Abstract
Ischemic stroke is the most common life-threatening neurological disease and has limited therapeutic options. One component of ischemic neuronal death is inflammation. Here we show that doxycycline and minocycline, which are broad-spectrum antibiotics and have antiinflammatory effects independent of their antimicrobial activity, protect hippocampal neurons against global ischemia in gerbils. Minocycline increased the survival of CA1 pyramidal neurons from 10.5% to 77% when the treatment was started 12 h before ischemia and to 71% when the treatment was started 30 min after ischemia. The survival with corresponding pre- and posttreatment with doxycycline was 57% and 47%, respectively. Minocycline prevented completely the ischemia-induced activation of microglia and the appearance of NADPH-diaphorase reactive cells, but did not affect induction of glial acidic fibrillary protein, a marker of astrogliosis. Minocycline treatment for 4 days resulted in a 70% reduction in mRNA induction of interleukin-1β-converting enzyme, a caspase that is induced in microglia after ischemia. Likewise, expression of inducible nitric oxide synthase mRNA was attenuated by 30% in minocycline-treated animals. Our results suggest that lipid-soluble tetracyclines, doxycycline and minocycline, inhibit inflammation and are neuroprotective against ischemic stroke, even when administered after the insult. Tetracycline derivatives may have a potential use also as antiischemic compounds in humans.
Tetracyclines are bacteriostatic agents with broad-spectrum antimicrobial activity (1). Semisynthetic, the so-called long-acting second-generation tetracyclines, doxycycline and minocycline, are absorbed rapidly and completely even in aged population (2–5) and have a superior tissue penetration into the brain and cerebrospinal fluid (1, 6). During recent years, doxycycline and minocycline have been shown to exert biological effects that are completely separate and distinct from their antimicrobial action. These effects include: inhibition of matrix metalloproteases (7), tumor-induced angiogenesis (8), malignant cell growth (9), and bone resorption (10) and depression of oxygen radical release from polymorphonuclear neutrophils (11, 12), inhibition of inducible nitric oxide synthase (iNOS) [a putative mediator of inflammation (13, 14)], and inhibition of protein tyrosine nitration by scavenging peroxynitrite (15). Experimental and clinical studies indicate that minocycline and doxycycline may be beneficial in treatment of rheumatoid arthritis and potentially in other inflammatory diseases (16–18).
Stroke is the most common life-threatening neurological disease. In westernized countries, stroke is the third leading cause of death after heart disease and cancer, and in the elderly it is a major source of disability leading to institutionalization (19, 20). While pharmacological therapy to reduce ischemic damage is being pursued, prevention and rehabilitation are still the only strategies, albeit relatively inefficient, to reduce the disability and lethality of the disease.
The brain damage produced by cerebral ischemia maturates over a period of several hours or days (21, 22). Especially in global ischemia, a delayed hippocampal damage is observed 3 to 5 days after the insult in CA1 pyramidal neurons (23), suggesting that mechanisms that develop slowly after ischemia have a role in ischemic cell death. Recent studies have shown that inflammatory cells infiltrate the ischemic brain area (24), and several proinflammatory genes or mediators, such as iNOS, cyclooxygenase-2, and cytokines are strongly expressed in the ischemic brain (25, 26). Inflammation is now recognized as a significant contributing mechanism in cerebral ischemia because antiinflammatory compounds or inhibitors of iNOS and cyclooxygenase-2 reduce ischemic damage and improve the outcome of animals after ischemic insult (25, 27, 28).
Because tetracyclines have antiinflammatory properties and are clinically well tolerated, we studied whether doxycycline and minocycline could serve as neuroprotective compounds against brain ischemia. In the present study, we report that in a gerbil model of forebrain ischemia, (i) doxycycline and minocycline, but not tetracycline, are neuroprotective even when the treatment is started 30 min after ischemia; (ii) minocycline prevents microglial activation but does not affect astrogliosis; and (iii) minocycline inhibits induction of interleukin-1β-converting enzyme mRNA (ICE), decreases induction of iNOS mRNA, and prevents nitric oxide synthase (NOS) protein expression.
MATERIALS AND METHODS
Animals and Surgery.
All animal experiments were conducted in accordance with National Institutes of Health and institutional guidelines. Male Mongolian gerbils (Tumblebrook, West Brookfield, MA) weighing 55–65 g were housed in standard temperature (22 ± 1°C) and light-controlled (light on 07:00–21:00) environment with ad libitum access to food and water. The animals were randomly divided into seven groups. Immediately before surgery, each subject was anesthetized with 5% halothane (70% N2O/30% O2). During surgery, halothane was lowered to 1.5–2%. A midline incision was made in the neck and surgical silk was loosely placed around isolated carotid arteries. Anesthesia was disconnected and atraumatic miniature aneurysm clips were attached to occlude both carotid arteries for 6 min. The body temperature was maintained at 36–37°C during the surgery with a heating pad.
