Abstract
The neuromodulator noradrenaline (NA) is released in almost all brain areas in a highly diffused manner. Its action is slow, as it acts through G protein-coupled receptors, but its wide release in the brain makes NA a crucial regulator for various fundamental brain functions such as arousal, attention and memory processes [102]. To understand how NA acts in the brain to promote such diverse actions, it is necessary to dissect the cellular actions of NA at the level of single neurons as well as at the level of neuronal networks. In the present article, we will provide a compact review of the main literatures concerning the NA actions on neuroplasticity processes. Depending on which subtype of adrenoceptor is activated, NA differently affects intrinsic membrane properties of postsynaptic neurons and synaptic plasticity. For example, β-adrenoceptor activation is mainly related to the potentiation of synaptic responses and learning and memory processes. α2-adrenoceptor activation may contribute to a high-order information processing such as executive function, but currently the direction of synaptic plasticity modification by α2-adrenoceptors has not been clearly determined. The activation of α1-adrenoceptors appears to mainly induce synaptic depression in the brain. But its physiological roles are still unclear: while its activation has been described as beneficial for cognitive functions, it may also exert detrimental effects in some brain structures such as the prefrontal cortex.
Key Words: Noradrenaline, LTP, LTD, neuromodulation, synaptic plasticity.
INTRODUCTION
Neuroplasticity can be defined as the potential of neural elements to react with adaptive changes to intrinsic or extrinsic inputs. It is the principal flexible property of neurons, or rather neuronal networks, through which they temporarily or permanently change their biochemical, physiological and morphological characteristics. These characteristics make neuroplasticity a good candidate for the basis of learning and memory.
Classically, NA is thought to act on three main classes of plasticity changes in the nervous system, which are the developmental (which we shall not address in this article), neuronal (or intrinsic), and synaptic plasticity. The literature highlights two points that 1) NA acts on the excitability of neurons, and that 2) NA modulates synaptic plasticity as well as itself inducing synaptic plasticity.
In the present article, we will first briefly introduce the NA system and adrenoceptor-related intracellular pathways. Second, we will review the literatures on how the stimulation of adrenoceptors modulates cellular excitability (i.e. neuronal/intrinsic plasticity). Then, we will introduce studies on short-term and long-term synaptic plasticity, which are induced or modulated by NA. Finally, the putative functional relevance of these plasticity processes will be briefly discussed.
NORADRENERGIC SYSTEM
NA is released in the entire brain areas with the exception of the basal ganglia, from the locus coeruleus (LC), the bilateral small nuclei located in the dorsal tegmentum. Cortical NA innervation is described as mostly non-synaptic, which may support the evenly diffused release of cortical NA to the extracellular space, compatible with both its neuromodulatory role and the multiplicity of its actions on diverse cellular targets in the cerebral cortex [111]. A single LC neuron may project to both cortical hemispheres of different cortical areas [34]. This way, the LC may innervate functionally diverse target areas simultaneously with a global and uniform activity. This may be one way in which the LC coordinates the activity of multiple brain systems [124].
There are three subtypes of adrenoceptors, β, α1 and α2 [134], which are all known to be metabotropic receptors, i.e. a class of receptors linked to G protein. The affinity of NA is higher for α2- than for α1-receptors, and both of these receptors have higher affinities to NA than β-receptors [8].
β-Adrenoceptors
Three types of β-receptors are described in the brain, i.e. β1-, β2- and β3-adrenoceptors [98, 118]. To date, these three β-receptors are not very well distinguished, since specific agonists/antagonists have not been extensively used in preceding studies.
The β-receptors are mainly located in postsynaptic neurons, although a small proportion may exist in presynaptic components in some regions such as the dentate gyrus [80] and prefrontal cortex (PFC) [50]. These receptors are associated with the activation of Gs that activates adenylate cyclase and produces cyclic adenosine monophosphate (cAMP), which can be further associated with CREB (cAMP response element-binding) protein activation. The three β-adrenoceptor subtypes are subject to a desensitization by means of uncoupling from their G proteins, the process being governed by G protein-coupled Receptor directed Kinases (GRKs) as well as by specific kinase like β-adrenergic receptor kinase (β-ARK; [24]).
α1-Adrenoceptors
α1-adrenoceptors are postsynaptic receptors and composed of three subtypes: α1A, α1B and α1D. These three subtypes are equally expressed in the hippocampus, the cerebral cortex and the brainstem, but in the thalamus and deep layers of parieto-frontal cortex, α1A-adrenoceptors are preferentially expressed [53, 94].
All three subtypes (α1A-, α1B- and α1D-adrenoceptor) are able to mobilize calcium ions from intracellular stores as well as to increase the calcium entry via voltage-gated calcium channels. Stimulation of all three α1-adrenoceptor subtypes leads to the hydrolysis of membrane phospholipids via G protein-mediated activation (Gq protein) of phospholipase Cβ. The resultant production of inositol triphosphate (IP3) mediates the α1-adrenoceptor-elicited calcium release from intracellular stores, thereby increasing cytosolic calcium concentrations. The simultaneously produced diacylglycerol (DAG) activates protein kinase C (PKC) [120], which is also activated by a group of calcium and calmodulin-sensitive protein kinases. Active PKC phosphorylates many cellular substrates including membrane channels, pumps, and ion-exchange proteins. The α1-adrenoceptors also have been reported to modulate other signaling pathways: their activation may result in an increased accumulation of cAMP and cGMP, a potentiation of cAMP responses elicited by Gs-linked receptors [51], the activation of phospholipase A2 and phospholipase D, the activation of cAMP phosphodiesterase, release of adenosine, and the stimulation of arachidonic acid release [134]. This class of receptors is also subject to desensitization by GRKs [97].
α2-Adrenoceptors
Three subtypes of α2-adrenoceptors are described, known as α2A-, α2B-, and α2C-adrenoceptors. Their mRNA shows a widespread distribution in the brain and is expressed primarily in regions of the brainstem that contain adrenaline- and NA-producing cells, but is also expressed in several other areas including the hippocampus and the cerebral cortex [90, 108]. PFC neurons express principally the α2A-subtype [6].
α2-adrenoceptors are located on both pre- and postsynaptic sites. The presynaptic localization indicates their functions as autoceptors, involved in the control of NA release by LC neuronal axons. Sub-cellularly, α2A-adrenoceptors in the LC and PFC are associated with synaptic and non-synaptic dendritic and perikarya membranes [5]. In addition, the cortical neurons, but not LC neurons, exhibit prominent immunoreactivity to α2A-adrenoceptors within dendritic spine heads [5].
α2-adrenoceptors are classically linked to Gi/o protein whose action is opposite to that of Gs. These receptors act through inhibiting adenylate cyclase via Gi protein and thereby inhibit the production of cAMP, while the βγ subunits of Gi protein increase potassium ion conductance. α2-adrenoceptors also suppress voltage-activated calcium channels via Go proteins, thus reducing the flow of extracellular calcium ions into target cells. Moreover, growing lines of evidence suggest that α2-adrenoceptors are linked not only to the activation of Gi/o cascade but also, for example, the activation of phospholipase C (PLC) and PKC at least in some cell types [20, 119]. As in the case of the other two classes of receptors, α2-adrenoceptors can be desensitized by GRKs, resulting in a functional uncoupling from their G-proteins [134].
INTRINSIC PLASTICITY
By the term neuronal or intrinsic plasticity, we shall refer to adaptive alterations of postsynaptic excitability, which are non-synaptic in nature and thus mechanistically internal to a given postsynaptic cell. Thus, NA activation of postsynaptic adrenoceptors results in the activation of various intracellular factors and triggers for example modifications of membrane ion channel properties (Fig. 1). This type of plasticity is crucial for neuronal function given that it directly modulates cellular characteristics such as ion channel opening. This class of plasticity may be temporally short-lived (observed only in the presence of agonists, for example) or may be long-lasting (observed even well after washout of the agonists or other induction agents or events). Importantly, as mentioned in the next section (see “Synaptic plasticity induced by NA”), increases in postsynaptic excitability through the induction of intrinsic plasticity, particularly after β-adrenoceptor activation, may constitute the mechanistic basis for long-lasting potentiation of the population spike. However, this potentiation is detected by means of synaptic stimulation. We will therefore list this potentiation under the next synaptic plasticity section.
