the neural dopamine deficient DTHgFS±; ple and the wild type rescue flies, DTHg; ple, show reduced circadian rhythmicity. As shown in Table I, the fraction of rhythmic flies drops from ~87% in the w1118 control, to 28 to 37%, respectively, in the two rescue lines, with a non-significant difference between these latter two lines. Thus, restoration of normal levels of brain dopamine is not sufficient to restore normal rhythmicity. Examining the DTHgFS± and the wild type rescue flies, DTHg in a heterozygous ple/+ background shows that a single copy of ple+ is sufficient to rescue rhythmicity, making it is extremely unlikely that the arrhymicity is due to effects of genetic background.
Table I.
Circadian Behavior and Activity Levels in DTHg Rescue Flies in ple and ple/+ Backgrounds
| Genotype: | w1118 (n=31) | DTHg; ple (N=30) | DTHg; ple/+ (n=10) | DTHgFS+/−; ple (n=32) | DTHgFS+/−; ple/+ (n=8) |
|---|---|---|---|---|---|
| Percent Rhythmic | 87.1 | 36.7 (P<0.001) | 80.0 | 28.1 (P<.001) | 87.5 |
| Period (Hours) ± SEM | 23.5 ± 0.05 | 23.7 ± 0.16 | 23.3 ± 0.10 | 24.2 ± .37 | 23.0 ± .07 (P<.001) |
| Activity Count/24 Hours | 919.2 ± 62.3 | 1208.3 ± 72.3 (P<0.004) | 1414.4 ± 133.0 (P<.001) | 689.4 ± 81.5 | 1117.8 ± 156.8 |
Circadian period in the rescue lines in ple backgrounds is not significantly different from the w1118 control, although there is a slight period shortening in DTHgFS±; ple/+ . Overall activity levels are increased in the DTHg rescue lines in ple or ple/+ backgrounds.
There is one behavioral alteration that is strikingly restricted to the dopamine deficient line. The neural dopamine deficient DTHgFS±; ple flies show a striking defect in low-light entrainment, as shown by actograms (Figure 1C), or by measuring the fraction of flies entrained by day 5 (Figure 2C). At the highest blue light intensity used, 3.3 nw/cm2, entrainment is near normal, but entrainment to the 6 hr phase delays at lower intensities is not observed. However, low light entrainment of the DTH;ple rescue flies is normal. This indicates that dopamine has a critical role in modulating the light sensitivity for circadian entrainment.
We also developed an assay to measure the non-clock dependent locomotor stimulating effects of light, positive behavioral masking, adapted from masking assays used in mice [12, 13]. The masking effect of light can be observed qualitatively in Figure 1, by the stimulation of locomotor activity at the beginning of the light phase, an effect seen even in flies lacking functional circadian clocks [8]. To more quantitatively measure behavioral masking, we subjected the flies to a 7 hr ultradian light cycle, with 3.5 hrs light followed by 3.5 hrs dark, varying the intensity of light every 7 cycles. Since seven hours is not a fractional harmonic of the normal 24 hr day length, the animals never entrain to this schedule.
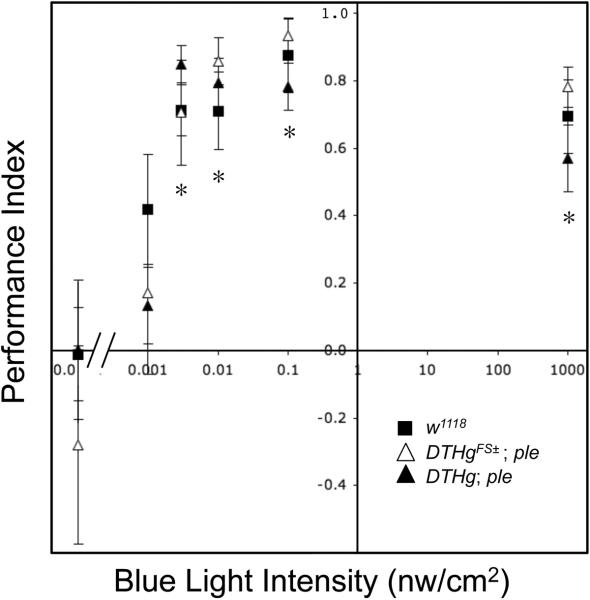
This behavioral masking data is analyzed by measuring the fraction of locomotor activity during the light phase of each 7 hr `day', converting this t o a Performance Index (PI) (see methods). A plot of this PI versus light intensity is shown in Figure 3. This plot shows significant masking for w1118, DTH;ple, and the neural dopamine deficient line DTHgFS±;ple, at or above blue light intensities of 0.003 nw/cm2. Thus, as with entrainment, flies show unexpected light sensitivity for behavioral masking. However, masking light sensitivity is indistinguishable in these lines. Thus, there is a selective role for dopamine in light dependent circadian entrainment, with no apparent role in the pathway leading to behavioral masking.