Drug Treatment.
Twelve hours before ischemia, group I was injected i.p. with 45 mg/kg (in 0.5 ml) of minocycline hydrochloride (Sigma), group II with the same dose of doxycycline hydrochloride (Sigma), and group III with 50 mg/kg of tetracycline hydrochloride (Sigma). Thereafter, the animals were injected twice a day, at a dose of 90 mg/kg during the first day after ischemia and 45 mg/kg starting 36 h after ischemia. The postischemic treatment was started 30 min after ischemia with 90 mg/kg of minocycline (group IV) or doxycycline (group V); the dose was reduced to 45 mg/kg 36 h after stroke and the animals were injected twice a day until sacrificed. Control groups were treated with saline starting 12 h before (group VI) or 30 min after (group VII) stroke.
Determination of Neuronal Damage.
Six days after ischemia, the animals were perfused with a 4% paraformaldehyde solution. A 5-mm coronal slice at hippocampal level was postfixed by immersion for 24 h, cryoprotected in 20% sucrose, and frozen in liquid nitrogen-cooled isopentane. Coronal sections (10 μm) were cut in a cryostat and mounted on slides, and alternate sections were stained with cresyl violet. The number of surviving neurons was counted by a blinded observer in CA1 pyramidal cell layer from three to four sections per animal at dorsal hippocampal level. Only whole neurons with visible nucleus were counted.
Histochemical Staining.
Ten-μm-thick cryosections or 50-μm-thick vibratome sections were cut from perfusion-fixed brains. Sections were reacted with a mouse monoclonal phosphotyrosine antibody (Upstate Biotechnology, Lake Placid, NY, diluted; 1:400) and a mouse monoclonal glial fibrillary acidic protein (GFAP) antibody (Boehringer Mannheim; 1:400) for 48 h. After being incubated with biotinylated antimouse serum and avidin–biotin complex (Vectastain Elite kit, Vector Laboratories) for 3 h each, the avidin–biotin complex was visualized with 0.05% diaminobenzidine and 0.02% H2O2, and the slides were rinsed and examined in a Leica 3000RB microscope. For lectin staining, the sections were incubated for 120 min with 40 mg/ml of isolectin B4 labeled with peroxidase (Sigma), and the staining was consequently visualized by using 0.05% diaminobenzidine and 0.02% H2O2. For NADPH-diaphorase histochemistry, the sections were rinsed in 0.1 M Tris buffer, pH 8.0, for 30 min and then incubated for at least 2 h in a reaction mixture modified from Scherer-Singler et al. (29) containing 0.2 mM nitro blue tetrazolium/1 mM NADPH dissolved in 0.2 mM sodium carbonate/bicarbonate buffer/0.2% Triton X-100 in 0.1 M Tris buffer, pH 8.0, at 37°C. After being rinsed in ice-cold phosphate buffer, the sections were coverslipped in buffered glycerol.
Northern Blotting.
Total RNA was isolated from frozen gerbil hippocampus by using the TRIzol reagent (GIBCO/BRL) according to the manufacturer’s instructions. An 8-μg sample was run through a formaldehyde/1.2% agarose gel and transferred to a Hybond N (Amersham) nylon membrane by capillary blotting. The 45-mer GFAP oligonucleotide was 3′ end-labeled by using [α-32P]dATP (Amersham) and terminal deoxynucleotidyl transferase (MBI Fermentas, Lithuania) to a specific activity of 4 × 108 cpm/ug. Hybridization was carried out in 5× SSC (standard saline citrate; 1× SSC = 0.15 M sodium chloride/0.015 M sodium citrate, pH 7);/5× Denhardt’s solution (1× Denhardt’s solution = 0.02% polyvinylpyrrolidone/0.02% Ficoll/0.02% BSA/50% formaldehyde)/1% SDS in the presence of 40 × 106 cpm of the probe at 42°C overnight. The membrane was washed twice in 2× SSC/0.1% SDS and once in 0.2× SSC/0.1% SDS at room temperature for 5 min each. The blots were kept wet and exposed to Fuji x-ray film at −80°C until the signal was detected.
Reverse Transcription–PCR (RT-PCR).