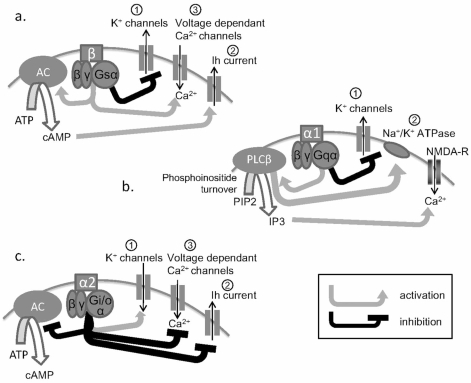
Fig. (1). Schematic representations of the action of adrenoceptors that induce intrinsic plasticity.
a. β-adrenoceptor activation generally induces depolarization of postsynaptic neurons with an increase of input resistance. It reduces K+ currents (1) and facilitates Ih current via cAMP pathway (2) and the entry of Ca2+ (3).
b. α1-adrenoceptors can also induce depolarization of neurons with an increase of input resistance. They reduce K+ currents (1) and act on Ca2+ entry via the activation of phosphoinositide turnover (2).
c. α2-adrenoceptors generally induce hyperpolarization coupled to an increase or decrease of input resistance via blockade of Ih current (2) or opening of K+ channels (1) respectively. They also inhibit Ca2+ channels (3).
AC, adenylate cyclase; ATP, adenosine triphosphate; Ca2+, calcium; cAMP, cyclic adenosine monophosphate; DAG, diacylglyrerol; Ih, hyperpolarization-activated currents; IP3, inositol (1,4,5)-trisphosphate; K+, potassium; PIP2, phosphatidylinositol biphosphate; PLCβ, phospholipase Cβ.
β-Adrenoceptor Cellular Effects
The activation of β-adrenoceptors acts on three cationic currents and induces intrinsic plasticity (Fig. 1a).
-
It may decrease the potassium conductance [41]. This effect results in a depolarization of postsynaptic membrane usually associated with an increase in the input resistance as shown in rat CA1 pyramidal neurons [65, 66] and layer II-III neurons of frontal cortex [28] (but see [76] for a decrease of input resistance in the thalamus). Potassium conductance can also be decreased through, for example, a block of calcium-dependent potassium channels as demonstrated in the hippocampus (dentate gyrus and CA1) [42].
This inhibition of potassium conductance may underlie the blockade of the slow after-hyperpolarization (AHP) current in the cortex [33], the thalamus (paratenial thalamic nucleus; [75, 76]), and the hippocampus [41, 42, 57, 65, 66].
β-adrenoceptor activation may enhance hyperpolarization-activated current (Ih) that is carried by sodium and potassium ions, due to an increase of intracellular concentration of cAMP as shown in guinea-pig dorsolateral geniculate nucleus [75, 95] and rat thalamic neurons [136].
Finally, β-adrenoceptor activation allows the enhancement of certain voltage-dependent calcium currents in the dentate gyrus [40], hippocampal CA3 [32], and the PFC [50].
α1-Adrenoceptor Cellular Effects
α1-adrenoceptors exert a general excitatory effect with a depolarization of resting membrane potential often associated with enhanced input resistance [2, 77, 93] (but see [99] for no change in input resistance) (Fig. 1b).
α1-adrenoceptor activation decreases potassium currents [74, 76]. The particular types of potassium currents reduced by α1-adrenoceptors include IA [2] and the leak potassium current (IKL) [77]. A decrease in the latter potassium current is associated with a change in the firing pattern of the neuron. A reduction of IKL induces a shift of firing mode from rhythmic oscillation to tonic single spike activity in the thalamus in guinea pigs and cats [74, 75] (see [77] for review). A similar result was found in γ-aminobutyric acid (GABAergic) cells of the thalamus, where NA produces a prolonged increase of cellular excitability due to a slow depolarization, which is surprisingly accompanied by a decrease in the input conductance. α1-adrenoceptor activation also prolongs after-hyperpolarization in dorsal raphe neurons [37].
Besides these potassium current modulations, α1-adrenoceptors activation and their consequential augmentation of intracellular calcium concentration may potentiate the activation of Na/K-ATPase [69].
α2-Adrenoceptor Cellular Effects
α2-adrenoceptors induce postsynaptic membrane hyperpolarization (Fig. 1c; [115]).
This hyperpolarization can be associated with decreases in input resistance, which may derive from the modulation of potassium channels, in spinal cord [92], hippocampal CA1 pyramidal neurons [65], and neocortical neurons [28]. The hyperpolarization may also be associated with a decrease in cAMP levels as shown in LC neurons [4], or can be due to an opening of ATP-dependent potassium channels (K-ATP channels) by Gi/o protein [137].
α2-adrenoceptors have also been seen to induce hyperpolarization with increases in the input resistance in medial PFC. In this case, opposite to β-adrenoceptors, α2-adrenoceptors block hyperpolarization-activated currents (Ih) [14] via blockade of hyperpolarization/cyclic nucleotide gated cation (HCN) channels. It is proposed that the overall effect of HCN channel inhibition is to suppress the response to isolated excitatory inputs while enhancing the response to a coherent burst of synaptic activity [20].
In addition, α2-adrenoceptor agonists inhibit voltage-activated calcium currents mediated by the N- or P-type calcium-channels [26, 122]. This suppression of voltage-sensitive calcium channels, as well as the inhibition of adenylate cyclase by Gi/o protein and the activation of potassium channels by α2-adrenoceptors, can all contribute to the reduction of neurotransmitter release [134].
SYNAPTIC PLASTICITY INDUCED BY NA
As described above, NA is generally thought to belong to the category of neuromodulators, which signifies functionally that NA acts on the neurons through modifications of currently ongoing events. From this aspect, NA has been extensively studied in the field of synaptic plasticity. But some evidence suggests also that NA itself can initiate synaptic plasticity. The majority of studies have been conducted in the hippocampus and sensory cortical areas. The term “synaptic plasticity” signifies that modifications occur at the level of the synapse. In this sense, it may be directly related to, or independent of, the intrinsic plasticity described in the preceding section.
At present, two examples of synaptic plasticity are well-known and have been extensively studied; i.e. long-term potentiation (LTP) and long-term depression (LTD). LTP is commonly defined as a lasting increase of synaptic efficacy and has been observed often in glutamatergic synapses in various brain regions such as the hippocampus and the PFC [39, 68]. LTD is, in contrast, a lasting decrease of synaptic efficacy and also observed in glutamatergic synapses across many brain areas [39, 68]. Modulation of LTP/LTD and the induction of LTP/LTD-like changes by NA are discussed in this section.
In addition, in the 1970’s, an important trend emerged which stated that NA might enhance the “signal-to-noise ratio”, which is in close relation with synaptic plasticity. The pioneers in this field were Foote et al. [35] and Segal and Bloom [109] who worked on auditory cortical and hippocampal neurons, respectively. This denomination suffers from the weakness that the basal or background activity cannot be considered simply as “noise”.
NA and Potentiation
A large part of the hippocampal literature describes the NA action of facilitating or inducing potentiation (i.e. LTP) through β-adrenoceptors. In vivo and in vitro studies conducted in the dentate gyrus and in area CA1 of the hippocampus show that the application of NA by itself induces a potentiation of the population spike, augmenting "E-S (EPSP-spike) coupling" (e.g. [23, 43, 88, 100, 133]). This effect seems to be mediated by β-adrenoceptors [43] and cAMP [30], and is likely to be mechanistically supported by the induction of intrinsic plasticity mentioned in the previous section [30]. This LTP of the population spike appears also to require concurrent synaptic activation of NMDA receptors [18], suggesting the existence of convergent action of β-adrenoceptors and NMDA receptors (but see [44] for NMDA receptor-independency of this NA effect). In the dentate gyrus in vivo, it was also shown that stimulation of β-adrenoceptors by endogenous NA induces LTP of the population spike [44, 84]. This NA-induced LTP was however shown to be mechanistically different from high-frequency stimulation-induced LTP of the population spike [58], although this latter tetanus-induced LTP depends on NMDA receptors [1].