Figure 3.
Neural dopamine is not required for low light masking behavior. Masking performance index is plotted as a function of blue light intensity. Flies were subjected to ultradian 7 hours days consisting of 3.5 hrs light, 3.5 dark. The fraction of locomotor activity in the light period was converted to a performance index according to the formula 2(L-0.5), where L is the fraction of total activity in the light period. The 3.5 hr light period is sufficiently long such that the ~1 hr burst of locomotor activity at the initiation of the light period only comprises a minor fraction of the total light period activity. Symbols: Squares:■ w1118; n= 7; Open triangles:△ DTHg; ple; n= 11 Filled triangles:▲ DTHgFS±; ple; n= 11. Asterisks indicate significant differences from performance index of given genotype in the dark, ANOVA, P<0.01.
Discussion
Sensitivity to extremely low levels of light is most commonly found in nocturnal animals. These animals, such as nocturnal geckos or insects such as nocturnal hawkmoths cannot only sense extremely low levels of light, but can discern colors at light intensities well below those to which diurnal animals are sensitive. Humans and diurnal vertebrates lose color vision at light intensities comparable to dim moonlight at irradiances of 3–10 nW/cm2 (reviewed in [1, 2]). In contrast, nocturnal hawkmoths and geckos can discern colors even at intensities of ~0.01– 0.3 nW/cm2, and normally function in starlight, ~0.001 nw/cm2 [3, 4]. Extreme light sensitivity in nocturnal insects commonly involves adaptations to their compound eyes to allow summation of photons from many individual ommatidia (reviewed in (Kelber and Roth, 2006)). These visual system adaptations are not seen in diurnal insects such as the fruit fly, Drosophila melanogaster. Accordingly, current data accords Drosophila with rather modest light sensitivity. For light-dependent entrainment of circadian rhythmicity, ~40 nW/cm2 blue light was required (Helfrich-Forster et al., 2002), although subsequent studies show entrainment by 1–5 nW/cm2 white light [5, 7]. A recent correction shows that the light intensity required to entrain wild type flies in Helfrich-Forster et al.(2002) was miscalculated, such that wild type flies are now thought to entrain at ~0.04 nW/cm2 blue light (C. Helfrich-Forster, personal communication). An intensity of ~0.5 nW/cm2 white light is reported to cause positive behavioral masking [6], the largely circadian clock-independent stimulation of locomotion [8]. For comparison, we find that a dark-adapted human observer loses the ability to perceive the diffuse planar blue light sources used in the present study at intensities of ~0.01–0.03 nW/cm2 (unpublished observations). This intensity is difficult to compare to published human perception studies which commonly use short duration flashes of focal light [14–16].
We find unexpectedly strong light sensitivity for Drosophila melanogaster, with behavioral masking and circadian entrainment at intensities as low as 0.001 nW/cm2, and at least two roles for dopamine in circadian rhythmicity. First, DTH rescue flies show poor behavioral rhythmicity in constant dark conditions, independent of whether dopamine levels are rescued in the nervous system. Second, we find that neuronal DTH rescue flies lacking neuronal dopamine show reduced light sensitivity for circadian entrainment, whereas light sensitivity of behavioral masking is unaffected. Dopamine has several roles in Drosophila neural function, from modulation of locomotor behaviors and arousal states [17–20](Riemensperger et al,, in preparation) to learning & memory [21–25](Riemensperger et al., in preparation), but a role for dopamine in insect light dependent circadian behavioral entrainment is novel.