Titan One Tube RT-PCR System (Boehringer Mannheim) was applied for the RT-PCR where 1 μg of DNase-treated (RQ1 RNase-free DNase; Promega) total RNA served as a template in each reaction. For iNOS amplification, the specific primers were 5′-TTT GAC CAG AGG ACC CAG AG-3′ and 5′-TTG GTG GCA AAG ATG AGC TC-3′ corresponding to bases 366–385 and 547–566 in the iNOS cDNA (AC#U03699), respectively and known to lie in separate exons at least in the rat iNOS gene. The RT-PCR profile was as follows: reverse transcription at 50°C for 30 min, initial denaturation at 94°C for 2 min, and then altogether 35 cycles composed of denaturation at 94°C for 30 sec, annealing at 55°C for 30 sec, and polymerization at 74°C for 1 min. The reaction was completed by an extended polymerization for 10 min. To detect the extent of ICE mRNA expression, the specific primers were 5′-CCA GAG CAC AAG ACC TCT GAC-3′ and 5′-TGG TGT GGA AGA GCA GAA AGC-3′ recognizing bases 661–681 (in exon 6) and 978–998 (in exon 7), respectively, in the human sequence. Reverse transcription was done at 50°C for 30 min and initial denaturation at 95°C for 2 min. Altogether, 35 cycles consisting of denaturation (95°C, 1 min), annealing (58°C, 1 min), and polymerization (72°C, 2 min) were carried out. The reaction was concluded with polymerization at 72°C for 10 min. As a reference for the iNOS and ICE expressions, RT-PCR of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was also done from each sample. The specific GAPDH primers were 5′-ACC ACA GTC CAT GCC ATC AC-3′ and 5′-TTC ACC ACC CTG TTG CTG TA-3′ (527–546 and 959–978 in AC#J02642, respectively). The same RT-PCR profile as for ICE was used, with the exception that only 25 cycles were done. The RT-PCR reactions were performed in PCT-100 Programmable Thermal Controller (MJ Research, Cambridge, MA), and amplification products were analyzed by running 10-μl samples from each reaction on a 2.5% agarose gel. The PCR products were analyzed by using a Bio-Rad Imaging Densitometer GS-700 and Multi-Analyst 1.02 program.
Data Analysis.
Data are expressed as mean ± standard error. Two group comparisons were evaluated by the paired or unpaired t test, as appropriate. Multiple comparisons were analyzed by ANOVA and Tukey’s test. Differences were considered statistically significant for P < 0.05.
RESULTS
Reduction of Ischemic Damage by Tetracyclines.
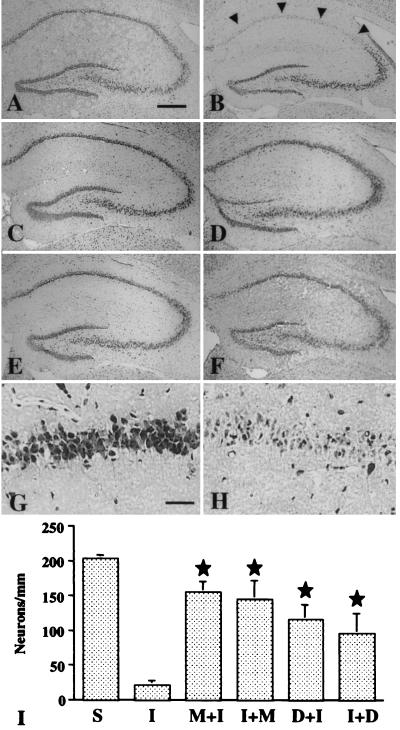
In sham-operated gerbils, the number of neurons in the CA1 pyramidal cell layer was 203.4 ± 5.14/mm (n = 8), whereas 6 days after 6-min global ischemia, the number was decreased to 10.5% (21.4 ± 5.71/mm; n = 6). Both minocycline and doxycycline treatments increased significantly the number of surviving neurons. The minocycline-pretreated gerbils had 76.7% (155.7 ± 15.14; n = 7), minocycline-posttreated gerbils had 71.4% (144.9 ± 27.1: n = 7), doxycycline-pretreated gerbils had 57.2% (116.28+21.4; n = 7), and doxycycline-posttreated gerbils had 47.1% (95.85 ± 28.6; n = 6) of the neuron profiles left in the CA1 pyramidal cell layer. (Fig. 1.) The neuroprotection was statistically significant in every animal group (P < 0.05). Pretreatment with the same dose of tetracycline did not provide any protection (not shown). Minocycline and doxycycline did not reduce the postoperative body temperatures, which were 36.3 ± 0.5 in saline-treated, 35.9 ± 0.3 in minocycline-pretreated, 36.7 ± 0.3 in minocycline-posttreated, 36.6 ± 0.3 in doxycycline-pretreated, and 36.2 ± 0.4 in doxycycline-posttreated gerbils; the differences are not statistically significant.
Figure 1.
