Abstract
Introduction
QT interval for a given heart rate differs between exercise and recovery (QT hysteresis) due to slow QT adaptation to changes in heart rate. We hypothesized that QT hysteresis is evident within stages of exercise and investigated which component of the QT contributes to hysteresis.
Methods and Results
Nineteen healthy volunteers performed a staged exercise test (four stages, 3 min each). Continuous telemetry was analyzed with software to compare QT intervals in a rate-independent fashion. QRST complexes during each minute were sorted by RR interval, and complexes in bins of 20 ms width were signal-averaged. QT and QTp (onset of QRS to peak T wave) were measured, and terminal QT calculated (peak to end of T wave, Tpe = QT − QTp). QT, QTp, and Tpe at the same heart rate were compared between the first and last minute of each stage. QT shortened from the first to last minute of exercise in each stage (Stage I: 358 ± 30 to 346 ± 25 ms, P < 0.001; Stage II: 342 ± 27 to 331 ± 24 ms, P = 0.003; Stage III: 329 ± 21 to 322 ± 18 ms, P = 0.03; Stage IV: 313 ± 22 to 303 ± 23 ms, P = 0.005). QTp also shortened in each stage, while Tpe was unchanged.
Conclusion
QT hysteresis occurs during exercise in normals, and the major determinant is shortening of the first component of the T wave. Terminal repolarization (peak to end of T wave), a surrogate for transmural dispersion of repolarization, does not shorten significantly with exercise.
Keywords: exercise, QT interval, QT hysteresis
Introduction
The QT interval at a given heart rate during recovery is shorter than the QT interval at the same heart rate during exercise, a phenomenon termed QT hysteresis.1 QT hysteresis is exaggerated in the congenital long-QT syndrome,2 a disease with increased risk for sudden cardiac death during exercise. Furthermore, beta-blockers, which reduce risk for cardiac events in long-QT syndrome,3 also normalize QT hysteresis in these patients.4 The mechanism of QT hysteresis in normal subjects is at least partly due to autonomic changes associated with exercise,5 and may provide insight into the risk of sudden death during and after exercise.
Terminal repolarization, measured as the interval from the peak to the end of the T wave, is another proposed indicator that may relate to sudden death risk. In arterially perfused wedge preparations, regional differences in repolarization across the ventricular wall give rise to heterogeneity of cardiac repolarization (transmural dispersion of repolarization) under physiologic and pathologic conditions.6 Subsequently, the interval from the peak to the end of the T wave was shown to correlate with transmural dispersion of repolarization,7 and to provide the substrate for torsade de pointes in the setting of a prolonged QT interval.8,9 The correlation of the peak and end of the T wave to the earliest and latest end of repolarization has been validated in a swine model.10 Clinical studies have demonstrated increased transmural dispersion of repolarization, as measured by the peak to end of the T wave, in patients with congenital LQTS;11–13 and the peak to end of the T wave as a fraction of the QT interval has been identified as the strongest predictor for torsade in a cohort of patients with drug-induced LQTS.14
The objective of this study is to: (1) investigate QT hysteresis during escalating bouts of exercise in normal subjects, and (2) investigate the effects of exercise on terminal repolarization.
Methods
Subjects
Subjects for this study were 19 healthy individuals (11 males), aged 18–40 years. Healthy was defined as no significant past medical history, no concurrent medications, normal baseline electrocardiogram, and normal prestudy complete blood count, serum electrolytes, and liver function tests. Female subjects were required to have a negative urine pregnancy test. Subjects refrained from alcohol or caffeine during the study period. Subjects were brought into sodium balance with 7 days of diet containing 100 mEq Na+/day and 100 mEq K+/day supplied by the Bionutrition Research Service of the Vanderbilt Clinical Research Center (CRC). Sodium balance is generally achieved on this diet in 4 days. After the 7-day dietary period, subjects came to the CRC after an overnight fast for the exercise study. The serum potassium was normal on the study day (mean 3.9 ± 0.1 mEq/L). All subjects gave written informed consent prior to entering the study. The protocol was approved by the Vanderbilt University Institutional Review Board.