The two circadian phenotypes likely represent separate roles for dopamine, presumably in different regions of the nervous system, since reduced amplitude of rhythmicity, as seen in our DTH rescue lines, is normally associated with higher rather than lower efficacy of reentrainment [26, 27]. The dopaminergic system in Drosophila is highly rhythmic, as evidenced by rhythmicity in responsiveness to dopamine agonists [28], and by the rhythmic transcription of the tyrosine hydroxylase gene [29–31], ple, which encodes the rate limiting enzyme in dopamine biosynthesis [32]. The rhythmicity of the ple transcript may explain the poor rhythmicity in ple rescue animals. These animals have near normal levels of brain dopamine in an apparently normal cellular pattern (Riemensperger et al, in preparation), but the inclusion of the GAL4 transcription factor into the regulatory cascade will almost certainly interfere with normal temporal cycling of the DTH transcript. Note that we have not detected significant diurnal variation in levels of brain dopamine in brain extracts [33], but this does not preclude diurnal variation in dopamine neuron subsets.
Low light circadian entrainment is disrupted in the brain dopamine deficient DTHgFS±; ple flies. The simplest mechanism for the disruption of low light circadian entrainment would be due to alterations in the photoreceptive pathway, which could be via cryptochrome (CRY), or visual photoreceptors. There is some support for dopaminergic involvement in the CRY pathway, since Sathyanarayanan et al (2008)[34] identified ple in a screen for genes that when targetted by RNAi have a strong inhibitory effect on light dependent degradation of CRY and TIM in cultured cells. This could indicate a positive role for dopamine in light dependent degradation of these molecules, providing a potential mechanism for the reduced light sensiivity for circadian entrainment that we observe in the absence of dopamine.
Atlernatively, it is known that visual photoreceptors are involved in dim-light entrainment, since genetic loss of all photoreceptive visual organs results in at least a 3 order of magnitude reduction in blue light sensitivity for circadian entrainment [11]. Analogous studies in mice show a ~60 fold reduction in dim light sensitivity for entrainment in rod/cone deficient animals [12].
A role for dopamine in fly visual function has some support, in that cAMP can slow the response to light in a preparation of isolated Drosophila photoreceptors, and this effect can be mimicked by application of octopamine or dopamine, an effect interpreted as enhanced adaptation to dark [35]. Dopamine signaling, via cAMP second messenger pathways, is not currently considered part of the main insect visual transduction pathway [36]. However, dopamine involvement could have been missed if it has an exclusive role in a neural pathway selectively required for circadian entrainment by dim light.
There is strong support of a role for dopamine functioning in the vertebrate retina, which makes visual involvement of dopamine in the fly all the more likely. The vertebrate retina contains autonomous circadian oscillators that are thought to allow the retina to prepare for the large difference in light intensity between day and night (reviewed in [37]. Central to this rhythmicity are opposing and rhythmic roles for melatonin and dopamine, with release of each modulator inhibiting synthesis and/or release of the other. The best defined role for dopamine in the vertebrate circadian oscillator is in entraining fetal rodents prior to light exposure, a capacity lost in adults [38–40]. This role of dopamine in could be related to the roles we have uncovered in adult Drosophila.
A selective role for dopamine in low light entrainment
The selective effect of neural dopamine on low light entrainment versus low light masking behavior implies separable pathways involved in modulating these behaviors, a novel finding, since previous studies have only identified circadian components with parallel effects on masking [41]. The best defined synaptic connections from eye to circadian neurons are the projections from the Drosophila eyelet, a remnant of the larval photoreceptive Bolwig's organ [11]. These authors show that this photoreceptive organ makes projections that terminate in close apposition to neurites from the s-LNv's and l-LNv's, neurons key to circadian rhythmicity [42, 43]. Connections from the main visual photoreceptors to these circadian neurons must be indirect, since the rod-like outer photoreceptor ommatidia terminate in the optic lamina, and the cone-like central ommatidia terminate in the optic medulla (reviewed in [44]). Nonetheless, dopamine could be acting as a neuromodulator in any of these pathways to increase sensitivity to a light dependent signal. The genetic tools available in Drosophila should prove useful to precisely identify these pathways.
Methods
Fly strains
Fly strains were as in Riemensperger et al (in preparation). Flies containing the wildtype rescuing UAS-DTHg, or the neural dopamine deficient UAS-DTHgFS± in ple background, were generated prior to each experiment by crossing a line containing the TH- and Ddc-GAL4 drivers with the respective UAS-transgene, each in a ple mutant background.
Assays of circadian rhythmicity and period
Flies were subject to 5 days of bright 12:12 LD conditions, at 450 μw/cm2 white light, then taken into constant darkness to measure circadian behaviors. Rhythmicity and period in constant darkness was determined over 7 days, using Clocklab software to perform chi-squared periodogram analyses (Coulbourne Instruments, Whitehall, PA, USA).