NA also facilitates LTP induced by high-frequency afferent stimulation. This was shown in area CA3 [46] and dentate gyrus [3, 18]. This NA facilitation of LTP is achieved through β-adrenoceptors [47] and can be mimicked by an adenylate cyclase activator (i.e. through the increase of cAMP; [52]) similar to the aforementioned case of LTP of the population spike induced by bath-application of NA. These examples of cAMP involvement in NA-induced or NA-facilitated LTP are consistent with another report suggesting that β-adrenoceptor stimulation facilitates LTP in area CA1 through protein kinase A (PKA) activation [132]. This PKA involvement was shown to depend on the stimulus pattern used to induce LTP, where the β-adrenergic facilitation of LTP induced by 5 Hz stimulation involves PKA [38, 132] whereas a similar facilitation of LTP triggered by 100 Hz stimulation does not [38]. Similarly, in area CA1, associative LTP induced by low-frequency paired stimulation was facilitated by PKA activated through β-adrenoceptors [63]. These cases of PKA-dependent LTP facilitation were shown to involve the extracellular signal regulated kinase (ERK) pathway [63, 132].
Depending on the strength of tetanus used to induce LTP, β-adrenoceptors also modulate the maintenance of the late phase of LTP (L-LTP) in the dentate gyrus [116]. A more recent report proposes that NA facilitates LTP induction in the hippocampus by phosphorylation of the GluR1 subunit, which facilitates the delivery of GluRs into synapses [48]. In the visual cortex also, NA facilitates LTP through β-adrenoceptors stimulation, and in this case, NA acts synergistically with muscarinic acetylcholine receptors [17]. Since the visual cortex LTP is dependent also on NMDA receptors [11], this result again indicates the convergent action of multiple neurotransmitters for LTP. In the medial amygdala, short-term potentiation induced by high-frequency stimulation was shown to convert to LTP in the presence of β-adrenoceptor agonist [128]. Interestingly, in the study of Watanabe et al. described above [48], it was shown that, although LTP is induced by tetanic stimulation combined with a β-adrenoceptor agonist in the medial part of the amygdala, the same protocol suppressed normal short-term potentiation in the lateral amygdala [128]. Similarly in the dentate gyrus, LTP or LTD can be facilitated depending on whether responses are evoked by medial or lateral perforant pathway stimulation [22, 96].
α1-adrenoceptors have been, shown to enhance the frequency of excitatory postsynaptic currents (EPSCs) in medial PFC neurons [73]. This class of receptors also enables the augmentation of synaptic density in rat visual cortex, which might be essential for the maintenance of synapses as well as for synaptic plasticity [86]. Moreover, Segal et al. [110] showed the enhancement of responses to NMDA in the presence of a α1-agonist in CA1 hippocampal neurons, an action achieved via the activation of phosphoinositide turnover.
NA and Depression
NA has also been described to induce synaptic depression. In transverse slices of rat visual cortex, it was shown that paired-pulse stimulation induces NMDA receptor-dependent acute and long-lasting homosynaptic depression in the presence of NA acting on α1-adrenoceptors [56]. A similar facilitation of synaptic depression was seen in the presence of acetylcholine [86]. These results parallel those reported by Scheiderer et al. [107] in the hippocampus, where muscarinic or adrenergic receptor activation induced LTD. In another study, Liu et al. [64] focused on NMDA receptor-mediated EPSCs in the PFC and showed that α1-adrenoceptor stimulation induces depression. We have also found that NA induces LTD of glutamatergic transmission in PFC slices via the activation of α1- and α2-adrenoceptors (Marzo et al., unpublished data). The majority of the examples of synaptic depression induced by NA involves α1-adrenoceptors, but not β-adrenoceptors [64, 78, 106]. Indeed, α- and β-adrenoceptors appear to exert opposite effects on synaptic transmission, i.e. facilitation of LTD and LTP, respectively. This was further suggested by the fact that NA induces LTP in dentate gyrus when applied with a α-adrenoceptor antagonist [22].
The locus of the induction of LTD has been shown to be postsynaptic in the PFC (Marzo et al., unpublished data). Also, the absence of changes in paired pulse facilitation supports the postsynaptic locus of induction for the acute depression of NMDA receptor-mediated synaptic responses in the PFC [64].
NA Effects on Inhibitory Transmission
The majority of studies on the NA effects on synaptic transmission focused on excitatory glutamatergic transmission. However, NA also acts on GABAergic transmission. Generally, increases of synaptic inhibition by NA are noted. For example, in the frontal cortex, NA induces an increase in the frequency of inhibitory postsynaptic currents (IPSC) recorded from pyramidal neurons, with NA acting through the enhancement of excitability of GABAergic neurons via α-adrenoceptor stimulation [54]. Similarly in the entorhinal cortex, α1-adrenoceptor activation increases the frequency of miniature IPSCs, suggesting a presynaptic effect [60]. In area CA1 of the hippocampus, on the other hand, NA decreases inhibitory postsynaptic potentials via α-adrenoceptor activation [67]. In the somatosensory cortex, it was shown that NA enhances GABA-induced inhibition [129] which in this case is mediated by β-adrenoceptor-inducing augmentation of cAMP [114]. Similar results were found in lateral hypothalamus [113] and cerebellum [21, 81]. In cerebellum, α-adrenoceptors are also involved in the increase in the IPSC with distinct roles played by α1- and α2-adrenoceptors [45]: thus, α1-adrenoceptor activation increases the spontaneous and evoked IPSC, but α2-adrenoceptors rather decrease the spontaneous IPSC without affecting the evoked IPSC.
FUNCTIONAL RELEVANCE OF NA INDUCED PLASTICITY
Different lines of evidence suggest that NA-induced plasticity may have multiple functional roles. For example, the induction of intrinsic plasticity would increase the probability of certain patterns or modes of neuronal discharge, and such changes may be related to the level of waking and arousal [12, 75]. NA system-related arousal in turn may participate in the information processing as indicated by the fact that LTP induction is modulated by appetitive and aversive stimuli [112]. In this respect, Seidenbecher et al. [112] showed a reinforcement of hippocampal LTP triggered by a sub-threshold tetanus in the presence of an event known to activate release of NA in the hippocampus. This LTP reinforcement was blocked by the administration of β-antagonist.
The crucial role of β-adrenoceptor activation for the maintenance of L-LTP [116] may be regarded as a basis for the consolidation of long-term memory. In fact, in behavioral studies, the NA system has been implicated in the consolidation and retrieval of memory [79, 101], partly from the effect of enhanced arousal [19]. It appears that β-adrenoceptors are the subtype involved in the memory consolidation, since, for example, β-adrenergic antagonists cause amnesia in spatial memory paradigms [101, 103]. β-adrenoceptors in the rat prelimbic area are also involved in a late phase of long-term olfactory memory consolidation [123]. Moreover, it has been suggested that memory consolidation is achieved by a long-term effect of NA on synaptic transmission, taking place during slow wave sleep (evidence demonstrates that the LC is transiently activated during this sleep phase after intensive learning [31]).
Retrieval is also an important step in memory processes during which NA appears to act. It was shown that mutant mice that cannot synthesize NA can still learn a contextual fear-conditioning task but are impaired in retention when tested 2 days later [85]. This retention deficit was rescued by the injection of a precursor of NA before the test, demonstrating that NA is necessary for the access to a memory trace at this time [85]. These results taken together suggest that NA is important for consolidation and retrieval of some types of memory. However, it should be pointed out also that in the case of amygdala-dependent fear memory, NA through β-adrenoceptors participates rather in memory re-consolidation but not its consolidation or retrieval [25, 85].
Another main function related to the NA system is the information processing from sensory collections to the high-order cognition [102]. Thus, NA has been shown to be crucial in tasks involving changes of strategy [82, 89], and this effect appears to depend on α-adrenoceptors. But there is still a controversy as to the role of α-adrenoceptors in cognitive functions [10]. For example, the activation of α1-adrenoceptors in the PFC is classically related to the impairment of cognitive performance [9], whereas α2-adrenoceptors are known to improve it [61]. Nevertheless, blockade of α1-adrenoceptors when NA levels are pharmacologically enhanced actually blocks the NA-induced enhancement of performance in the strategy switching [58].
Mechanistically, the above flexibility for strategy adoption may be related to LTD induction as shown in the hippocampus [29, 91]. It is thus possible that LTD generally weakens network efficacy so that it enables the neural network to select and encode new representations through the readily potentiable synapses.
Another line of evidence relates to novelty exploration. It is known that LC neurons show enhanced discharge in response to novel stimuli and rapidly habituate after a few encounters with the same stimuli [125]. This characteristic of LC neurons suggests that NA may participate in the process of information acquisition [13, 27, 104, 105], perhaps through the induction of synaptic plasticity. Both directions of synaptic plasticity, i.e. potentiation and depression, can be enhanced by novelty exploration [62, 70, 135], and the enhancement effects depend on the activation of β-adrenoceptors [55, 117].