Exercise Tests and Electrocardiographic Recording
Subjects were positioned on a recumbent bicycle and ECG leads were placed. After a 10-min rest period, subjects performed exercise on an electrically braked bicycle ergometer, using a staged protocol. The exercise protocol consisted of four stages, 3 min each (12 min total) with workloads of 25, 50, 75, and 100 W. After 12 min, subjects stopped exercise and remained on the recumbent bicycle. Continuous recording of the ECG (surface lead II) was obtained throughout the protocol at 500 Hz sampling frequency using a Gould ECG amplifier (Valley View, OH, USA) and Windaq acquisition equipment (Akron, OH, USA).
In order to study hysteresis within exercise, we chose to compare QRST complexes from the first minute of an exercise stage with complexes from the last minute of an exercise stage. At the beginning of an exercise stage, when the workload is increased, the RR interval shortens rapidly (within 1 min) then remains relatively stable through the remainder of the exercise stage. Thus, in the first minute of an exercise stage, a fairly wide range of RR intervals is observed (Fig. 1, top panel), while in the last minute of an exercise stage, there is a relatively narrow range of RR intervals (Fig. 1, bottom panel). RR intervals of the same length can be selected from both the first and last minute of the exercise stage, and the QT intervals of those complexes compared, avoiding the need for a rate correction formula. Using custom-designed software written in the Matlab programming environment (Natick, MA, USA), automated algorithms to identify peak R waves were applied to the data with investigator overreading.15 All QRST complexes during the first and last minute of each exercise stage were sorted according to RR interval into bins of 20 ms width (see Fig. 1). For each stage and subject, an identical RR interval bin with at least 10 complexes in both the first and last minute of the exercise stage was selected for comparison. QRST complexes in the selected bins were aligned by the peak of the R wave, and then signal-averaged (see Fig. 2). The signal averaged tracing was magnified, and measurements made with digital calipers. The start of the QT interval was defined as the earliest deflection of the QRS complex and the end was defined as the point at which the maximal downward slope of the T wave crossed the isoelectric point of the corresponding QRS complex. The absolute QT and the QTp (onset to peak of T wave) were measured, and the terminal QT calculated (Tpe [peak to end of T wave] = QT − QTp). Absolute QT, QTp, and Tpe at the same heart rate (i.e., identical RR bin) were compared between the first and last minute of each exercise stage. QTc was also calculated with Bazett’s correction by dividing the absolute QT by the square root of the RR interval in the mid-point of the bin (i.e., 660 ms for bin spanning 650–670 ms). Subjects in whom no single bin contained at least 10 complexes in both the first and last minute of an exercise stage were excluded from analysis for that stage. Due to differences in heart rate profiles among subjects, the individual RR interval bin was not identical across subjects within a stage, nor was it identical across stages for an individual subject. These methods allow for comparison of repolarization indices (QT, QTp, and Tpe) with a fixed RR interval at the beginning and end of an exercise stage within individuals.
Figure 1.