Low light assays
Light controlled chambers were constructed from light-tight wooden boxes, fitted at either end with light tight baffles to allow ambient ventilation (Mill Cabinets, Bridgewater, VA). Diffuse monochromatic light was provided from eight 5 mm discrete 470 nm LEDs with serial resistors (Shenzhen Sheng Nan Electronics, Shenzhen, PR China, http://www.sn-led.com/), mounted in a plastic sheet pointing away from the interior of the chamber, and separated from the main chamber by a white plastic diffuser. Light intensity measured at the surface of the diffuser varied by no more than 30% over the surface of the diffuser when measured by a UDT 350 Photometer (United Detector Technologies, Baltimore, MD). LED intensity was controlled with constant voltage power supplies (Mastech, HY1803D), modified to allow finer control by replacing the supplied voltage control potentiometer with a 10 turn 5K wirewound potentiometer.
Absolute light levels down to 1 nW/cm2 were measured with the UDT 350 Photometer. Lower intensities were measured using low light tandem silicon cells (HP-5520-8, Nuoqun (Happy) Microelectronics, Ghuangdong, PR China), which provide a voltage linearly related to irradiance at the low end of their output range, and which provide good signal-to-noise down to 1 pW/cm2 blue light (unpublished data).
The light tight chambers were housed in a temperature/humidity controlled room, at 18–20°C, 50–70% relative humidity. Fly activity levels were monitored in Trikinetics activity monitors (Waltham, MA), using male flies with a plug of standard yeast agar fly food at one end. Since the activity monitors output high levels of infrared radiation which is efficiently detected by the photocells, the photocells were mounted pointing away from the monitors, and adjacent to the LED light source. Data was collected, and light schedules were controlled using DAM software (Trikinetics).
Ultradian masking assays were performed by subjecting animals to 7 cycles of 7 hour `days', of 3.5 hours L/3.5 hours D at each light intensity. The fraction of locomotor activity during the light phase (L) of each 7 hr `day' was converted this to a Performance Index (PI) using the formula 2(L- 0.5).
Acknowledgements
We thank Carla Green, Ignacio Provencio, Michael Menaker, and Herman Wijnen for helpful comments throughout this work. JH is supported by a grant from the NIH, and was supported by intramural funds from the University of Virginia. TR was supported by a fellowship from the Fondation pour la Recherche Médicale, and SB was supported by a research grant from the Fondation de France.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Kelber A, Roth LS. Nocturnal colour vision--not as rare as we might think. J Exp Biol. 2006;209:781–788. doi: 10.1242/jeb.02060. [DOI] [PubMed] [Google Scholar]
- 2.Roth LS, Balkenius A, Kelber A. The absolute threshold of colour vision in the horse. PLoS ONE. 2008;3:e3711. doi: 10.1371/journal.pone.0003711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roth LS, Kelber A. Nocturnal colour vision in geckos. Proceedings. 2004;271(Suppl 6):S485–487. doi: 10.1098/rsbl.2004.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnsen S, Kelber A, Warrant E, Sweeney AM, Widder EA, Lee RL, Jr., Hernandez-Andres J. Crepuscular and nocturnal illumination and its effects on color perception by the nocturnal hawkmoth Deilephila elpenor. J Exp Biol. 2006;209:789–800. doi: 10.1242/jeb.02053. [DOI] [PubMed] [Google Scholar]
- 5.Bachleitner W, Kempinger L, Wulbeck C, Rieger D, Helfrich-Forster C. Moonlight shifts the endogenous clock of Drosophila melanogaster. Proc Natl Acad Sci U S A. 2007;104:3538–3543. doi: 10.1073/pnas.0606870104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kempinger L, Dittmann R, Rieger D, Helfrich-Forster C. The nocturnal activity of fruit flies exposed to artificial moonlight is partly caused by direct light effects on the activity level that bypass the endogenous clock. Chronobiology international. 2009;26:151–166. doi: 10.1080/07420520902747124. [DOI] [PubMed] [Google Scholar]
