Abstract
Laryngeal edema is a frequent complication of intubation. It often presents shortly after extubation as post-extubation stridor and results from damage to the mucosa of the larynx. Mucosal damage is caused by pressure and ischemia resulting in an inflammatory response. Laryngeal edema may compromise the airway necessitating reintubation. Several studies show that a positive cuff leak test combined with the presence of risk factors can identify patients with increased risk for laryngeal edema. Meta-analyses show that pre-emptive administration of a multiple-dose regimen of glucocorticosteroids can reduce the incidence of laryngeal edema and subsequent reintubation. If post-extubation edema occurs this may necessitate medical intervention. Parenteral administration of corticosteroids, epinephrine nebulization and inhalation of a helium/oxygen mixture are potentially effective, although this has not been confirmed by randomized controlled trials. The use of non-invasive positive pressure ventilation is not indicated since this will delay reintubation. Reintubation should be considered early after onset of laryngeal edema to adequately secure an airway. Reintubation leads to increased cost, morbidity and mortality.
Introduction
Laryngeal edema is a common cause of airway obstruction after extubation in intensive care patients and is thought to arise from direct mechanical trauma to the larynx by the endotracheal tube [1,2]. The severity of airway obstruction due to laryngeal edema varies. In more severe cases, the edema can lead to acute respiratory compromise necessitating emergency reintubation [2-8]. Reintubation itself is associated with increased mechanical ventilation days and length of stay in the intensive care unit, higher costs, morbidity and mortality [5,9-12]. These associations, however, do not all apply to reintubation due to laryngeal edema.
The increased attention for post-extubation laryngeal edema is reflected by several recent studies [3,4,6-8,13-16]. Some of these studies have attempted to identify risk factors, whereas others studied the potential preventive effect of corticosteroids on the development of clinically relevant laryngeal edema [3,4,6,7,13].
The present review will provide an overview of the etiology, incidence, risk factors, prevention and treatment of post-extubation laryngeal edema and extubation failure in adult critically ill patients admitted to the intensive care unit. Since the incidence, pathophysiological mechanisms, consequences and management of laryngeal edema in children differ considerably from those in adults, discussing laryngeal edema in children is beyond the scope of this article.
Etiology and pathogenesis
Endotracheal intubation can cause damage to the oropharynx, larynx and trachea [2,17-21]. Laryngeal edema and mucosal ulcerations occur in almost all patients intubated for 4 days or more [2,19,20]. Vocal cord ulceration and granulation tissue are also found in most cases, usually located posterior to the level of the vocal cords, where the tube exerts the highest pressure [2,18,21,22]. These injuries are usually reversible, with most of the lesions resolving within 1 month [2,17,21]. Pressure and ischemia are thought to contribute to mucosal edema, which may subsequently progress and present as inspiratory stridor within hours of extubation (Figure 1) [18,22,23]. Quantitative data on lumen narrowing pertaining to laryngeal edema and respiratory distress are not available: however, respiratory distress develops in those patients with >50% narrowing of the tracheal lumen [24].
Figure 1.
Laryngeal edema. Courtesy of L. Baijens.
Incidence
Although laryngeal edema occurs in nearly all intubated patients, only some of them develop clinical symptoms. Laryngeal edema is therefore usually transient and self-limiting. Clinical signs associated with laryngeal edema develop rapidly following extubation [25]. About 15% of all reintubations are performed because of post-extubation laryngeal edema [1]. In a study by Francois and colleagues, 87 out of 611 patients (12%) developed laryngeal edema requiring reintubation. From these 87 cases, 70 (80%) patients developed symptoms within 30 minutes after extubation, whilst almost one-half (47%) of the patients developed symptoms within 5 minutes [6].
Post-extubation stridor (PES) is accepted as a clinical marker of laryngeal edema following extubation [1-3,6-9,14,25-30]. Stridor is commonly defined as a high-pitched sound produced by airflow through a narrowed airway. The ease of clinically detecting PES, without the need for further diagnostic techniques, makes PES a widely used outcome measure for post-extubation laryngeal edema.
The incidence of PES varies. Reported incidences vary from 3.5 to 30.2% (see Table 1) [2,3,7,8,14,25-30]. Differences in definition complicate a comparison of studies, especially since the outcome measures used (laryngeal injury, laryngeal edema and PES) partially overlap. In the randomized controlled trial of Francois and colleagues, 46% of the patients with PES or visualized laryngeal edema in whom no medical intervention was performed did not require reintubation. The occurrence of stridor is therefore not a very sensitive marker for clinically relevant laryngeal edema requiring reintubation [6].
Table 1.