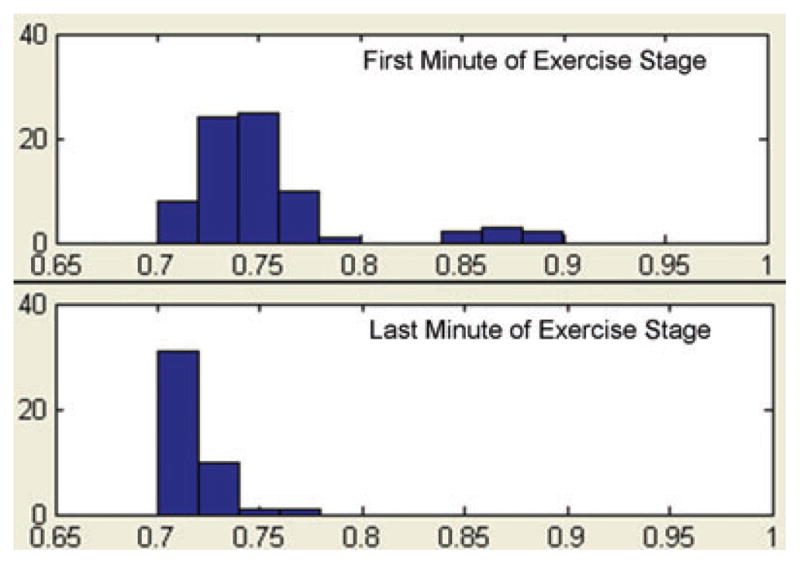
Histograms of RR intervals during exercise for one subject. All QRST complexes during the first (top panel) and last (bottom panel) minute of a 3-min exercise stage are sorted by RR interval (X-axis represents RR interval in seconds, Y-axis represents # of intervals). Bins are of 20 ms width. In the first minute, there are a few complexes in the three bins spanning 0.84 and 0.9 s, but most occur in the five bins spanning 0.7–0.8 s. During the last minute of the exercise stage, all complexes occur in the four bins spanning 0.7–0.78 ms. For the analysis, identical bins containing at least 10 complexes in both the first and last minute of the stage are selected.
Figure 2.
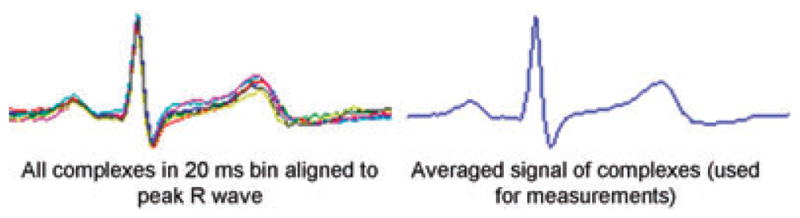
Superimposed and signal averaged complexes. All QRST complexes in a selected bin are superimposed, and aligned by the peak of the R wave (left-hand side). These signals are then averaged (right-hand side), the tracing magnified, and measurements of QT and QTp made with digital calipers.
Data Analysis
QT, QTc, QTp, and Tpe at first and last minute of each exercise stage were compared with a paired t-test. A two-tailed P-value < 0.05 was considered significant. Data were analyzed using the statistical software SPSS for Windows version 11.5 (SPSS Inc., Chicago, IL, USA).
Results
QT Hysteresis is Evident Within Exercise Stages
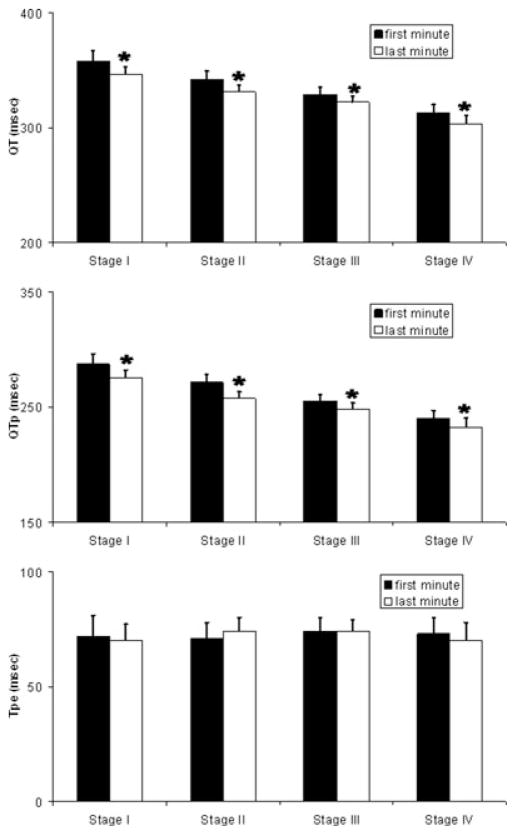
Identical RR interval bins with at least 10 complexes in both the first and last minute of exercise stage were found in 11 subjects for stage I, 15 subjects for stage II, 12 subjects for stage III, and 9 subjects for stage IV. There were a total of 58 bins with 10–90 complexes per bin (median 23 complexes per bin) in the analyzed dataset. The absolute QT intervals at the same heart rate during the first and last minute of each stage of exercise are shown in Figure 3(A). It should be noted that RR interval bins differ between stages for a single individual, so comparison of QT interval between stages was not performed. Within stage I, comparing QT interval at the identical RR interval bin (same heart rate) from the first to last minute of exercise, QT shortens from 358 ± 30 to 346 ± 25 ms (P < 0.001). Similarly, QT shortens significantly in stage II (342 ± 27 to 331 ± 24 ms, P = 0.003), stage III (329 ± 21 to 322 ± 18 ms, P = 0.03), and stage IV (313 ± 22 to 