- 7.Klarsfeld A, Malpel S, Michard-Vanhee C, Picot M, Chelot E, Rouyer F. Novel features of cryptochrome-mediated photoreception in the brain circadian clock of Drosophila. J Neurosci. 2004;24:1468–1477. doi: 10.1523/JNEUROSCI.3661-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wheeler DA, Hamblen-Coyle MJ, Dushay MS, Hall JC. Behavior in light-dark cycles of Drosophila mutants that are arrhythmic, blind, or both. J Biol Rhythms. 1993;8:67–94. doi: 10.1177/074873049300800106. [DOI] [PubMed] [Google Scholar]
- 9.Blaschke I, Lang P, Hofbauer A, Engelmann W, Helfrich-Forster C. Preliminary action spectra suggest that the clock cells of Drosophila are synchronized to the external LD-cycle by the compound eyes plus extraretinal photoreceptors. Brain and evolution; Proceedings of the 24th Gottingen neurobiology conference I.1996. [Google Scholar]
- 10.Frank KD, Zimmerman WF. Action spectra for phase shifts of a circadian rhythm in Drosophila. Science. 1969;163:688–689. doi: 10.1126/science.163.3868.688. [DOI] [PubMed] [Google Scholar]
- 11.Helfrich-Forster C, Edwards T, Yasuyama K, Wisotzki B, Schneuwly S, Stanewsky R, Meinertzhagen IA, Hofbauer A. The extraretinal eyelet of Drosophila: development, ultrastructure, and putative circadian function. J Neurosci. 2002;22:9255–9266. doi: 10.1523/JNEUROSCI.22-21-09255.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mrosovsky N, Hattar S. Impaired masking responses to light in melanopsin-knockout mice. Chronobiology international. 2003;20:989–999. doi: 10.1081/cbi-120026043. [DOI] [PubMed] [Google Scholar]
- 13.Redlin U, Hattar S, Mrosovsky N. The circadian Clock mutant mouse: impaired masking response to light. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005;191:51–59. doi: 10.1007/s00359-004-0570-z. [DOI] [PubMed] [Google Scholar]
- 14.Warrant E, Allan IB, Akimichi K, Gordon MS, Gerald W, Thomas DA, Richard HM, Peter D, Donata O, Stuart F. The Senses: A Comprehensive Reference. Academic Press; New York: 2008. Nocturnal Vision; pp. 53–86. [Google Scholar]
- 15.Hecht S, Shlaer S, Pirenne MH. Energy at the Threshold of Vision. Science. 1941;93:585–587. doi: 10.1126/science.93.2425.585. [DOI] [PubMed] [Google Scholar]
- 16.Hood DCF, M.A. Sensitivity to light. In Handbook of perception and human performance. In: Boff KR, Kaufman L, Thomas JP, editors. Sensory Processes and Perception. Volume 1. Wiley; New York: 1986. [Google Scholar]
- 17.Friggi-Grelin F, Coulom H, Meller M, Gomez D, Hirsh J, Birman S. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J Neurobiol. 2003;54:618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- 18.Andretic R, van Swinderen B, Greenspan RJ. Dopaminergic modulation of arousal in Drosophila. Curr Biol. 2005;15:1165–1175. doi: 10.1016/j.cub.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 19.Kume K, Kume S, Park SK, Hirsh J, Jackson FR. Dopamine is a regulator of arousal in the fruit fly. J Neurosci. 2005;25:7377–7384. doi: 10.1523/JNEUROSCI.2048-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lima SQ, Miesenbock G. Remote Control of Behavior through Genetically Targeted Photostimulation of Neurons. Cell. 2005;121:141–152. doi: 10.1016/j.cell.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riemensperger T, Voller T, Stock P, Buchner E, Fiala A. Punishment prediction by dopaminergic neurons in Drosophila. Curr Biol. 2005;15:1953–1960. doi: 10.1016/j.cub.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 23.Kim YC, Lee HG, Han KA. D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila. J Neurosci. 2007;27:7640–7647. doi: 10.1523/JNEUROSCI.1167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Claridge-Chang A, Roorda RD, Vrontou E, Sjulson L, Li H, Hirsh J, Miesenbock G. Writing memories with light-addressable reinforcement circuitry. Cell. 2009;139:405–415. doi: 10.1016/j.cell.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krashes MJ, DasGupta S, Vreede A, White B, Armstrong JD, Waddell S. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell. 2009;139:416–427. doi: 10.1016/j.cell.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Busza A, Murad A, Emery P. Interactions between circadian neurons control temperature synchronization of Drosophila behavior. J Neurosci. 