Effect of minocycline and doxycycline treatment on the CA1 pyramidal neurons after global brain ischemia. The hippocampus is shown in a cresyl violet-stained section from a sham-operated gerbil (A); and from ischemia-operated gerbils treated with saline (B, H); minocycline, starting 12 h before operation (C, G); minocycline, starting 30 min after operation (D); doxycycline, starting 12 h before operation (E); and doxycycline, starting 30 min after operation (F). A substantial number of the stained pyramidal neurons remain intact in the CA1 pyramidal cell layer in minocycline- and doxycycline-treated gerbils compared to the CA1 pyramidal cells in saline-treated gerbils (arrowheads). Bars = 400 μm (A–F) and 40 μm (G, H). The graph (I) summarizes the quantitative results of CA1 neuronal counts: S, sham-operated; I, saline-treated; M+I/D+I, minocycline/doxycycline treatment started before ischemia; I+M/I+D, minocycline/doxycycline treatment started after ischemia. star, P < 0.05, when compared with saline-treated ischemic (I) gerbils.
Activation of Non-Neuronal Cells.
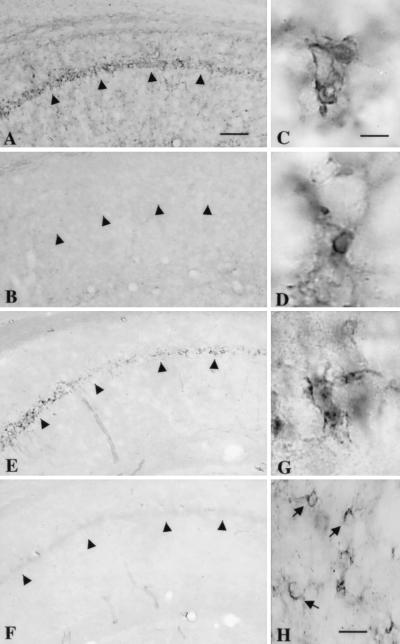
To determine whether neuroprotection by minocycline is associated with activation of nonneuronal cells, we studied GFAP expression, a marker of astrogliosis, and phosphotyrosine immunoreactivity and isolectin B4 binding, which are markers of activated microglia. OX-42 antibody was not used, because the antibody does not react with gerbil antigens. Northern blotting run with the samples from gerbils 7 days after ischemia showed that GFAP expression in the hippocampus was increased to the same extent in saline- and minocycline-treated gerbils (Fig. 2A). The number and distribution of GFAP-immunoreactive astrocytes was similar in saline- and minocycline-treated gerbils after ischemia (Fig. 2 B and C). Instead, while both lectin binding and phosphotyrosine immunostaining were induced in saline-treated ischemic gerbils, no clear lectin binding or phosphotyrosine immunostaining were seen in minocycline-treated ischemic gerbils (Fig. 3 A and B). High-magnification micrographs showed phosphotyrosine and lectin-positive cells in the CA1 subfield of the hippocampus with the most intense staining in the CA1 pyramidal cell layer. The cells were small and occasionally sent out short processes, thus having a typical morphology of activated microglia (Fig. 3 C and D).
Figure 2.
Minocycline treatment does not alter GFAP expression in the hippocampus of gerbils exposed to global ischemia. Northern blotting (A) demonstrates a similar induction of GFAP mRNA in saline-treated (I) and minocycline-pretreated (M+I) gerbils when compared to sham-operated controls (C). Samples from two different animals in each group are shown. A 32P-dATP-labeled cyclophilin oligonucleotide probe (CP) was used to demonstrate equal amounts of loaded RNAs. GFAP immunostaining (B, C) in the CA1 hippocampal field shows approximately the same distribution and number of positively labeled astrocytes (arrows) in saline- (B) and minocycline-pretreated (C) ischemic gerbils. PCL = pyramidal cell layer. Bar = 30 μm.
Figure 3.
Activation of microglial cells (A–D) and induction of NADPH-diaphorase (NOS) reactivity (E–H) in the CA1 hippocampus 6 days after global ischemia are prevented by minocycline pretreatment. Phosphotyrosine immunoreactivity (A–C) and isolectin B4-binding (D) were used as markers of microglial activation. In a saline-treated gerbil, phosphotyrosine immunoreactivity is evident, especially in the CA1 pyramidal cell layer (A), while in a minocycline-treated gerbil (B), no clear staining is seen. High magnification of phosphotyrosine staining (C) and isolectin B4 binding (D) reveals a cell morphology typical of microglia. NADPH-diaphorase reactivity is induced in the CA1 pyramidal cell layer of a saline-treated (E) but not in a minocycline-treated (F) gerbil. The NADPH-diaphorase-stained cells in the pyramidal cell layer send out short processes with an irregular shape, resembling microglia (G). In the stratum radiatum, the NADPH-diaphorase-reactive cells show staining in the cell body and send out thick processes, which is typical of astrocyte morphology (H, arrows). Arrowheads point to the CA1 pyramidal cell layer. Bars = 250 μm (A, B, E, F); 8 μm (C, D, G) and 25 μm (H).
Induction of ICE and iNOS Is Reduced in Minocycline-Treated Ischemic Animals.
