Abstract
The N-dicyclopropylmethyl (Dcpm) residue, introduced into amino acids via reaction of dicyclopropylmethanimine hydrochloride with an amino acid ester followed by sodium cyanoborohydride or triacetoxyborohydride reduction, can be used as an amide bond protectant for peptide synthesis. Examples which demonstrate the amelioration of aggregation effects include syntheses of the alanine decapeptide and the prion peptide (106–126). Avoidance of cyclization to the aminosuccinimide followed substitution of Fmoc-(Dcpm)Gly-OH for Fmoc-Gly-OH in the assembly of sequences containing the sensitive Asp-Gly unit.
We describe a new backbone protectant for the synthesis of so-called “difficult peptides”, namely those peptides for which the presence of specific sequences of amino acids, often hydrophobic amino acids, lead to slow deblocking and incomplete coupling processes. Several approaches to overcome these problems have been devised. These include the use of more potent coupling reagents,1 pseudoproline insertions,2 the depsipeptide technique3 and the use of backbone protection.4 Recently dicyclopropylmethyl (Dcpm) and dimethylcyclopropylmethyl (Dmcp) groups have been described as amide protectants for Asn, Gln and the C-terminal position of linear peptide amides.5 In view of their utility as amide protectants, consideration has now been given to their possible use as peptide bond protectants. Of these two alkyl residues the Dcpm group was chosen due to its lesser steric requirements and the fact that the appropriate N-substituted amino acids could be synthesized via standard reductive alkylation techniques. Finally, deblocking of the Dcpm residue from an internal (tertiary amide) position is expected to be enhanced and perhaps as rapid as the removal of the Dmcp residue from the terminal amide position. This expectation follows from the curious fact recorded in the literature6 that secondary amide, N-t-butylbenzamide 1, is stable toward TFA whereas the analogous tertiary amide N-t-butyl-N-methylbenzamide 2 loses the t-butyl residue upon treatment with the same acid. It has long been recognized that N-t-butyl amides such as 1 differ from the corresponding oxygen analogs (t-butyl esters) in being stable toward acidic deblocking.7 The sensitivity of 2 could perhaps be explained on the basis of its being a tertiary alkyl-based tertiary amide which might be subject to more facile N- relative to O-protonation with subsequent ready loss of the t-butyl cation in TFA.8
In the present work the high acid sensitivity of the internal Dcpm residue was verified, thus allowing for its use as backbone protectant. Regarding the susceptibility of an N-Dcpm-substituted amino acid or amino acid ester to coupling reactions, it is well known that coupling to N-methyl amino acids is difficult9 and coupling to N-Dcpm amino acids is expected to be even less facile. However, the steric requirements of the Dcpm group may be unique since in its coupling chemistry the Dcpm-bearing model amino acid α,α-dicyclopropylgycine resembles its simple α,α-dimethyl analog, H-Aib-OH, more than its extremely hindered analog, α,α-diisopropylglycine.10 Since H-Aib-OH is an easily handled amino acid for peptide incorporation similar effects are to be expected for the N-Dcpm residue.10 Indeed as shown in the present work, for the relatively unhindered amino acids glycine and alanine, coupling to the N-Dcpm derivatives is readily achievable. On the other hand for the more hindered amino acids such as valine, isoleucine and threonine special acylating techniques may be required.
The currently most commonly used backbone protectant, the Hmb group, differs4 from the Dcpm residue in being acylated via prior reaction at the o-hydroxyl group followed by an O → N acyl shift.11
Prior to preparation of appropriate amino acid derivatives a model experiment was carried with the previously described imine N-n-propyl dicyclopropylketimine. 13 Reduction and benzoylation gave the corresponding tertiary amide which was tested for its stability toward TFA under conditions normally used for the final deprotection step during Fmoc/t-Bu-based solid phase peptide assembly. As expected, the Dcpm residue was readily removed with the formation of N-(n-propyl) benzamide.