303 ± 23 ms, P = 0.005). Thus, a difference in QT interval at the same heart rate (QT hysteresis) can be observed not only when comparing exercise and recovery, but also when comparing early and late exercise within the same stage. QTc also shortens significantly in stage I (438 ± 25 to 422 ± 19 ms, P < 0.001), stage II (436 ± 23 to 422 ± 17 ms, P = 0.003), stage III (433 ± 21 to 424 ± 16 ms, P = 0.03), and stage IV (437 ± 16 to 422 ± 13 ms, P = 0.007).
Figure 3.
QT interval, QTp, and Tpe at a fixed heart rate during staged exercise. The absolute QT interval (Panel A), QTp (Panel B), and Tpe (Panel C) are plotted for both the first and last minute of each exercise stage. Within each stage absolute QT and QTp (at the same heart rate) shorten significantly, while the Tpe does not shorten. Y-axis in ms. Data are displayed as mean + 1 SEM. * Indicates P < 0.05.
Terminal Repolarization Does Not Shorten with Exercise
QTp and Tpe intervals at the same heart rate during the first and last minute of each stage of exercise are displayed in Figure 3(B and C). As with absolute QT interval, QTp shortens significantly with a fixed RR interval within each exercise stage (Stage I, 287 ± 26 to 275 ± 22 ms, P < 0.001; Stage II, 271 ± 23 to 257 ± 21 ms, P < 0.001; Stage III, 255 ± 18 to 248 ± 14 ms, P = 0.008; Stage IV, 240 ± 14 to 232 ± 16 ms, P = 0.008). In contrast, terminal repolarization (Tpe) does not change significantly with exercise when comparing Tpe at identical RR intervals (Stage I, 72 ± 12 to 70 ± 11 ms, P = 0.2; Stage II, 71 ± 10 to 74 ± 10 ms, P = 0.15; Stage III, 74 ± 16 to 74 ± 7 ms, P = 0.9; Stage IV, 73 ± 13 to 70 ± 14 ms, P = 0.2).
Discussion
Previous studies of QT hysteresis have predominantly compared exercise to recovery.1,2,4,16 This study demonstrated significant differences in QT interval at a given heart rate within stages of an exercise protocol in normal subjects. Our methods allowed assessment of effects of continued exercise on QT interval independent of heart rate by selecting only QRST complexes within a very narrow RR interval bin (20 ms) during both the first and last minute of each exercise stage. Furthermore, we demonstrate that the major component of the QT interval changing with exercise is the interval from onset of the QRS complex to the peak of the T wave (QTp). Terminal repolarization (peak to end of T wave) does not shorten with exercise.