Finally, these behavioral modulations by NA are closely related to stress and anxiety, since NA is known to be released during acute stressful episodes [87]. Following chronic stress, large cognitive impairments are observed, and they can be improved by antidepressants such as desipramine that blocks NA reuptake [15], suggesting down-regulation of NA transmission after chronic stress exposure.
CONCLUDING REMARKS
NA participates in the modulation of a large spectrum of behaviors. NA appears to be related to the capacity of the organism to reach different levels of waking states, to integrate sensory information, and to engage in central cognitive processes such as memory. These different functions seem to be associated with the release of NA in various distinct areas.
From a cellular point of view, NA has been described to act as a “gating” agent [129-131]. This action consists of its capacity to change the threshold of postsynaptic neurons necessary to induce a sensory response. For example, iontophoretically applied NA induces a decrease in the discharge threshold in the majority of auditory cells and a global increase in the “signal-to-noise ratio” [71]. Another similar concept of NA action is “tuning”. NA participates in the selection of sensory responses to a specific class of sensory stimulation via inhibition of the response to adjacent classes of sensory stimuli [49, 72]. These effects were shown to last for more than 15 min [72] and were mediated by α-adrenoceptors [121]. NA’s ability to enhance the “signal-to-noise ratio” can be interpreted as a mechanism to select the most salient information within a neural network. Moreover, NA also modulates the integration of information by an improvement of spike timing precision [59]. These two last cellular actions of NA may be related to high cognitive functions such as the selection of pertinent behavior, which sometimes needs behavioral switches to flexibly meet contextual demands [16].
Considering the diverse actions induced by NA, the importance of the concentrations used should be pointed out [36]. Armstrong-James and Fox [7] found an inhibitory effect with an elevated quantity of NA whereas with a lower level, it exerted an excitatory effect on the spontaneous activity of somatosensory cortex. A similar effect was found in the hippocampus [83]. Globally, however, a general major action of NA in the brain seems to be inhibitory [35, 64, 106, 126, 127, 129], as we have observed in PFC synaptic transmission (Marzo et al., unpublished data). But some biphasic actions are also described, where the excitatory effect of NA was transient and followed by an inhibition [16, 126].
NA effects may also depend on layers within a single structure. In somatosensory cortex, middle layer neuronal activity was decreased by NA in the majority of cases, whereas neurons in deep and superficial layers showed NA-induced excitation for both evoked and spontaneous activities [127]. Our laboratory also observed differential effects of NA in PFC slices where synaptic depression was observed in layer I-II to layer V pyramidal neuron synapses after NA application whereas layer VI synapses showed little change (Marzo et al., unpublished data).
A recent trend in the plasticity field highlights the importance of neuroplasticity not only in physiological regulations but also in pathological regulations of brain networks. In this respect, we note that another catecholamine dopamine plays a major functional role in the PFC [39], through up- or down-regulation of PFC glutamatergic synapses depending on developmental and behavioral conditions. A main difference of the manner of dopaminergic regulation of PFC synaptic transmission from that of NA is that dopamine has to act temporally together with high-frequency conditioning input to glutamatergic synapses in order to exert its long-term modulatory effects on synaptic efficacy. NA, in contrast, depresses, or in some cases potentiates (Marzo et al., unpublished data), PFC synapses without coincidental enhanced glutamatergic activity, suggesting global effects of NA on the signal-to-noise ratio. How these NA effects are related to behavior and whether there is any interaction between the dopamine-induced and NA-induced synaptic changes remain as important future questions.
In conclusion, the activation of noradrenergic system is able to induce and modulate intrinsic and synaptic plasticity in two different directions, depending on the concentration, the structure, and the engaged pathway. Further studies, giving more attention to these details, should reveal yet more specific links between behavior and NA modulation of brain networks.
REFERENCES
- 1.Abraham WC, Mason SE. Effects of the NMDA receptor/channel antagonists CPP and MK801 on hippocampal field potentials and long-term potentiation in anesthetized rats. Brain Res. 1988;462:40–46. doi: 10.1016/0006-8993(88)90582-3. [DOI] [PubMed] [Google Scholar]
- 2.Aghajanian GK. Modulation of a transient outward current in serotonergic neurones by alpha 1-adrenoceptors. Nature. 1985;315:501–503. doi: 10.1038/315501a0. [DOI] [PubMed] [Google Scholar]
- 3.Almaguer-Melian W, Rojas-Reyes Y, Alvare A, Rosillo JC, Frey JU, Bergado JA. Long-term potentiation in the dentate gyrus in freely moving rats is reinforced by intraventricular application of norepinephrine, but not oxotremorine. Neurobiol. Learn. Memory. 2005;83:72–78. doi: 10.1016/j.nlm.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Andrade R, Aghajanian GK. Opiate- and alpha 2-adrenoceptor-induced hyperpolarizations of locus ceruleus neurons in brain slices: reversal by cyclic adenosine 3':5'-monophosphate analogues. J. Neurosci. 1985;5:2359–2364. doi: 10.1523/JNEUROSCI.05-09-02359.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aoki C, Go C G, Venkatesan C, Kurose H. Perikaryal and synaptic localization of alpha 2A-adrenergic receptor-like immunoreactivity. Brain Res. 1994;650:181–204. doi: 10.1016/0006-8993(94)91782-5. [DOI] [PubMed] [Google Scholar]
- 6.Aoki C, Venkatesan C, Go CG, Forman R, Kurose H. Cellular and subcellular sites for noradrenergic action in the monkey dorsolateral prefrontal cortex as revealed by the immunocytochemical localization of noradrenergic receptors and axons. Cereb. Cortex. 1998;8:269–277. doi: 10.1093/cercor/8.3.269. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong-James M, Fox K. Effects of ionophoresed noradrenaline on the spontaneous activity of neurones in rat primary somatosensory cortex. J. Physiol. 1983;335:427–447. doi: 10.1113/jphysiol.1983.sp014542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnsten AF. Through the looking glass: differential noradenergic modulation of prefrontal cortical function. Neural. Plasticity. 2000;7:133–146. doi: 10.1155/NP.2000.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnsten A F, Mathew R, Ubriani R, Taylor JR, Li BM. Alpha-1 noradrenergic receptor stimulation impairs prefrontal cortical cognitive function. Biol. Psychiatry. 1999;45:26–31. doi: 10.1016/s0006-3223(98)00296-0. [DOI] [PubMed] [Google Scholar]
- 10.Arnsten AF, Steere JC, Jentsch DJ, Li BM. Noradrenergic influences on prefrontal cortical cognitive function: opposing actions at postjunctional alpha 1 versus alpha 2-adrenergic receptors. Adv. Pharmacol. 1998;42:764–767. doi: 10.1016/s1054-3589(08)60859-5. [DOI] [PubMed] [Google Scholar]
- 11.Artola A, Brocher S, Singer W. Different voltage-dependent thresholds for inducing long-term depression and long-term potentiation in slices of rat visual cortex. Nature. 1990;347:69–72. doi: 10.1038/347069a0. [DOI] [PubMed] [Google Scholar]