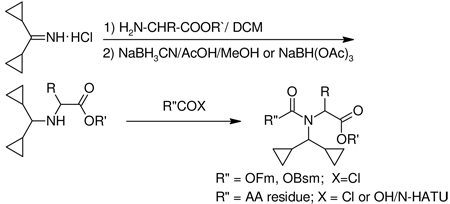
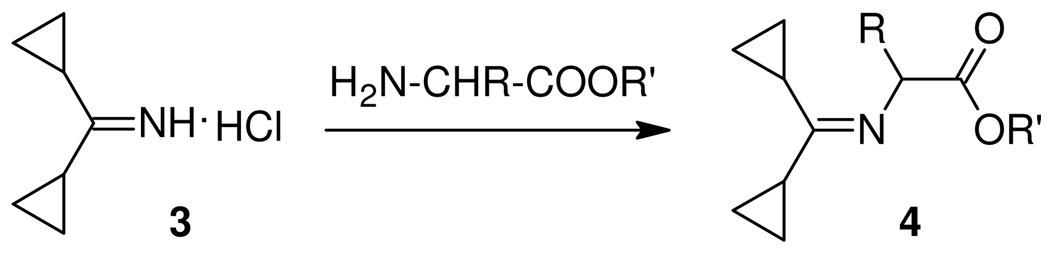
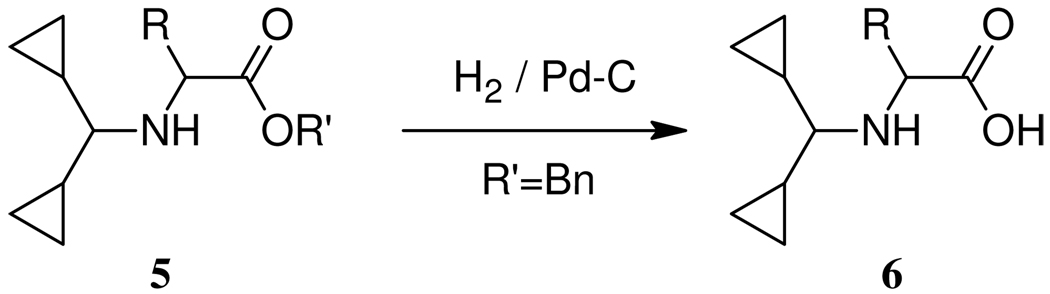
A general method for the synthesis of N-Dcpm amino acids was then developed. A key intermediate 3, obtainable by treatment of the commercially available, or easily synthesized dicyclopropyl ketone, with ammonia in the presence of titanium tetrachloride, has been described in the patent literature.14 Reaction of 3 with an amino acid ester gave imine 4 which upon reduction with sodium cyanoborohydride or triacetoxyborohydride in the presence of acetic acid gave the amino acid derivative 5 which in the case of the benzyl ester (5, R` = Bn) underwent standard catalytic hydrogenolysis to give the free amino acid 6.
For relatively unhindered amino acids (e.g. 6, R = H, Me) the amino function is readily attacked by an appropriate acylating agent. Thus the Fmoc15 and Bsmoc16 derivatives 7 (R″ = Fm, Bsm) are readily obtained via the corresponding chloroformates using the
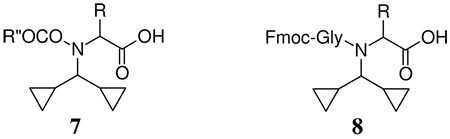 |
Bolin technique.17 Analogously treatment with Fmoc-Gly-Cl18 or Fmoc-Gly-F19 yields the corresponding protected dipeptide 8 (R = H, Me).
It was demonstrated that in the synthesis of 5 (R = Me R′ = Bn) via 3 no significant loss of configuration occurred at the alanine residue as shown by conversion to 7 (R = Me, R``= Fm). Coupling of the Fmoc-protected acid to proline atached to a Rink amide resin by means of N-HATU/DIEA in DMF gave the corresponding dipeptide resin which was Dcpm-deblocked and cleaved from the resin by treatment with TFA to give Fmoc-Ala-Pro-NH2 20 which was contaminated by only 0.95 % of the DL-isomer showing that no more than this amount of contamination could have been present in the sample 5 used or could have been formed during the coupling process. Further work will be required in order to determinete the exact source of the contamination but the amount is small enough not to impinge upon the present work.