QT hysteresis is observed because the QT interval “lags,” or slowly responds to changes in heart rate;17,18 however, the precise mechanisms of QT hysteresis are unclear. Both autonomic and nonautonomic factors affect the QT/heart rate relationship during exercise and recovery.1,5,19–21 Magnano et al. demonstrated that QT intervals at a heart rate of 100 bpm differed depending on whether the heart rate was achieved with exercise, isoproterenol, or atropine.22 This suggests that there are heart rate-independent autonomic effects on QT interval. Our finding of heart rate independent exercise-induced QT shortening is consistent with this and other previous studies. Fananapazir et al. studied patients with fixed rate ventricular pacing during exercise, revealing mild QT shortening, which was abolished with beta-blockers.23 Taken together, it seems that circulating catecholamines offer the best explanation for the heart rate-independent autonomic effects on QT interval. Our finding that terminal repolarization does not shorten with exercise is also consistent with previous studies. Zabel et al. demonstrated no relationship between Tpe and heart rate during either exercise or atrial pacing.24 Sundqvist and Slyven observed only a 10 ms change in Tpe from rest to maximal exercise in normal subjects.25 This small difference was observed with a large change in heart rate (peak heart rates near 170 bpm), and accompanied by changes in QTp six to sevenfold larger than the change in Tpe. We observed no exercise effect on Tpe when comparing complexes at the same heart rate.
Implications
Exercise and recovery from exercise represent high-risk cardiovascular states, as the risk for sudden death is increased nearly 17-fold during and after vigorous exercise.26,27 QT hysteresis, while present in normal individuals, is exaggerated in patients with congenital long-QT syndrome, a population at particularly high risk for sudden death during exercise.2 The lag in QT shortening in response to changes in heart rate result in relatively prolonged QT intervals for a given heart rate during exercise, especially, as we have shown during initial periods of increased workload during exercise. QT prolongation is a predictor of sudden death in the general population,28,29 thus relatively long QT intervals during exercise may predispose to sudden death. Furthermore, recent studies suggest that increased transmural dispersion of repolarization underlies the risk for ventricular arrhythmias in the setting of a prolonged QT interval,8,9 and Tpe is a marker of transmural dispersion. Our finding that Tpe does not shorten with exercise in normals suggests that transmural dispersion remains long even after the QT interval shortens in response to heart rate changes. This could also contribute to the increased risk of sudden death during and after exercise. Viitasalo et al. recently demonstrated abrupt increases in Tpe at elevated heart rates during ambulatory monitoring in patients with congenital LQTS.13 Furthermore, beta-blockers, which are protective in LQTS, prevented these abrupt increases in Tpe in patients with LQTS.30
Limitations
Significant differences in QT, QTp, and Tpe can be observed in different leads,31 but we assessed QT interval only in lead II for this study. The rate of change of QT interval during exercise and recovery differs between highly trained athletes and relatively sedentary individuals.32 Our subjects were healthy volunteers, in sodium balance, free from cardiovascular disease or drugs that affect the QT interval; however, we did not control for baseline level of physical activity. While women are known to have longer QT intervals and greater QT hysteresis than men, we studied both male and female subjects. Our analysis was restricted to intrasubject comparisons, however, minimizing the importance of these gender differences for our study. We used a single staged exercise protocol for all subjects, and QT/RR response differs depending on the exercise protocol.33 Our 3 min per stage protocol is similar to those used in clinical practice. We studied subjects exercising on a recumbent bicycle ergometer to improve the quality of electrocardiographic tracings, but subjects exercising in an upright position may have different QT responses due to posture.34 The QT interval is influenced not only by its antecedent RR interval, but by several preceding RR intervals. Thus, the QT intervals in the first minute of an exercise stage are partly influenced by the slower heart rates preceding the increase in exercise intensity. Finally, due to our relatively small sample size, we may be under-powered to detect small changes in terminal repolarization with exercise.
Conclusion
Exercise is associated with a heart rate-independent shortening of the QT interval, resulting in QT hysteresis within stages of exercise in normal subjects. The predominant portion of the QT interval that shortens with exercise is QTp (onset of QRS to peak of T wave). Terminal repolarization (peak to end of T wave), a surrogate for transmural dispersion of repolarization, does not shorten with exercise, perhaps contributing to the increased risk of sudden death during exercise.