- 12.Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J. Neurosci. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aston-Jones G, Rajkowski J, Kubiak P. Conditioned responses of monkey locus coeruleus neurons anticipate acquisition of discriminative behavior in a vigilance task. Neuroscience. 1997;80:697–715. doi: 10.1016/s0306-4522(97)00060-2. [DOI] [PubMed] [Google Scholar]
- 14.Barth AM, Vizi ES, Zelles T, Lendvai B. Alpha2-adrenergic receptors modify dendritic spike generation via HCN channels in the prefrontal cortex. J. Neurophysiol. 2008;99:394–401. doi: 10.1152/jn.00943.2007. [DOI] [PubMed] [Google Scholar]
- 15.Bondi C O, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008;33:320–331. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- 16.Bouret S, Sara SJ. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 2005;28:574–582. doi: 10.1016/j.tins.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Brocher S, Artola A, Singer W. Agonists of cholinergic and noradrenergic receptors facilitate synergistically the induction of long-term potentiation in slices of rat visual cortex. Brain Res. 1992;573:27–36. doi: 10.1016/0006-8993(92)90110-u. [DOI] [PubMed] [Google Scholar]
- 18.Burgard EC, Decker G, Sarvey JM. NMDA receptor antagonists block norepinephrine-induced long-lasting potentiation and long-term potentiation in rat dentate gyrus. Brain Res. 1989;482:351–355. doi: 10.1016/0006-8993(89)91199-2. [DOI] [PubMed] [Google Scholar]
- 19.Cahill L, McGaugh JL. The neurobiology of memory for emotional events: adrenergic activation and the amygdala. Proc. West. Pharmacol. Soc. 1996;39:81–84. [PubMed] [Google Scholar]
- 20.Carr DB, Andrews GD, Glen WB, Lavin A. alpha2-Noradrenergic receptors activation enhances excitability and synaptic integration in rat prefrontal cortex pyramidal neurons via inhibition of HCN currents. J. Physiol. 2007;584:437–450. doi: 10.1113/jphysiol.2007.141671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheun JE, Yeh HH. Noradrenergic potentiation of cerebellar Purkinje cell responses to GABA: cyclic AMP as intracellular intermediary. Neuroscience. 1996;74:835–844. doi: 10.1016/0306-4522(96)00130-3. [DOI] [PubMed] [Google Scholar]
- 22.Dahl D, Sarvey JM. Norepinephrine induces pathway-specific long-lasting potentiation and depression in the hippocampal dentate gyrus. Proc. Natl. Acad Sci. USA. 1989;86:4776–4780. doi: 10.1073/pnas.86.12.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dahl D, Winson J. Action of norepinephrine in the dentate gyrus. I. Stimulation of locus coeruleus. Exp. Brain Res. 1985;59:491–496. doi: 10.1007/BF00261339. [DOI] [PubMed] [Google Scholar]
- 24.Dawson TM, Arriza JL, Jaworsky DE, Borisy FF, Attramadal H, Lefkowitz RJ, Ronnett GV. Beta-adrenergic receptor kinase-2 and beta-arrestin-2 as mediators of odorant-induced desensitization. Science. 1993;259:825–829. doi: 10.1126/science.8381559. [DOI] [PubMed] [Google Scholar]
- 25.Debiec J, Ledoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129:267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 26.DeBock F, Kurz J, Azad SC, Parsons CG, Hapfelmeier G, Zieglgansberger W, Rammes G. Alpha2-adrenoreceptor activation inhibits LTP and LTD in the basolateral amygdala: involvement of Gi/o-protein-mediated modulation of Ca2+-channels and inwardly rectifying K+-channels in LTD. Eur. J. Neurosci. 2003;17:1411–1424. doi: 10.1046/j.1460-9568.2003.02544.x. [DOI] [PubMed] [Google Scholar]
- 27.Devilbiss DM, Page ME, Waterhouse BD. Locus ceruleus regulates sensory encoding by neurons and networks in waking animals. J. Neurosci. 2006;26:9860–9872. doi: 10.1523/JNEUROSCI.1776-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dodt HU, Pawelzik H, Zieglgansberger W. Actions of noradrenaline on neocortical neurons in vitro. Brain Res. 1991;545:307–311. doi: 10.1016/0006-8993(91)91303-i. [DOI] [PubMed] [Google Scholar]
- 29.Duffy S, Labrie V, Roder JC. D-serine augments NMDA-NR2B receptor-dependent hippocampal long-term depression and spatial reversal learning. Neuropsychopharmacology. 2008;33:1004–1018. doi: 10.1038/sj.npp.1301486. [DOI] [PubMed] [Google Scholar]
- 30.Dunwiddie TV, Taylor M, Heginbotham LR, Proctor WR. Long-term increases in excitability in the CA1 region of rat hippocampus induced by beta-adrenergic stimulation: possible mediation by cAMP. J. Neurosci. 1992;12:506–517. doi: 10.1523/JNEUROSCI.12-02-00506.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eschenko O, Sara SJ. Learning-dependent, transient increase of activity in noradrenergic neurons of locus coeruleus during slow wave sleep in the rat: brain stem-cortex interplay for memory consolidation? Cereb. Cortex. 2008;18:2596–2603. doi: 10.1093/cercor/bhn020. [DOI] [PubMed] [Google Scholar]
- 32.Fisher R, Johnston D. Differential modulation of single voltage-gated calcium channels by cholinergic and adrenergic agonists in adult hippocampal neurons. J. Neurophysiol. 1990;64:1291–1302. doi: 10.1152/jn.1990.64.4.1291. [DOI] [PubMed] [Google Scholar]
- 33.Foehring RC, Schwindt PC, Crill WE. Norepinephrine selectively reduces slow Ca2+- and Na+-mediated K+ currents in cat neocortical neurons. J. Neurophysiol. 1989;61:245–256. doi: 10.1152/jn.1989.61.2.245. [DOI] [PubMed] [Google Scholar]
- 34.Foote SL, Bloom FE, Aston-Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol. Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- 35.Foote SL, Freedman R, Oliver AP. Effects of putative neurotransmitters on neuronal activity in monkey auditory cortex. Brain Res. 1975;86:229–242. doi: 10.1016/0006-8993(75)90699-x. [DOI] [PubMed] [Google Scholar]
- 36.Foote SL, Morrison JH. Extrathalamic modulation of cortical function. Ann. Rev. Neurosci. 1987;10:67–95. doi: 10.1146/annurev.ne.10.030187.000435. [DOI] [PubMed] [Google Scholar]
- 37.Freedman JE, Aghajanian GK. Role of phosphoinositide metabolites in the prolongation of afterhyperpolarizations by alpha 1-adrenoceptors in rat dorsal raphe neurons. J. Neurosci. 1987;7:3897–3906. doi: 10.1523/JNEUROSCI.07-12-03897.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gelinas JN, Tenorio G, Lemon N, Abel T, Nguyen PV. Beta-adrenergic receptor activation during distinct patterns of stimulation critically modulates the PKA-dependence of LTP in the mouse hippocampus. Learn. Mem. 2008;15:281–289. doi: 10.1101/lm.829208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goto Y, Yang CR, Otani S. Functional and dysfunctional synaptic plasticity in prefrontal cortex: roles in psychiatric disorders. Biol. Psychiatry in press. 2009 doi: 10.1016/j.biopsych.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 40.Gray R, Johnston D. Noradrenaline and beta-adrenoceptor agonists increase activity of voltage-dependent calcium channels in hippocampal neurons. Nature. 1987;327:620–622. doi: 10.1038/327620a0. [DOI] [PubMed] [Google Scholar]
- 41.Haas HL, Konnerth A. Histamine and noradrenaline decrease calcium-activated potassium conductance in hippocampal pyramidal cells. Nature. 1983;302:432–434. doi: 10.1038/302432a0. [DOI] [PubMed] [Google Scholar]
- 42.Haas HL, Rose GM. Noradrenaline blocks potassium conductance in rat dentate granule cells in vitro. Neurosci. Lett. 1987;78:171–174. doi: 10.1016/0304-3940(87)90628-8. [DOI] [PubMed] [Google Scholar]
- 43.Harley CW, Milway JS. Glutamate ejection in the locus coeruleus enhances the perforant path-evoked population spike in the dentate gyrus. Exp. Brain Res. 1986;63:143–150. doi: 10.1007/BF00235656. [DOI] [PubMed] [Google Scholar]
- 44.Heginbotham LR, Dunwiddie TV. Long-term increases in the evoked population spike in the CA1 region of rat hippocampus induced by beta-adrenergic receptor activation. J. Neurosci. 1991;11:2519–2527. doi: 10.1523/JNEUROSCI.11-08-02519.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirono M, Obata K. Alpha-adrenoceptive dual modulation of inhibitory GABAergic inputs to Purkinje cells in the mouse cerebellum. J. Neurophysiol. 2006;95:700–708. doi: 10.1152/jn.00711.2005. [DOI] [PubMed] [Google Scholar]