The methodology described was also applied to the synthesis of the valine derivatives 5 (R = iPr, R′ = Bn) and 6 (R = iPr) however this more hindered amino acid could not be acylated via Fmoc-Cl or Bsmoc-Cl to give 7 (R = iPr, R″ = Fm or Bsm). Similarly the coupling of a simple protected amino acid (e.g. Z-Phe-OH) to 5 (R = iPr, R′ = Bn) did not succeed with a variety of standard coupling reagents (e.g. N-HBTU,21,23 N-HATU,22,23 TFFH,24 etc.). However, with the more potent acylating agent Phth-Phe-Cl coupling in the presence of BSA25 gave the dipeptide in 56–62% yield. Details will be provided in a subsequent publication.
As a first sequence in which to examine the utility of the Dcpm residue as a backbone protectant the difficult deca-alanine sequence built onto arginine was examined.26 Previously this system has been shown to be subject to the effects of aggregation leading to both deblocking and coupling deficiencies. An automated synthesis of the deca-alanine sequence using 4 equiv of Fmoc amino acid with coupling via N-HATU gave material contaminated by several des-Ala units as well as undeblocked Fmoc-containing segments (Fig. 1, Supporting Information). An attempt to improve the synthesis was made by substituting Fmoc(Dcpm)-Ala-OH for Fmoc-Ala-OH in order to introduce the 5th alanine unit and at the same time reduce the effect of aggregation for all subsequent coupling steps. The remainder of the synthesis was carried out in the normal manner but the only material obtained following work-up and removal of the peptide from the resin was the N-Dcpm-penta-alanine derivative (Fig 2, Supporting Information) showing that the system shut down following introduction of the Dcpm-Ala unit.
However, since previous studies had suggested that Bsmoc amino acids were more reactive than their Fmoc counterparts, 3c it was simply necessary to substitute Bsmoc-Ala-OH for the Fmoc analog in this synthesis and, remarkably, there was obtained about 50% of the desired deca-alanine product. The 11-mer is accompanied by about 50% of the N-Dcpm-substituted deletion sequence which had been the sole product formed in the all Fmoc case (Fig. 3, Supporting Information). If HOAt/DIC was used in place of N-HATU and coupling continued for a longer time the reaction could be pushed toward completion (Fig. 8, Supporting Information).
A sequence which is expected to be subject to difficulties due to the presence of the base-sensitive Asp-Gly unit27 is dodecapeptide, 9 which on attempted synthesis under standard conditions via N-HBTU gave only the aminosuccinimide cyclization product. With Fmoc-(Dcpm)-Gly-OH substituted for Fmoc-Gly-OH
ADGSLDDYNHLV-amide 9
under the same conditions (N-HBTU) the result was still unsatisfactory (only 17% of 9) but if N-HATU was substituted for N-HBTU the desired dodecapeptide amide was obtained in a yield of 91% along with only 8% of the des-Asp deletion peptide (see Figs. 9a,b, Supporting Information).
A classical “difficult sequence” was then examined, namely the prion peptide (106– 126) 10 which was previously examined by Jobling, et al.28 Using Fmoc
106 114 119 126 KTNMKHMAGAAAAGAVVGGLG 10
chemistry with N-HBTU coupling and introducing the Hmb residue at positions 114 and 119 these workers obtained a low yield of the desired peptide (7.3%) along with a number of deletion sequences. With Fmoc (Dcpm)-Gly-OH introduced at the same two positions and using N-HATU for coupling using normal Fmoc amino acids except for positions 113 and 118 which were introduced by Bsmoc-Ala-OH there was obtained a relatively clean sample of the desired peptide (crude yield 41%, purity 89%) (see Figs. 10a,b, Supporting Information). The only major by-product was a small amount of the methionine sulfoxide derivative.
In conclusion, it has been shown that the N-dicyclopropylmethyl residue can be used as an amide backbone protectant in the case of relatively unhindered amino acids. N-Dcpm amino acids can be readily synthesized via treatment of dicyclopropyl ketone imine hydrochloride with an amino acid ester followed by sodium cyanoborohydride or sodium triacetoxyborohydride reduction.
Supplementary Material
Scheme 1.
Scheme 2.