2007;27:10722–10733. doi: 10.1523/JNEUROSCI.2479-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vitaterna MH, Ko CH, Chang AM, Buhr ED, Fruechte EM, Schook A, Antoch MP, Turek FW, Takahashi JS. The mouse Clock mutation reduces circadian pacemaker amplitude and enhances efficacy of resetting stimuli and phase-response curve amplitude. Proc Natl Acad Sci U S A. 2006;103:9327–9332. doi: 10.1073/pnas.0603601103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andretic R, Hirsh J. Circadian modulation of dopamine receptor responsiveness in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2000;97:1873–1878. doi: 10.1073/pnas.97.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDonald MJ, Rosbash M. Microarray analysis and organization of circadian gene expression in Drosophila. Cell. 2001;107:567–578. doi: 10.1016/s0092-8674(01)00545-1. [DOI] [PubMed] [Google Scholar]
- 30.Ceriani MF, Hogenesch JB, Yanovsky M, Panda S, Straume M, Kay SA. Genome-wide expression analysis in Drosophila reveals genes controlling circadian behavior. J Neurosci. 2002;22:9305–9319. doi: 10.1523/JNEUROSCI.22-21-09305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boothroyd CE, Wijnen H, Naef F, Saez L, Young MW. Integration of light and temperature in the regulation of circadian gene expression in Drosophila. PLoS Genet. 2007;3:e54. doi: 10.1371/journal.pgen.0030054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neckameyer WS. Multiple roles for dopamine in Drosophila development. Dev Biol. 1996;176:209–219. doi: 10.1006/dbio.1996.0128. [DOI] [PubMed] [Google Scholar]
- 33.Hardie SL, Hirsh J. An improved method for the separation and detection of biogenic amines in adult Drosophila brain extracts by high performance liquid chromatography. J Neurosci Methods. 2005 doi: 10.1016/j.jneumeth.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Sathyanarayanan S, Zheng X, Kumar S, Chen CH, Chen D, Hay B, Sehgal A. Identification of novel genes involved in light-dependent CRY degradation through a genome-wide RNAi screen. Genes Dev. 2008;22:1522–1533. doi: 10.1101/gad.1652308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chyb S, Hevers W, Forte M, Wolfgang WJ, Selinger Z, Hardie RC. Modulation of the light response by cAMP in Drosophila photoreceptors. J Neurosci. 1999;19:8799–8807. doi: 10.1523/JNEUROSCI.19-20-08799.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montell C. Visual transduction in Drosophila. Annual review of cell and developmental biology. 1999;15:231–268. doi: 10.1146/annurev.cellbio.15.1.231. [DOI] [PubMed] [Google Scholar]
- 37.Green CB, Besharse JC. Retinal circadian clocks and control of retinal physiology. J Biol Rhythms. 2004;19:91–102. doi: 10.1177/0748730404263002. [DOI] [PubMed] [Google Scholar]
- 38.Viswanathan N, Weaver DR, Reppert SM, Davis FC. Entrainment of the fetal hamster circadian pacemaker by prenatal injections of the dopamine agonist SKF 38393. J Neurosci. 1994;14:5393–5398. doi: 10.1523/JNEUROSCI.14-09-05393.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weaver DR, Reppert SM. Definition of the developmental transition from dopaminergic to photic regulation of c-fos gene expression in the rat suprachiasmatic nucleus. Brain Res Mol Brain Res. 1995;33:136–148. doi: 10.1016/0169-328x(95)00117-b. [DOI] [PubMed] [Google Scholar]
- 40.Duffield GE, Hastings MH, Ebling FJ. Investigation into the regulation of the circadian system by dopamine and melatonin in the adult Siberian hamster (Phodopus sungorus) J Neuroendocrinol. 1998;10:871–884. doi: 10.1046/j.1365-2826.1998.00274.x. [DOI] [PubMed] [Google Scholar]
- 41.Mazzoni EO, Desplan C, Blau J. Circadian pacemaker neurons transmit and modulate visual information to control a rapid behavioral response. Neuron. 2005;45:293–300. doi: 10.1016/j.neuron.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 42.Helfrich-Forster C. Robust circadian rhythmicity of Drosophila melanogaster requires the presence of lateral neurons: a brain-behavioral study of disconnected mutants. Journal of comparative physiology. 1998;182:435–453. doi: 10.1007/s003590050192. [DOI] [PubMed] [Google Scholar]
- 43.Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- 44.Meinertzhagen IA HT. The development of the optic lobe. In: M Bate AM-A, editor. The Development of Drosophila Melanogaster. Cold Spring Harbor Press; New York: 1993. pp. 1363–1491. [Google Scholar]