- 46.Hopkins WF, Johnston D. Frequency-dependent noradrenergic modulation of long-term potentiation in the hippocampus. Science. 1984;226:350–352. doi: 10.1126/science.6091272. [DOI] [PubMed] [Google Scholar]
- 47.Hopkins WF, Johnston D. Noradrenergic enhancement of long-term potentiation at mossy fiber synapses in the hippocampus. J. Neurophysiol. 1988;59:667–687. doi: 10.1152/jn.1988.59.2.667. [DOI] [PubMed] [Google Scholar]
- 48.Hu H, Real E, Takamiya K, Kang MG, Ledoux J, Huganir RL, Malinow R. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131:160–173. doi: 10.1016/j.cell.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 49.Hurley LM, Devilbiss DM, Waterhouse BD. A matter of focus: monoaminergic modulation of stimulus coding in mammalian sensory networks. Curr. Opin. Neurobiol. 2004;14:488–495. doi: 10.1016/j.conb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 50.Ji XH, Cao XH, Zhang CL, Feng ZJ, Zhang XH, Ma L, Li BM. Pre- and postsynaptic beta-adrenergic activation enhances excitatory synaptic transmission in layer V/VI pyramidal neurons of the medial prefrontal cortex of rats. Cereb. Cortex. 2008;18:1506–1520. doi: 10.1093/cercor/bhm177. [DOI] [PubMed] [Google Scholar]
- 51.Jiao X, Gonzalez-Cabrera PJ, Xiao L, Bradley ME, Abel PW, Jeffries WB. Tonic inhibitory role for cAMP in alpha(1a)-adrenergic receptor coupling to extracellular signal-regulated kinases 1/2. J. Pharmacol. Exp. Therap. 2002;303:247–256. doi: 10.1124/jpet.102.037747. [DOI] [PubMed] [Google Scholar]
- 52.Johnston D, Hopkins WF, Gray R. Cellular mechanisms of noradrenergic enhancement of long-term synaptic potentiation in hippocampus. NIDA Res. Monograph. 1987;78:95–107. [PubMed] [Google Scholar]
- 53.Jones LS, Gauger LL, Davis JN. Anatomy of brain alpha 1-adrenergic receptors: in vitro autoradiography with [125I]-heat. J. Comparative Neurol. 1985;231:190–208. doi: 10.1002/cne.902310207. [DOI] [PubMed] [Google Scholar]
- 54.Kawaguchi Y, Shindou T. Noradrenergic excitation and inhibition of GABAergic cell types in rat frontal cortex. J. Neurosci. 1998;18:6963–6976. doi: 10.1523/JNEUROSCI.18-17-06963.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kemp A, Manahan-Vaughan D. Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proc. Natl. Acad. Sci. USA. 2004;101:8192–8197. doi: 10.1073/pnas.0402650101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kirkwood A, Rozas C, Kirkwood J, Perez F, Bear MF. Modulation of long-term synaptic depression in visual cortex by acetylcholine and norepinephrine. J. Neurosci. 1999;19:1599–1609. doi: 10.1523/JNEUROSCI.19-05-01599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lancaster B, Hu H, Ramakers GM, Storm JF. Interaction between synaptic excitation and slow afterhyperpolarization current in rat hippocampal pyramidal cells. J. Physiol. 2001;536:809–823. doi: 10.1111/j.1469-7793.2001.00809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lapiz MD, Morilak DA. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience. 2006;137:1039–1049. doi: 10.1016/j.neuroscience.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 59.Lecas JC. Locus coeruleus activation shortens synaptic drive while decreasing spike latency and jitter in sensorimotor cortex. Implications for neuronal integration. Eur. J.Neurosci. 2004;19:2519–2530. doi: 10.1111/j.0953-816X.2004.03341.x. [DOI] [PubMed] [Google Scholar]
- 60.Lei S, Deng PY, Porter JE, Shin HS. Adrenergic facilitation of GABAergic transmission in rat entorhinal cortex. J. Neurophysiol. 2007;98:2868–2877. doi: 10.1152/jn.00679.2007. [DOI] [PubMed] [Google Scholar]
- 61.Li BM, Mao ZM, Wang M, Mei ZT. Alpha-2 adrenergic modulation of prefrontal cortical neuronal activity related to spatial working memory in monkeys. Neuropsychopharmacology. 1999;21:601–610. doi: 10.1016/S0893-133X(99)00070-6. [DOI] [PubMed] [Google Scholar]
- 62.Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat. Neurosci. 2003;6:526–531. doi: 10.1038/nn1049. [DOI] [PubMed] [Google Scholar]
- 63.Lin YW, Min MY, Chiu TH, Yang HW. Enhancement of associative long-term potentiation by activation of beta-adrenergic receptors at CA1 synapses in rat hippocampal slices. J. Neurosci. 2003;23:4173–4181. doi: 10.1523/JNEUROSCI.23-10-04173.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu W, Yuen EY, Allen PB, Feng J, Greengard P, Yan Z. Adrenergic modulation of NMDA receptors in prefrontal cortex is differentially regulated by RGS proteins and spinophilin. Proc. Natl. Acad. Sci. USA. 2006;103:18338–18343. doi: 10.1073/pnas.0604560103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Madison DV, Nicoll RA. Actions of noradrenaline recorded intracellularly in rat hippocampal CA1 pyramidal neurones, in vitro. J. Physiol. 1986;372:221–244. doi: 10.1113/jphysiol.1986.sp016006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Madison DV, Nicoll RA. Cyclic adenosine 3',5'-monophosphate mediates beta-receptor actions of noradrenaline in rat hippocampal pyramidal cells. Journal of Physiology. 1986;372:245–259. doi: 10.1113/jphysiol.1986.sp016007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Madison DV, Nicoll RA. Norepinephrine decreases synaptic inhibition in the rat hippocampus. Brain Res. 1988;442:131–138. doi: 10.1016/0006-8993(88)91440-0. [DOI] [PubMed] [Google Scholar]
- 68.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 69.Mallick BN, Adya HV, Faisal M. Norepinephrine-stimulated increase in Na+, K+-ATPase activity in the rat brain is mediated through alpha1A-adrenoceptor possibly by dephosphorylation of the enzyme. J. Neurochem. 2000;74:1574–1578. doi: 10.1046/j.1471-4159.2000.0741574.x. [DOI] [PubMed] [Google Scholar]
- 70.Manahan-Vaughan D, Braunewell KH. Novelty acquisition is associated with induction of hippocampal long-term depression. Proc. Natl. Acad. Sci. USA. 1999;96:8739–8744. doi: 10.1073/pnas.96.15.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Manunta Y, Edeline JM. Effects of noradrenaline on rate-level function of auditory cortex neurons: is there a "gating" effect of noradrenaline? Exp.Brain Res. 1998;118:361–372. doi: 10.1007/s002210050290. [DOI] [PubMed] [Google Scholar]
- 72.Manunta Y, Edeline JM. Noradrenergic induction of selective plasticity in the frequency tuning of auditory cortex neurons. J. Neurophysiol. 2004;92:1445–1463. doi: 10.1152/jn.00079.2004. [DOI] [PubMed] [Google Scholar]
- 73.Marek GJ, Aghajanian GK. 5-HT2A receptor or alpha1-adrenoceptor activation induces excitatory postsynaptic currents in layer V pyramidal cells of the medial prefrontal cortex. Eur. J. Pharmacol. 1999;367:197–206. doi: 10.1016/s0014-2999(98)00945-5. [DOI] [PubMed] [Google Scholar]
- 74.McCormick DA. Cellular mechanisms underlying cholinergic and noradrenergic modulation of neuronal firing mode in the cat and guinea pig dorsal lateral geniculate nucleus. J. Neurosci. 1992;12:278–289. doi: 10.1523/JNEUROSCI.12-01-00278.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McCormick DA, Pape HC, Williamson A. Actions of norepinephrine in the cerebral cortex and thalamus: implications for function of the central noradrenergic system. Prog. Brain Res. 1991;88:293–305. doi: 10.1016/s0079-6123(08)63817-0. [DOI] [PubMed] [Google Scholar]
- 76.McCormick DA, Prince DA. Noradrenergic modulation of firing pattern in guinea pig and cat thalamic neurons, in vitro. J. Neurophysiol. 1988;59:978–996. doi: 10.1152/jn.1988.59.3.978. [DOI] [PubMed] [Google Scholar]