Acknowledgments
Supported in part by grants #RR-00095, RR024975, and HL076264, National Institutes of Health, and HL65962, the Pharmacogenomics of Arrhythmia Therapy site of the Pharmacogenetics Research Network.
References
- 1.Sarma JS, Venkataraman SK, Samant DR, Gadgil U. Hysteresis in the human RR-QT relationship during exercise and recovery. Pacing Clin Electrophysiol. 1987;10:485–491. doi: 10.1111/j.1540-8159.1987.tb04510.x. [DOI] [PubMed] [Google Scholar]
- 2.Krahn AD, Klein GJ, Yee R. Hysteresis of the RT interval with exercise: A new marker for the long-QT syndrome? Circulation. 1997;96:1551–1556. doi: 10.1161/01.cir.96.5.1551. [DOI] [PubMed] [Google Scholar]
- 3.Moss AJ, Zareba W, Hall WJ, Schwartz PJ, Crampton RS, Benhorin J, Vincent GM, Locati EH, Priori SG, Napolitano C, Medina A, Zhang L, Robinson JL, Timothy K, Towbin JA, Andrews ML. Effectiveness and limitations of beta-blocker therapy in congenital long-QT syndrome. Circulation. 2000;101:616–623. doi: 10.1161/01.cir.101.6.616. [DOI] [PubMed] [Google Scholar]
- 4.Krahn AD, Yee R, Chauhan V, Skanes AC, Wang J, Hegele RA, Klein GJ. Beta blockers normalize QT hysteresis in long QT syndrome. Am Heart J. 2002;143:528–534. doi: 10.1067/mhj.2002.120408. [DOI] [PubMed] [Google Scholar]
- 5.Lecocq B, Lecocq V, Jaillon P. Physiologic relation between cardiac cycle and QT duration in healthy volunteers. Am J Cardiol. 1989;64:481–486. doi: 10.1016/0002-9149(89)90425-6. [DOI] [PubMed] [Google Scholar]
- 6.Antzelevitch C, Shimizu W, Yan GX, Sicouri S. Cellular basis for QT dispersion. J Electrocardiol. 1998;30(Suppl):168–175. doi: 10.1016/s0022-0736(98)80070-8. [DOI] [PubMed] [Google Scholar]
- 7.Antzelevitch C. T peak-Tend interval as an index of transmural dispersion of repolarization. Eur J Clin Invest. 2001;31:555–557. doi: 10.1046/j.1365-2362.2001.00849.x. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu W, Antzelevitch C. Sodium channel block with mexiletine is effective in reducing dispersion of repolarization and preventing torsade des pointes in LQT2 and LQT3 models of the long-QT syndrome. Circulation. 1997;96:2038–2047. doi: 10.1161/01.cir.96.6.2038. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu W, Antzelevitch C. Differential effects of beta-adrenergic agonists and antagonists in LQT1, LQT2 and LQT3 models of the long QT syndrome. J Am Coll Cardiol. 2000;35:778–786. doi: 10.1016/s0735-1097(99)00582-3. [DOI] [PubMed] [Google Scholar]
- 10.Xia Y, Liang Y, Kongstad O, Liao Q, Holm M, Olsson B, Yuan S. In vivo validation of the coincidence of the peak and end of the T wave with full repolarization of the epicardium and endocardium in swine. Heart Rhythm. 2005;2:162–169. doi: 10.1016/j.hrthm.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Lubinski A, Lewicka-Nowak E, Kempa M, Baczynska AM, Romanowska I, Swiatecka G. New insight into repolarization abnormalities in patients with congenital long QT syndrome: The increased transmural dispersion of repolarization. Pacing Clin Electrophysiol. 1998;21:172–175. doi: 10.1111/j.1540-8159.1998.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 12.Tanabe Y, Inagaki M, Kurita T, Nagaya N, Taguchi A, Suyama K, Aihara N, Kamakura S, Sunagawa K, Nakamura K, Ohe T, Towbin JA, Priori SG, Shimizu W. Sympathetic stimulation produces a greater increase in both transmural and spatial dispersion of repolarization in LQT1 than LQT2 forms of congenital long QT syndrome. J Am Coll Cardiol. 2001;37:911–919. doi: 10.1016/s0735-1097(00)01200-6. [DOI] [PubMed] [Google Scholar]