Acknowledgment
We are indebted to the National Science Foundation (NSF CHE-9003192) and the National Institutes of Health (GM-09706) for support of the work in Amherst.
Footnotes
Abbreviations used: ACN = acetonitrile; BSA = bistrimethylsilylacetamide; Bsmoc = benzothiophenesulfonyl-2-methyloxycarbonyl; Dcpm = dicyclopropylmethyl; DIC = diisopropylcarbodiimide; Dmcp = dimethylcyclopropyl-methyl; DODT = 3,6-dioxa-1,8-octanedithiol; Fmoc = 9-fluorenemethyloxycarbonyl; N-HATU = 1-[bis(dimethylamino)methylene]-1-H-1,2,3-triazolo[4,5-b]pyridinium hexafluorophosphate 3-oxide; N-HBTU = 1-[bis(dimethylamino)methylene]-1-H-benzotriazolium hexafluorophosphate 3-oxide; Hmb = 2-hydroxy-4-methoxybenzyl; HOAt = 1-hydroxy-7-azabenzotriazole; Phth = o-phthaloyl; TFA = trifluoroacetic acid; TFFH = tetramethyl fluoroformamidinium hexafluorophosphate.
Supporting Information Available:
Experimental procedures and compound characterization data.
References
- 1.(a) El-Agnaf OMA, Goodwin H, Sheridan JM, Frears ER, Austen BM. Prot. Pept. Lett. 2000;7:1. [Google Scholar]; (b) Milton SCF, Milton RCL, Kates SA, Glàbe C. Lett. Pept. Sci. 1999;6:151. [Google Scholar]
- 2.Mutter M, Nefzi A, Sato T, Sun X, Wahl F, Wuhr T. Pept. Res. 1995;8:145. [PubMed] [Google Scholar]
- 3.(a) Sohma Y, Hayashi Y, Chiyomori Y, Kimura M, Sasaki M, Taniguchi A, Fukue K, Kimura T, Kiso Y. Pept. Sci. 2005;41:175. doi: 10.1002/psc.649. [DOI] [PubMed] [Google Scholar]; (b) Mutter M, Chandravarkar A, Boyat C, Lopez J, Dos Santos S, Mandal B, Mimna R, Murat K, Patiny L, Saucēde L, Tuchscherer G. Angew. Chem. Int. Ed. 2004;43:4172. doi: 10.1002/anie.200454045. [DOI] [PubMed] [Google Scholar]; (c) Carpino LA, Krause E, Sferdean CD, Schümann M, Fabian H, Bienert M, Beyermann M. Tetrahedron Lett. 2004;45:7519. [Google Scholar]
- 4.(a) Johnson T, Quibell M, Owen D, Sheppard RC. J. Chem. Soc. Chem. Commun. 1993:369. [Google Scholar]; (b) Johnson T, Quibell M. Tetrahedron Lett. 1994;35:463. [Google Scholar]; (c) Johnson T, Quibell M, Sheppard RC. J. Pept. Sci. 1995;1:11. doi: 10.1002/psc.310010104. [DOI] [PubMed] [Google Scholar]; (d) Ede NJ, Ang KH, James IW, Bray AW. Tetrahedron Lett. 1996;37:9097. [Google Scholar]
- 5.Carpino LA, Chao HG, Ghassemi S, Mansour EME, Riemer C, Warrass R, Sadat-Aalaee D, Truran GA, Imazumi H, El-Faham A, Ionescu D, Ismail H, Kowalski T, Han CH. J. Org. Chem. 1995;60:7718. [Google Scholar]
- 6.Catt JD, Matier WD. J. Org. Chem. 1974;39:566. [Google Scholar]
- 7.Callahan FM, Anderson GW, Paul R, Zimmerman JE. J [Google Scholar]
- 8.Compare Guiheneuf G, Abboud J-LM, Lachkar A. Can. J. Chem. 1988;66:1032..