- 77.McCormick DA, Wang Z. Serotonin and noradrenaline excite GABAergic neurones of the guinea-pig and cat nucleus reticularis thalami. J. Physiol. 1991;442:235–255. doi: 10.1113/jphysiol.1991.sp018791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McElligott ZA, Winder DG. Alpha1-adrenergic receptor-induced heterosynaptic long-term depression in the bed nucleus of the stria terminalis is disrupted in mouse models of affective disorders. Neuropsychopharmacology. 2008;33:2313–2323. doi: 10.1038/sj.npp.1301635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McGaugh JL, Roozendaal B. Drug enhancement of memory consolidation: historical perspective and neurobiological implications. Psychopharmacology (Berlin) 2009;202:3–14. doi: 10.1007/s00213-008-1285-6. [DOI] [PubMed] [Google Scholar]
- 80.Milner TA, Shah P, Pierce JP. beta-adrenergic receptors primarily are located on the dendrites of granule cells and interneurons but also are found on astrocytes and a few presynaptic profiles in the rat dentate gyrus. Synapse. 2000;36:178–193. doi: 10.1002/(SICI)1098-2396(20000601)36:3<178::AID-SYN3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 81.Moises HC, Burne RA, Woodward DJ. Modification of the visual response properties of cerebellar neurons by norepinephrine. Brain Res. 1990;514:259–275. doi: 10.1016/0006-8993(90)91421-c. [DOI] [PubMed] [Google Scholar]
- 82.Moore TL, Schettler SP, Killiany RJ, Herndon JG, Luebke JI, Moss MB, Rosene DL. Cognitive impairment in aged rhesus monkeys associated with monoamine receptors in the prefrontal cortex. Behav. Brain Res. 2005;160:208–221. doi: 10.1016/j.bbr.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 83.Mueller AL, Hoffer BJ, Dunwiddie TV. Noradrenergic responses in rat hippocampus: evidence for medication by alpha and beta receptors in the in vitro slice. Brain Res. 1981;214:113–126. doi: 10.1016/0006-8993(81)90442-x. [DOI] [PubMed] [Google Scholar]
- 84.Munro CA, Walling SG, Evans JH, Harley CW. Beta-adrenergic blockade in the dentate gyrus in vivo prevents high frequency-induced long-term potentiation of EPSP slope, but not long-term potentiation of population spike amplitude. Hippocampus. 2001;11:322–328. doi: 10.1002/hipo.1046. [DOI] [PubMed] [Google Scholar]
- 85.Murchison CF, Zhang XY, Zhang WP, Ouyang M, Lee A, Thomas SA. A distinct role for norepinephrine in memory retrieval. Cell. 2004;117:131–143. doi: 10.1016/s0092-8674(04)00259-4. [DOI] [PubMed] [Google Scholar]
- 86.Nakadate K, Matsukawa M, Okado N. Identification of adrenoceptor subtype-mediated changes in the density of synapses in the rat visual cortex. Neuroscience. 2006;138:37–46. doi: 10.1016/j.neuroscience.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 87.Nakane H, Shimizu N, Hori T. Stress-induced norepinephrine release in the rat prefrontal cortex measured by microdialysis. Am. J. Physiol. 1994;267:R1559–1566. doi: 10.1152/ajpregu.1994.267.6.R1559. [DOI] [PubMed] [Google Scholar]
- 88.Neuman RS, Harley CW. Long-lasting potentiation of the dentate gyrus population spike by norepinephrine. Brain Res. 1983;273:162–165. doi: 10.1016/0006-8993(83)91106-x. [DOI] [PubMed] [Google Scholar]
- 89.Newman LA, Darling J, McGaughy J. Atomoxetine reverses attentional deficits produced by noradrenergic deafferentation of medial prefrontal cortex. Psychopharmacology (Berlin) 2008;200:39–50. doi: 10.1007/s00213-008-1097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nicholas AP, Pieribone V, Hokfelt T. Distributions of mRNAs for alpha-2 adrenergic receptor subtypes in rat brain: an in situ hybridization study. J. Comparative Neurol. 1993;328:575–594. doi: 10.1002/cne.903280409. [DOI] [PubMed] [Google Scholar]
- 91.Nicholls RE, Alarcon JM, Malleret G, Carroll RC, Grody M, Vronskaya S, Kandel ER. Transgenic mice lacking NMDAR-dependent LTD exhibit deficits in behavioral flexibility. Neuron. 2008;58:104–117. doi: 10.1016/j.neuron.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 92.North RA, Yoshimura M. The actions of noradrenaline on neurones of the rat substantia gelatinosa in vitro. J . Physiol. 1984;349:43–55. doi: 10.1113/jphysiol.1984.sp015141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ogata N, Matsuo T. The effects of catecholamines on electrical activity of neurons in the guinea pig supraoptic nucleus in vitro. Brain Res. 1986;385:122–135. doi: 10.1016/0006-8993(86)91553-2. [DOI] [PubMed] [Google Scholar]
- 94.Palacios JM, Hoyer D, Cortes R. alpha 1-Adrenoceptors in the mammalian brain: similar pharmacology but different distribution in rodents and primates. Brain Res. 1987;419:65–75. doi: 10.1016/0006-8993(87)90569-5. [DOI] [PubMed] [Google Scholar]
- 95.Pape HC, McCormick DA. Noradrenaline and serotonin selectively modulate thalamic burst firing by enhancing a hyperpolarization-activated cation current. Nature. 1989;340:715–718. doi: 10.1038/340715a0. [DOI] [PubMed] [Google Scholar]
- 96.Pelletier MR, Kirkby RD, Jones SJ, Corcoran ME. Pathway specificity of noradrenergic plasticity in the dentate gyrus. Hippocampus. 1994;4:181–188. doi: 10.1002/hipo.450040208. [DOI] [PubMed] [Google Scholar]
- 97.Price RR, Morris DP, Biswas G, Smith MP, Schwinn DA. Acute agonist-mediated desensitization of the human alpha 1a-adrenergic receptor is primarily independent of carboxyl terminus regulation implications for regulation of alpha 1aAR splice variants. J. Biol. Chem. 2002;277:9570–9579. doi: 10.1074/jbc.M111762200. [DOI] [PubMed] [Google Scholar]
- 98.Rainbow TC, Parsons B, Wolfe BB. Quantitative autoradiography of beta 1- and beta 2-adrenergic receptors in rat brain. Proc. Natl. Acad. Sci. USA. 1984;81:1585–1589. doi: 10.1073/pnas.81.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Randle JC, Bourque CW, Renaud LP. Alpha 1-adrenergic receptor activation depolarizes rat supraoptic neurosecretory neurons in vitro. Am. J. Physiol. 1986;251:R569–574. doi: 10.1152/ajpregu.1986.251.3.R569. [DOI] [PubMed] [Google Scholar]
- 100.Richter-Levin G, Segal M, Sara S. An alpha 2 antagonist, idazoxan, enhances EPSP-spike coupling in the rat dentate gyrus. Brain Res. 1991;540:291–294. doi: 10.1016/0006-8993(91)90521-v. [DOI] [PubMed] [Google Scholar]
- 101.Sara SJ. Retrieval and reconsolidation: toward a neurobiology of remembering. Learn. Memory. 2000;7:73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- 102.Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- 103.Sara SJ, Roullet P, Przybyslawski J. Consolidation of memory for odor-reward association: beta-adrenergic receptor involvement in the late phase. Learn. Memory. 1999;6:88–96. [PMC free article] [PubMed] [Google Scholar]
- 104.Sara SJ, Segal M. Plasticity of sensory responses of locus coeruleus neurons in the behaving rat: implications for cognition. Prog. Brain Res. 1991;88:571–585. doi: 10.1016/s0079-6123(08)63835-2. [DOI] [PubMed] [Google Scholar]
- 105.Sara SJ, Vankov A, Herve A. Locus coeruleus-evoked responses in behaving rats: a clue to the role of noradrenaline in memory. Brain Res. Bull. 1994;35:457–465. doi: 10.1016/0361-9230(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 106.Scheiderer CL, Dobrunz LE, McMahon LL. Novel form of long-term synaptic depression in rat hippocampus induced by activation of alpha 1 adrenergic receptors. J. Neurophysiol. 2004;91:1071–1077. doi: 10.1152/jn.00420.2003. [DOI] [PubMed] [Google Scholar]
- 107.Scheiderer CL, Smith CC, McCutchen E, McCoy PA, Thacker EE, Kolasa K, Dobrunz LE, McMahon LL. Coactivation of M(1) muscarinic and alpha1 adrenergic receptors stimulates extracellular signal-regulated protein kinase and induces long-term depression at CA3-CA1 synapses in rat hippocampus. J. Neurosci. 2008;28:5350–5358. doi: 10.1523/JNEUROSCI.5058-06.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Scheinin M, Lomasney JW, Hayden-Hixson DM, Schambra UB, Caron MG, Lefkowitz RJ, Fremeau R. T., Jr. Distribution of alpha 2-adrenergic receptor subtype gene expression in rat brain. Mol. Brain Res. 1994;21:133–149. doi: 10.1016/0169-328x(94)90386-7. [DOI] [PubMed] [Google Scholar]