- 13.Viitasalo M, Oikarinen L, Swan H, Vaananen H, Glatter K, Laitinen PJ, Kontula K, Barron HV, Toivonen L, Scheinman MM. Ambulatory electrocardiographic evidence of transmural dispersion of repolarization in patients with long-QT syndrome type 1 and 2. Circulation. 2002;106:2473–2478. doi: 10.1161/01.cir.0000036369.16112.7d. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi M, Shimizu M, Ino H, Terai H, Uchiyama K, Oe K, Mabuchi T, Konno T, Kaneda T, Mabuchi H. T-peak to end interval and QT dispersion in acquired long QT syndrome. New index for arrhythmogenicity. Clin Sci. 2003;105:671–676. doi: 10.1042/CS20030010. [DOI] [PubMed] [Google Scholar]
- 15.Darbar D, Hardin B, Harris P, Roden DM. A rate-independent method of assessing QT-RR slope following conversion of atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:636–641. doi: 10.1111/j.1540-8167.2007.00817.x. [DOI] [PubMed] [Google Scholar]
- 16.Chauhan VS, Krahn AD, Walker BD, Klein GJ, Skanes AC, Yee R. Sex differences in QTc interval and QT dispersion: Dynamics during exercise and recovery in healthy subjects. Am Heart J. 2002;144:858–864. doi: 10.1067/mhj.2002.125619. [DOI] [PubMed] [Google Scholar]
- 17.Vainer J, Van Der SB, Smeets JL, Gorgels AP, Sreeram N, Wellens HJ. Beat-to-beat behavior of QT interval during conducted supraventricular rhythm in the normal heart. Pacing Clin Electrophysiol. 1994;17:1469–1476. doi: 10.1111/j.1540-8159.1994.tb01511.x. [DOI] [PubMed] [Google Scholar]
- 18.Lang CC, Flapan AD, Neilson JM. The impact of QT lag compensation on dynamic assessment of ventricular repolarization: Reproducibility and the impact of lead selection. Pacing Clin Electrophysiol. 2001;24:366–373. doi: 10.1046/j.1460-9592.2001.00366.x. [DOI] [PubMed] [Google Scholar]
- 19.Arrowood JA, Kline J, Simpson PM, Quigg RJ, Pippin JJ, Nixon JV, Mohanty PK. Modulation of the QT interval: Effects of graded exercise and reflex cardiovascular stimulation. J Appl Physiol. 1993;75:2217–2223. doi: 10.1152/jappl.1993.75.5.2217. [DOI] [PubMed] [Google Scholar]
- 20.Cuomo S, De Caprio L, Di Palma A, Lirato C, Lombardi L, De Rosa ML, Vetrano A, Rengo F. Influence of autonomic tone on QT interval duration. Cardiologia. 1997;42:1071–1076. [PubMed] [Google Scholar]
- 21.Davey P, Bateman J. Heart rate and catecholamine contribution to QT interval shortening on exercise. Clin Cardiol. 1999;22:513–518. doi: 10.1002/clc.4960220805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magnano AR, Holleran S, Ramakrishnan R, Reiffel JA, Bloomfield DM. Autonomic nervous system influences on QT interval in normal subjects. J Am Coll Cardiol. 2002;39:1820–1826. doi: 10.1016/s0735-1097(02)01852-1. [DOI] [PubMed] [Google Scholar]