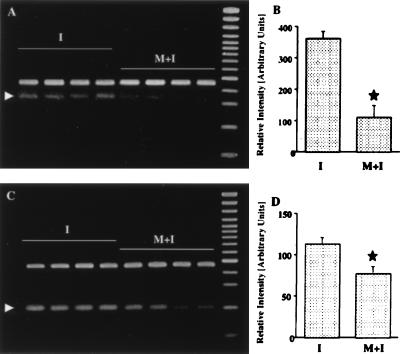
Because microglia induction was blocked by minocycline treatment, we studied whether induction of ICE, an apoptosis-promoting gene that is strongly induced in microglia after global ischemia (30), or iNOS, an enzyme suggested to produce toxic concentration of nitric oxide in nonneuronal cells after ischemia (31), are affected by neuroprotective minocycline treatment. The semiquantitative RT-PCR (n = 4 + 4) showed that 4 days after ischemia, expression of ICE mRNA was attenuated by approximately 70% and expression of iNOS mRNA by 30% in the hippocampus of minocycline-treated gerbils (Fig. 4). In addition, 6 days after ischemia, NADPH-diaphorase-reactive cells were seen in the hippocampi of saline-treated, but not in minocycline-treated, ischemic gerbils (Fig. 3 E and F). Most of the NADPH-diaphorase-reactive cells were located in the pyramidal cell layer of the CA1 subfield and had small cell bodies with short ramified processes (Fig. 3G). Some NADPH-diaphorase-reactive cells with a morphology typical of astrocytes were detected in the stratum radiatum of the CA1 subfield, (Fig. 3H). Therefore, minocycline inhibited NOS activity also in astrocytes, even though it did not block astrogliosis.
Figure 4.
RT-PCR analysis of ICE mRNA (A, B) and iNOS mRNA (C, D) in the hippocampus of saline-treated (I) and minocycline-treated (M+I) gerbils 4 days after ischemia. Arrowheads in A and C point to the amplified ICE and iNOS mRNA products. mRNA isolated from the same tissues was subjected to RT-PCR by using primers for the GAPDH gene. No significant alteration in mRNA expression is observed for this gene (upper bands in A and C). B and D represent quantitation of amplified mRNA products, corrected by corresponding GADPH hybridization signals. (mean ± standard error). Star, statistically significant difference (P < 0.05).
DISCUSSION
The major finding in this study is that treatment with minocycline and doxycycline provide a significant neuroprotection against global brain ischemia even when the treatment is started after the ischemic insult. The finding is in agreement with previous studies showing that doxycycline protects against mesenteric (32) and hepatic (33) ischemia-reperfusion injury. While this study was in progress, Clark et al. (34) reported that doxycycline treatment reduces ischemic brain damage in transient focal brain ischemia when the treatment is started before ischemia. Our results show that minocycline is an even more potent neuroprotective compound than doxycycline and that minocycline blocks activation of microglia, the cells believed to have an active role in brain inflammatory and degenerative processes.
The doses used, 180 mg/kg for the first day after ischemia followed by 90 mg/kg per day, are very high. In mice, doxycycline at the dose of 128 mg/kg per 24 h is well tolerated, and LD50 for minocycline when given intracranially is as high as 38 mg/kg (35). Rats receiving 100–125 mg/kg minocycline per 24 h for 3–4 weeks show no side effects (18, 36, 37), and also dogs have been treated with 55 mg/kg daily for 7 days for eradication of Brucella canis (37). In the present study, the dose of 180/90 mg/kg per day was selected because the treatment did not result in any side effects, no increase in mortality was observed, and the maximal penetration of the drugs to the brain cerebrospinal fluid was desired. We have also found that significantly lower doses of the compounds are neuroprotective in both global and focal brain ischemia (unpublished data). In humans, doxycycline and minocycline are used at a dose of 200 mg per 24 h, and daily tetracycline doses even higher than 2 g are still relatively safe in healthy nonpregnant people (1). Minocycline is not routinely used in human infections because of its potential vestibular toxicity (1). However, minocycline has been shown recently to be beneficial in some cases of rheumatoid arthritis, and its clinical use in this disease has been suggested (16–18). It is conceivable that in severe life-threatening diseases such as stroke, inconvenient side effects of treatment are acceptable.
Minocycline proved to be a more potent neuroprotective compound than doxycycline in a gerbil model of global brain ischemia. Both minocycline and doxycycline are lipid soluble and penetrate tissues such as the brain more efficiently than other tetracyclines (1, 5). Minocycline is still twice as lipid soluble as doxycycline (5), which may partially explain the better neuroprotection observed by minocycline. Doxycycline is more potent than minocycline in inhibiting NOS in human osteoarthritis-affected cartilage in vitro, whereas minocycline is a more potent iNOS inhibitor than doxycycline in stimulated murine macrophages (13). We found that minocycline decreased induction of iNOS mRNA by 30% and inhibited completely NOS protein production, as judged by NADPH-diaphorase activity. It is possible that minocycline and doxycycline differ in affinity to several enzymes or cell structures responsible for neuronal damage in ischemia. Because iNOS is implicated in brain ischemia (25, 27, 31), more complete inhibition of the enzyme by minocycline than by doxycycline could contribute to the difference seen in their neuroprotective capacity.