- 9.Humphrey JM, Chamberlin AR. Chem. Rev. 1997;97:2243. doi: 10.1021/cr950005s. [DOI] [PubMed] [Google Scholar]
- 10.(a) Yamada T, Sano A, Yanagihara R, Miyazawa T. In: Kitada C, editor. Peptide Chemistry 1996, Proc. of the 34th Japanese Peptide Symposium; Osaka: Protein Research Foundation; 1997. p. 357. [Google Scholar]; (b) Yamada T, Gohda S, Sano A, Yanagihara R, Miyazawa T, Kuwata S. In: Ramage R, Epton R, editors. Peptides 1996, Proc. of the 24th European Peptide Symposium; p. 927. [Google Scholar]
- 11.To make it more suitable for use with hindered amino acids Miranda and Alewood and co-workers12 have modified the Hmb residue by the introduction of a nitro group into the benzylic ring. While this change has the desired effect, the resulting protectant cannot be removed by acid-catalysed deblocking.
- 12.Miranda LP, Meutermans WDF, Smythe ML, Alewood PF. J. Org. Chem. 2000;65:5460. doi: 10.1021/jo991340+. [DOI] [PubMed] [Google Scholar]
- 13.Pocar D, Stradi R, Trimarco P. Tetrahedron. 1975;31:2427. [Google Scholar]
- 14.Kurono M, Kondo Y, Matsumoto Y, Sawai K. 5, 110, 945. U.S.P. 1992 [Chem. Abstr.1992, 117, 111466].
- 15.Carpino LA, Han GY. J. Org. Chem. 1972;37:3404. [Google Scholar]
- 16.Carpino LA, Ismail M, Truran GA, Mansour EME, Iguchi S, Ionescu D, El-Faham A, Riemer C, Warrass R. J Org. Chem. 1999;64:4324. [Google Scholar]
- 17.Bolin RR, Sytwu I, Humeic F, Meienhofer Int. J. Pept. Protein Res. 1983;33:353. [Google Scholar]
- 18.Carpino LA, Cohen BJ, Stephens KE, Jr, Sadat-Aalaee SY, Tien J-H, Langridge J. Org. Chem. 1986;51:3732. [Google Scholar]
- 19.Carpino LA, Sadat-Aalaee D, Chao HG, DeSelms RH. J. Am. Chem. Soc. 1990;112:9651. [Google Scholar]
- 20.Authentic samples of the two diastereomeric dipeptides were prepared by normal solution techniques and shown to be base line-separated on a C-18 HPLC coulmn (see Supporting Information). For a prior report on the Ala/Pro dipeptide amide without experimental data see: Li J, Wilk E, Wilk S. Arch. Biochem. Biophys. 1995;323:148. doi: 10.1006/abbi.1995.0020..
- 21.Dourtoglou V, Gross B, Lambropoulou V, Zioudrou C. Synthesis. 1984:572. [Google Scholar]
- 23.Carpino LA, Imazumi H, El-Faham A, Ferrer FJ, Zhang C, Lee Y, Foxman BM, Henklein P, Hanay C, Mügge C, Wenschuh H, Klose J, Beyermann M, Bienert M. Angew. Chem., Int. Ed. 2002;41:441. doi: 10.1002/1521-3773(20020201)41:3<441::aid-anie441>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 24.Carpino LA, El-Faham A. J. Am. Chem. Soc. 1995;117:5401. [Google Scholar]
- 25.Compare: Carpino LA, Beyermann M, Wenschuh H, Bienert M. Acc. Chem. Res. 1996;29:268..
- 26.Compare: Larsen D, Holm A. Int. J. Pept. Protein. Res. 1994;43:1. doi: 10.1111/j.1399-3011.1994.tb00368.x.Kates SA, Sole NA, Beyermann M, Barany G, Albericio F. Peptide Res. 1996;9:106..
- 27.For a series of papers dealing with this general problem see Mergler M, Dick F, Sax B, Weiler P, Vorherr T. J. Pept. Sci. 2003;9:36. doi: 10.1002/psc.430.Mergler M, Dick F, Sax B, Stähelin C, Vorherr T. J. Pept. Sci. 2003;9:518. doi: 10.1002/psc.473.Mergler M, Dick F. J. Pept. Sci. 2005;11:650. doi: 10.1002/psc.668..
- 28.Jobling MF, Barrow CJ, White AR, Masters CL, Collins SJ, Cappai R. Lett. Pept. Sci. 1999;6:129. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.