- 23.Fananapazir L, Bennett DH, Faragher EB. Contribution of heart rate to QT interval shortening during exercise. Eur Heart J. 1983;4:265–271. doi: 10.1093/oxfordjournals.eurheartj.a061458. [DOI] [PubMed] [Google Scholar]
- 24.Zabel M, Franz MR, Klingenheben T, Mansion B, Schultheiss HP, Hohnloser SH. Rate-dependence of QT dispersion and the QT interval: Comparison of atrial pacing and exercise testing. J Am Coll Cardiol. 2000;36:1654–1658. doi: 10.1016/s0735-1097(00)00921-9. [DOI] [PubMed] [Google Scholar]
- 25.Sundqvist K, Sylven C. Cardiac repolarization properties during standardized exercise test as studied by QT, QT peak and terminated T-wave intervals. Clin Physiol. 1989;9:419–425. doi: 10.1111/j.1475-097x.1989.tb00996.x. [DOI] [PubMed] [Google Scholar]
- 26.Albert CM, Mittleman MA, Chae CU, Lee IM, Hennekens CH, Manson JE. Triggering of sudden death from cardiac causes by vigorous exertion. N Engl J Med. 2000;343:1355–1361. doi: 10.1056/NEJM200011093431902. [DOI] [PubMed] [Google Scholar]
- 27.Mittleman MA, Siscovick DS. Physical exertion as a trigger of myocardial infarction and sudden cardiac death. Cardiol Clin. 1996;14:263–270. doi: 10.1016/s0733-8651(05)70279-4. [DOI] [PubMed] [Google Scholar]
- 28.Schouten EG, Dekker JM, Meppelink P, Kok FJ, Vandenbroucke JP, Pool J. QT interval prolongation predicts cardiovascular mortality in an apparently healthy population. Circulation. 1991;84:1516–1523. doi: 10.1161/01.cir.84.4.1516. [DOI] [PubMed] [Google Scholar]
- 29.de Bruyne MC, Hoes AW, Kors JA, Hofman A, van Bemmel JH, Grobbee DE. Prolonged QT interval predicts cardiac and all-cause mortality in the elderly. The Rotterdam Study. Eur Heart J. 1999;20:278–284. doi: 10.1053/euhj.1998.1276. [DOI] [PubMed] [Google Scholar]
- 30.Viitasalo M, Oikarinen L, Swan H, Vaananen H, Jarvenpaa J, Hietanen H, Karjalainen J, Toivonen L. Effects of beta-blocker therapy on ventricular repolarization documented by 24-h electrocardiography in patients with type 1 long-QT syndrome. J Am Coll Cardiol. 2006;48:747–753. doi: 10.1016/j.jacc.2006.04.084. [DOI] [PubMed] [Google Scholar]
- 31.Merri M. QT Variability. In: Moss AJ, Stern S, editors. Noninvasive Electrocardiology: Clinical Aspects of Holter Monitoring. London: WB Saunders; 1996. [Google Scholar]
- 32.Rajappan K, O’Connell C, Sheridan DJ. Changes in QT interval with exercise in elite male rowers and controls. Int J Cardiol. 2003;87:217–222. doi: 10.1016/s0167-5273(02)00326-1. [DOI] [PubMed] [Google Scholar]
- 33.Chauhan VS, Krahn AD, Mitoff P, Klein GJ, Skanes AC, Yee R. Sudden intense exercise increases QT heart rate slope and T wave complexity in long QT syndrome and normal subjects. Pacing Clin Electrophysiol. 2004;27:1415–1423. doi: 10.1111/j.1540-8159.2004.00647.x. [DOI] [PubMed] [Google Scholar]
- 34.Walker BD, Krahn AD, Klein GJ, Skanes AC, Yee R, Wang J, Hegele RA. Effect of change in posture and exercise on repolarization in patients with long QT syndrome with HERG channel mutations. Can J Cardiol. 2005;21:33–38. [PubMed] [Google Scholar]