Minocycline and doxycycline prevented microglial activation. In response to brain injury, including brain ischemia, both astroglia (38) and microglia (39) are activated. To a certain extent, glial activation is triggered secondarily to injury, but the triggering mechanism does not need to be lethal since minor alterations in ionic homeostasis or strong depolarization can lead to micro- and astrogliosis (38). In the present study, astrogliosis, as demonstrated with GFAP mRNA and protein expression, was similar in treated and nontreated ischemic hippocampi, indicating that minocycline did not protect the brain from injurious challenge. Prevention of microglial activation but not astrogliosis shows that astrogliosis does not indicate neuronal cell death and that activation of astroglial and microglial cells in response to ischemic damage are mediated through different pathways. This finding is in agreement with a previous study showing distinct regulation of microglial and astroglial activation after spreading depression (40). In fact, the finding that minocycline is capable of blocking NOS (NADPH-diaphorase) activity in astrocytes without affecting astrogliosis also indicates that astrogliosis is not necessarily, if at all, associated with activation of proinflammatory pathways in these cells. Even though both astrocytes and microglia may be capable of producing pro- and antiinflammatory cytokines and neurotoxins, such as nitric oxide (31), activated microglia are generally considered as neuronal damage-promoting cells and activated astrocytes as neuronal survival-supporting cells (41). This idea of the role of different glial cells is a simplification, but the specific correlation of microglial activation with neuronal death supports the hypothesis that activated microglia, but not necessarily astrogliosis, may contribute to ischemic neuronal death.
Minocycline treatment reduced the induction of ICE (caspase-1) to 30% when estimated by a semiquantitative RT-PCR 4 days after ischemia. ICE is a member of the caspase-family proteases and cleaves pro-interleukin-1β (pro-IL-1β), an inactive precursor, to the mature form of IL-1β, which is a proinflammatory cytokine (42, 43). After global ischemia in gerbils, ICE mRNA and protein have been reported to be strongly expressed in microglia (30). This result is in line with our findings that both microglial activation and ICE induction are inhibited by minocycline. Mature IL-1β is secreted by activated microglia and participates in leukocyte adhesion and edema formation by disrupting the blood–brain barrier and recruiting neutrophils (44–46). Previous studies have shown that inhibitors of ICE-like proteases (47) and IL-1 receptor antagonists (48) are strongly neuroprotective in brain ischemia when administered intracerebroventricularly, suggesting that inhibition of ICE induction contributes to neuroprotection by minocycline treatment.
Induction of iNOS mRNA, protein, and enzyme activity is well documented in focal and global brain ischemia (25). Inhibitors of iNOS protect against focal brain ischemia, and mice deficient in iNOS have smaller infarcts than wild-type mice, indicating that iNOS is an important mediator in focal ischemic injury (25, 27). In global ischemia, iNOS induction is detected in nonneuronal cells, especially in astrocytes (31), and nonspecific NOS inhibitors provide protection against global ischemia (25). iNOS is thought to contribute to neuronal damage in global ischemia, even though contradictory results have been reported (25). In agreement with previous studies, we detected induction of iNOS mRNA and NADPH-diaphorase reactivity in astrocytes and microglia-like cells. Minocycline treatment reduced iNOS mRNA induction by 30% and blocked completely the induction of NADPH-diaphorase reactivity in the hippocampus, suggesting that minocycline inhibits iNOS at levels of transcription and translation. Because nonneuronal cells expressing iNOS produce large quantities of NO with neurotoxic consequences, it is likely that the inhibitory effect of minocycline is a mechanism reducing ischemic damage in global-brain ischemia.
These data suggest that doxycycline and minocycline, the second-generation tetracycline derivatives, but not tetracycline itself, provide neuroprotection against brain ischemia. Because tetracyclines have long been applied clinically, the compounds might provide a novel approach to stroke protection in humans. Further studies in other ischemia models are necessary to determine the optimal dose and administration of these compounds in brain ischemia.
Acknowledgments
The study was supported by Bioabsorbable Concepts, Ltd., Finland.