- 109.Segal M, Bloom F E. The action of norepinephrine in the rat hippocampus. I. Iontophoretic studies. Brain Res. 1974;72:79–97. doi: 10.1016/0006-8993(74)90652-0. [DOI] [PubMed] [Google Scholar]
- 110.Segal M, Markram H, Richter-Levin G. Actions of norepinephrine in the rat hippocampus. Prog. Brain Res. 1991;88:323–330. doi: 10.1016/s0079-6123(08)63819-4. [DOI] [PubMed] [Google Scholar]
- 111.Seguela P, Watkins K C, Geffard M, Descarries L. Noradrenaline axon terminals in adult rat neocortex: an immunocytochemical analysis in serial thin sections. Neuroscience. 1990;35:249–264. doi: 10.1016/0306-4522(90)90079-j. [DOI] [PubMed] [Google Scholar]
- 112.Seidenbecher T, Reymann K G, Balschun D. A post-tetanic time window for the reinforcement of long-term potentiation by appetitive and aversive stimuli. Proc. Natl. Acad. Sci. USA. 1997;94:1494–1499. doi: 10.1073/pnas.94.4.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sessler FM, Cheng JT, Waterhouse BD. Electrophysiological actions of norepinephrine in rat lateral hypothalamus. I. Norepinephrine-induced modulation of LH neuronal responsiveness to afferent synaptic inputs and putative neurotransmitters. Brain Res. 1988;446:77–89. doi: 10.1016/0006-8993(88)91298-x. [DOI] [PubMed] [Google Scholar]
- 114.Sessler FM, Liu W, Kirifides ML, Mouradian RD, Lin RC, Waterhouse BD. Noradrenergic enhancement of GABA-induced input resistance changes in layer V regular spiking pyramidal neurons of rat somatosensory cortex. Brain Res. 1995;675:171–182. doi: 10.1016/0006-8993(95)00060-4. [DOI] [PubMed] [Google Scholar]
- 115.Shen KZ, North RA, Surprenant A. Potassium channels opened by noradrenaline and other transmitters in excised membrane patches of guinea-pig submucosal neurones. J. Physiol. 1992;445:581–599. doi: 10.1113/jphysiol.1992.sp018941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Straube T, Frey JU. Involvement of beta-adrenergic receptors in protein synthesis-dependent late long-term potentiation (LTP) in the dentate gyrus of freely moving rats: the critical role of the LTP induction strength. Neuroscience. 2003;119:473–479. doi: 10.1016/s0306-4522(03)00151-9. [DOI] [PubMed] [Google Scholar]
- 117.Straube T, Korz V, Balschun D, Frey JU. Requirement of beta-adrenergic receptor activation and protein synthesis for LTP-reinforcement by novelty in rat dentate gyrus. J. Physiol. 2003;552:953–960. doi: 10.1113/jphysiol.2003.049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Summers RJ, Papaioannou M, Harris S, Evans BA. Expression of beta 3-adrenoceptor mRNA in rat brain. Br. J. Pharmacol. 1995;116:2547–2548. doi: 10.1111/j.1476-5381.1995.tb17205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Talaia C, Queiroz G, Pinheiro H, Moura D, Goncalves J. Involvement of G-protein betagamma subunits on the influence of inhibitory alpha2-autoreceptors on the angiotensin AT1-receptor modulation of noradrenaline release in the rat vas deferens. Neurochem. Int. 2006;49:698–707. doi: 10.1016/j.neuint.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 120.Tanaka C, Nishizuka Y. The protein kinase C family for neuronal signaling. Ann.Rev. Neurosci. 1994;17:551–567. doi: 10.1146/annurev.ne.17.030194.003003. [DOI] [PubMed] [Google Scholar]
- 121.Thiel CM. Pharmacological modulation of learning-induced plasticity in human auditory cortex. Restorative Neurol. Neurosci. 2007;25:435–443. [PubMed] [Google Scholar]
- 122.Timmons SD, Geisert E, Stewart AE, Lorenzon NM, Foehring RC. alpha2-Adrenergic receptor-mediated modulation of calcium current in neocortical pyramidal neurons. Brain Res. 2004;1014:184–196. doi: 10.1016/j.brainres.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 123.Tronel S, Feenstra MG, Sara SJ. Noradrenergic action in prefrontal cortex in the late stage of memory consolidation. Learn. Memory. 2004;11:453–458. doi: 10.1101/lm.74504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Valentino R, Aston-Jones G. Physiological and Anatomical Determinants of Locus Coeruleus Discharge: Behavioral and Clinical Implications in Psychopharmacology - 4th Generation of Progress, A.C.o. Neuropsychopharmacology, Psychopharmacology. (Berl): Nashville, TN: 2000. [Google Scholar]
- 125.Vankov A, Herve-Minvielle A, Sara SJ. Response to novelty and its rapid habituation in locus coeruleus neurons of the freely exploring rat. Eur. J. Neurosci. 1995;7:1180–1187. doi: 10.1111/j.1460-9568.1995.tb01108.x. [DOI] [PubMed] [Google Scholar]
- 126.Videen TO, Daw NW, Rader RK. The effect of norepinephrine on visual cortical neurons in kittens and adult cats. J. Neurosci. 1984;4:1607–1617. doi: 10.1523/JNEUROSCI.04-06-01607.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Warren RA, Dykes RW. Transient and long-lasting effects of iontophoretically administered norepinephrine on somatosensory cortical neurons in halothane-anesthetized cats. Can. J. Physiol. Pharmacol. 1996;74:38–57. [PubMed] [Google Scholar]
- 128.Watanabe Y, Ikegaya Y, Saito H, Abe K. Opposite regulation by the beta-adrenoceptor-cyclic AMP system of synaptic plasticity in the medial and lateral amygdala in vitro. Neuroscience. 1996;71:1031–1035. doi: 10.1016/0306-4522(95)00498-x. [DOI] [PubMed] [Google Scholar]
- 129.Waterhouse BD, Moises HC, Woodward DJ. Noradrenergic modulation of somatosensory cortical neuronal responses to iontophoretically applied putative neurotransmitters. Exp. Neurol. 1980;69:30–49. doi: 10.1016/0014-4886(80)90141-7. [DOI] [PubMed] [Google Scholar]
- 130.Waterhouse BD, Moises HC, Woodward DJ. Phasic activation of the locus coeruleus enhances responses of primary sensory cortical neurons to peripheral receptive field stimulation. Brain Res. 1998;790:33–44. doi: 10.1016/s0006-8993(98)00117-6. [DOI] [PubMed] [Google Scholar]
- 131.Waterhouse BD, Sessler FM, Cheng JT, Woodward DJ, Azizi SA, Moises HC. New evidence for a gating action of norepinephrine in central neuronal circuits of mammalian brain. Brain Res. Bull. 1988;21:425–432. doi: 10.1016/0361-9230(88)90154-2. [DOI] [PubMed] [Google Scholar]
- 132.Winder DG, Martin KC, Muzzio IA, Rohrer D, Chruscinski A, Kobilka B, Kandel ER. ERK plays a regulatory role in induction of LTP by theta frequency stimulation and its modulation by ß-adrenergic receptors. Neuron. 1999;24:715–726. doi: 10.1016/s0896-6273(00)81124-1. [DOI] [PubMed] [Google Scholar]
- 133.Winson J, Dahl D. Action of norepinephrine in the dentate gyrus. II. Iontophoretic studies. Exp. Brain Res. 1985;59:497–506. doi: 10.1007/BF00261340. [DOI] [PubMed] [Google Scholar]
- 134.Wozniak M, Schramm N, Limbird L. The Noradrenergic Receptor Subtypes in Psychopharmacology - The Fourth Generation of Progress A.C.o. Neuropsychopharmacology, Psychopharmacology. (Berl): Nashville, TN: 2000. [Google Scholar]
- 135.Xu L, Anwyl R, Rowan MJ. Spatial exploration induces a persistent reversal of long-term potentiation in rat hippocampus. Nature. 1998;394:891–894. doi: 10.1038/29783. [DOI] [PubMed] [Google Scholar]
- 136.Yue BW, Huguenard JR. The role of H-current in regulating strength and frequency of thalamic network oscillations. Thalamus Relat. Syst. 2001;1:95–103. doi: 10.1016/S1472-9288(01)00009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhao Y, Fang Q, Straub SG, Sharp GW. Both Gi and Go heterotrimeric G proteins are required to exert the full effect of norepinephrine on the beta-cell K ATP channel. J. Biol. Chem. 2008;283:5306–5316. doi: 10.1074/jbc.M707695200. [DOI] [PubMed] [Google Scholar]