ABBREVIATIONS
- GFAP
glial fibrillary acidic protein
- ICE
interleukin-1β converting enzyme
- NOS
nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- IL-1β
interleukin-1β
- RT-PCR
reverse transcription–PCR
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
References
- 1.Klein N C, Cunha B A. Med Clin N Am. 1995;79:789–801. doi: 10.1016/s0025-7125(16)30039-6. [DOI] [PubMed] [Google Scholar]
- 2.Cunha B A, Sibley C, Ristuccia P A. Ther Drug Monit. 1982;4:115–130. doi: 10.1097/00007691-198206000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Sande M A, Mandell G L. In: The Pharmacological Basis of Therapeutics. Goodman L S, Gilman A, editors. New York: Macmillan; 1985. pp. 1170–1192. [Google Scholar]
- 4.Kramer P A, Chapron D J, Benson J, Mercik S A. Clin Pharmacol Ther. 1978;23:467–472. doi: 10.1002/cpt1978234467. [DOI] [PubMed] [Google Scholar]
- 5.Barza M, Brown R B, Shanks C, Gamble C, Weinstein L. Antimicrob Agents Chemother. 1975;8:713–720. doi: 10.1128/aac.8.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aronson A L. J Am Vet Med Assoc. 1980;176:1061–1068. [PubMed] [Google Scholar]
- 7.Golub L M, Ramamurthy N S, McNamara T F. Crit Rev Oral Biol Med. 1991;2:297–322. doi: 10.1177/10454411910020030201. [DOI] [PubMed] [Google Scholar]
- 8.Maragoudakis M E, Peristeris P, Missirls P, Aletras A, Anriopoulou P, Haralapoulos G. Ann N Y Acad Sci. 1994;732:280–293. doi: 10.1111/j.1749-6632.1994.tb24743.x. [DOI] [PubMed] [Google Scholar]
- 9.Masumori N, Tsukamoto T, Miyao N, Mumamoto Y, Saiki I, Yoneda J. J Urol. 1994;151:1400–1404. doi: 10.1016/s0022-5347(17)35268-0. [DOI] [PubMed] [Google Scholar]
- 10.Rifkin B R, Vernillo A T, Golub L M, Ramamurthy N S. Ann N Y Acad Sci. 1994;732:165–180. doi: 10.1111/j.1749-6632.1994.tb24733.x. [DOI] [PubMed] [Google Scholar]
- 11.Gabler W, Creamer H. J Periodontal Res. 1991;26:52–58. doi: 10.1111/j.1600-0765.1991.tb01626.x. [DOI] [PubMed] [Google Scholar]
- 12.Gabler W, Smith J, Tsukuda N. Res Commun Chem Pathol Pharmacol. 1992;8:151–160. [PubMed] [Google Scholar]
- 13.Amin A R, Attur M G, Thakker G D, Patel P D, Vyas P R, Patel R N, Patel I R, Abramson S B. Proc Natl Acad Sci USA. 1996;93:14014–14019. doi: 10.1073/pnas.93.24.14014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amin A R, Patel R N, Thakker G D, Lowenstein C J, Attur M G, Abramson S B. FEBS Lett. 1997;410:259–264. doi: 10.1016/s0014-5793(97)00605-4. [DOI] [PubMed] [Google Scholar]
- 15.Whiteman M, Halliwell B. Free Radical Res. 1997;26:49–56. doi: 10.3109/10715769709097783. [DOI] [PubMed] [Google Scholar]
- 16.Furst D E. Curr Opin Rheumatol. 1998;10:123–128. doi: 10.1097/00002281-199803000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Nordstrom D, Lindy O, Lauhio A, Sorsa T, Santavirta S, Konttinen Y T. Rheumatol Int. 1998;17:175–180. doi: 10.1007/s002960050030. [DOI] [PubMed] [Google Scholar]
- 18.Greenwald R A, Moak S A, Ramamurthy N S, Golub L M. J Rheumatol. 1992;19:927–938. [PubMed] [Google Scholar]
- 19.Wolf P A, Cobb J L, D’Agostino R B. In: Stroke, Pathophysiology, Diagnosis and Management. Barnett H J M, Mohr J P, Stein B M, Yatsu F M, editors. New York: Churchill Livingstone; 1997. pp. 3–27. [Google Scholar]
- 20.Centers for Disease Control and Prevention, Division of Chronic Disease Control and Community. Cardiovascular Disease Surveillance: Stroke 1980–1989. Atlanta: Centers Dis. Control; 1994. [Google Scholar]
- 21.Dereski M O, Chopp M, Knight R A, Rodolosi L C, Garcia J H. Acta Neuropathol. 1993;85:327–333. doi: 10.1007/BF00227730. [DOI] [PubMed] [Google Scholar]
- 22.Marshall R S, Mohr J P. J Neurol Neurosurg Psychiatry. 1993;56:6–16. doi: 10.1136/jnnp.56.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siesjö B K. Brain Energy Metabolism. New York: Wiley; 1978. [Google Scholar]
- 24.Feuerstein G Z, Wang X, Barone F C. In: Cerebrovascular Diseases. Ginsberg M D, Bogousslavsky J, editors. Oxford: Blackwell Scientific; 1997. pp. 856–862. [Google Scholar]
- 25.Iadecola C. Trends Neurosci. 1997;20:132–139. doi: 10.1016/s0166-2236(96)10074-6. [DOI] [PubMed] [Google Scholar]
- 26.Koistinaho J, Hökfelt T. NeuroReport. 1997;8:i–viii. [PubMed] [Google Scholar]
- 27.Iadecola C, Zhang F M, Casey R, Nagayama M, Ross M E. J Neurosci. 1997;17:9157–9164. doi: 10.1523/JNEUROSCI.17-23-09157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nogawa S, Zhang F, Ross M E, Iadecola C. J Neurosci. 1997;17:2746–2755. doi: 10.1523/JNEUROSCI.17-08-02746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scherer-Singler U, Vincent S R, Kimura H, McGeer E G. J Neurosci Methods. 1983;9:229–234. doi: 10.1016/0165-0270(83)90085-7. [DOI] [PubMed] [Google Scholar]
- 30.Bhat R V, DiRocco R, Marcy V R, Flood D G, Zhu Y, Dobrzanski P, Siman R, Scott R, Contreras P C, Miller M. J Neurosci. 1996;16:4146–4154. doi: 10.1523/JNEUROSCI.16-13-04146.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Endoh M, Maiese K, Wagner J. Brain Res. 1994;651:92–100. doi: 10.1016/0006-8993(94)90683-1. [DOI] [PubMed] [Google Scholar]
- 32.Smith J R, Gabler W L. Res Commun Mol Pathol Pharmacol. 1995;88:303–315. [PubMed] [Google Scholar]
- 33.Smith J R, Gabler W L. Inflammation. 1994;18:193–201. doi: 10.1007/BF01534560. [DOI] [PubMed] [Google Scholar]
- 34.Clark W M, Lessov N, Lauten J D, Hazel K. J Mol Neurosci. 1997;9:103–108. doi: 10.1007/BF02736854. [DOI] [PubMed] [Google Scholar]
- 35.Tsai Y H, Hirth R S, Leitner F. Exp Chemother. 1980;26:196–206. doi: 10.1159/000237905. [DOI] [PubMed] [Google Scholar]
- 36.Noble J F, Kanegis L A, Hallesy D W. Toxicol Appl Pharmacol. 1967;11:128–149. doi: 10.1016/0041-008x(67)90034-8. [DOI] [PubMed] [Google Scholar]
- 37.Breedveld F C, Trentham D E. Arthritis Rheum. 1988;31:R3. [Google Scholar]
- 38.Lascola C D, Kraig R P. In: Primer on Cerebrovascular Diseases. Welch K M A, Caplan L R, Reis D J, Siesjö B K, Weir B, editors. New York: Academic; 1997. pp. 114–116. [Google Scholar]
- 39.Giulian D. In: Primer on Cerebrovascular Diseases. Welch K M A, Caplan L R, Reis D J, Siesjö B K, Weir B, editors. New York: Academic; 1997. pp. 117–123. [Google Scholar]
- 40.Caggiano A O, Kraig R P. J Comp Neurol. 1996;369:93–108. doi: 10.1002/(SICI)1096-9861(19960520)369:1<93::AID-CNE7>3.0.CO;2-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minghetti L, Levi G. Prog Neurobiol. 1998;54:99–125. doi: 10.1016/s0301-0082(97)00052-x. [DOI] [PubMed] [Google Scholar]
- 42.Cerretti D P, Kozlosky C J, Mosley B, Nelson N, Ness K V, Greenstreet T A, March C J, Kronheim S R, Druck T, Cannizzarro L A, et al. Science. 1992;256:97–100. doi: 10.1126/science.1373520. [DOI] [PubMed] [Google Scholar]
- 43.Thornberry N A, Bulll H G, Calaycay J R, Chapman K T, Howard A D, Kostura M J, Miller D K, Molineaux S M, Weidner J R, Aunins J, et al. Nature (London) 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 44.Dinarello C A. Blood. 1991;77:1627–1645. [PubMed] [Google Scholar]
- 45.Plantais L C, Vogelzang N J. Am J Med. 1990;89:621–629. doi: 10.1016/0002-9343(90)90181-c. [DOI] [PubMed] [Google Scholar]
- 46.Qualiarello V J, Wispelway B, Long W J, Scheld W M. J Clin Invest. 1991;87:1360–1366. doi: 10.1172/JCI115140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hara H, Friedlander R M, Gagliardini V, Auata C, Fink K, Huang Z, Shimizu-Sasamata M, Yuan J, Moskowitz M A. Proc Natl Acad Sci USA. 1997;94:2007–2012. doi: 10.1073/pnas.94.5.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Realton J K, Rothwell N J. Brain Res Bull. 1992;29:243–246. doi: 10.1016/0361-9230(92)90033-t. [DOI] [PubMed] [Google Scholar]