Incidence of post-extubation stridor and laryngeal edema
| Study | Year | Extubations or participants (n) | Cases (n) | % | Definition |
|---|---|---|---|---|---|
| Post-extubation stridor | |||||
| Epstein and colleagues [1] | 1998 | 74 | 11 | 15 | Stridor with resolution upon reintubation |
| Maury and colleagues [25] | 2004 | 115 | 4 | 3.5 | High-pitched inspiratory wheeze within 24 hours of extubation with respiratory rate >30/minute |
| Sandhu and colleagues [26] | 2000 | 110 | 13 | 11.8 | High-pitched inspiratory wheeze requiring medical intervention |
| Miller and Cole [27] | 1996 | 100 | 6 | 6 | High-pitched inspiratory wheeze requiring medical intervention |
| Kriner and colleagues [8] | 2005 | 462 | 20 | 4.3 | Inspiratory grunting, whistling or wheezing requiring medical intervention within 24 hours after extubation |
| Ding and colleagues [28] | 2006 | 51 | 4 | 7.8 | High-pitched inspiratory wheeze associated with respiratory distress |
| Colice and colleagues [2] | 1989 | 82 | 5 | 6 | Post-extubation stridor or hoarseness |
| Ho and colleagues [7] | 1996 | 38a | 10 | 26 | Crowing sound on inspiration |
| Jaber and colleagues [30] | 2003 | 112 | 13 | 12 | High-pitched inspiratory wheeze requiring medical intervention |
| Cheng and colleagues [3] | 2006 | 43 | 13 | 30.2 | High-pitched inspiratory wheeze requiring medical intervention (in control group of intervention arm with positive cuff leak test) |
| de Bast and colleagues [36] | 2002 | 76 | 10 | 13 | Inspiratory wheezing |
| Lee and colleagues [13] | 2007 | 40 | 11 | 27.5 | Stridor heard with stethoscope |
| Laryngeal edema | |||||
| Francois and colleagues [6] | 2007 | 343a | 76 | 22 | Stridor with respiratory distress with need for medical intervention (minor) or severe respiratory distress needing reintubation <24 hours after extubation (major) |
| Darmon and colleagues [4] | 1992 | 663 | 28 | 4.2 | Laryngeal dyspnea and/or stridor (minor laryngeal edema) or the need for reintubation due to laryngeal edema as confirmed by endoscopy (major laryngeal edema) |
| de Bast and colleagues [36] | 2002 | 76 | 8 | 11 | Stridor with respiratory distress requiring reintubation within 24 hours, confirmed by fiberoptic examination or direct view |
| Chung and colleagues [55] | 2006 | 95 | 35 | 36.8 | Near total occlusion of the airway as seen on video bronchoscopy |
aPlacebo group.
Complications
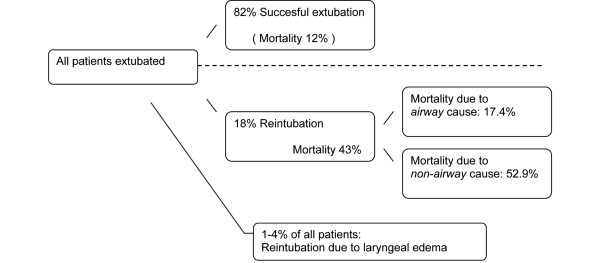
The main complication of post-extubation laryngeal edema is reintubation. The incidence of extubation failure, however, varies widely - incidences up to 18% are reported [5,10,27]. Extubation failure is often defined as reintubation within a certain time after extubation. The need for reintubation may also result from other causes, however, such as pulmonary failure, heart failure, aspiration or abundant secretions [1]. Several studies found reintubation rates of 1 to 4% specifically due to post-extubation laryngeal edema in general intensive care unit populations [4-8]. Reintubation rates among patients with PES are higher than in the general post-extubation population, varying from 18 to 69% [4,6,8,14,25,27,30].
Reintubation in general is associated with mortality [5,9-12]. A prospective study found mortality rates of 43% in re-intubated patients, compared with a mortality of 12% in the total study group (n = 17/40 vs. n = 29/247, P < 0.00001) [10]. Morbidity is also increased due to an increased duration of mechanical ventilation and an increased length of both intensive care unit stay and hospital stay increasing the risk for infections and other complications [10,12,31]. This unfavorable outcome is reflected in the number of patients transferred to a long-term care facility (38% vs. 21%, P < 0.0001) [10]. The cause of extubation failure, however, has also been shown to be independently related to mortality [1]. Airway obstruction as a cause for reintubation is associated with significantly lower mortality rates compared with nonairway reintubation causes (17.4% vs. 52.9%, P < 0.01) (Figure 2) [1].
Figure 2.
Incidence of reintubation and mortality.
Risk factors
Early identification of patients with an increased risk of developing laryngeal edema evolving into respiratory failure would be useful. This early identification would facilitate prevention and/or early treatment. Early recognition is crucial, since delay to reintubation is a predictor of hospital mortality [1].
Several studies have identified risk factors for laryngeal injury, the development of laryngeal edema or PES and the associated need for reintubation (Table 2). In general, few studies identified risk factors using multivariate analysis or correction for confounding factors [4,15,19]. Female gender is a risk factor for both laryngeal edema and PES [3,4,6-8,14,25]. This predisposition has been hypothesized to be due to the female mucous membrane being less resistant to trauma and thinner than that in men [4,32,33]. A relatively large tube to trachea ratio in women may also facilitate mucosal injury [4,7,21,33,34], although this is not universally reported to be gender dependent [6,8].
Table 2.
Risk factors for extubation complications
| Outcome measure | Study | Year | Risk factors |
|---|---|---|---|
| Laryngeal injury | Colice and colleagues [2] | 1989 | Persistent laryngeal neuromotor activity, tracheostomy |
| Kastanos and colleagues [17] | 1983 | Severe respiratory failure, high cuff pressure, duration of endotracheal intubation, secretion infection | |
| Esteller and colleagues [19] | 2005 | Longer duration of intubation, tracheostomy, number of days in the intensive care unit | |
| Laryngeal edema | Darmon and colleagues [4] | 1992 | Duration of intubation (>36 hours), gender (female) |
| Francois and colleagues [6] | 2007 | Trauma at admission, gender (female), short duration of intubation (<7 days), smaller height to tube diameter ratio, absence of methylprednisolone pre treatment | |
| Post-extubation stridor | Cheng and colleagues [3] | 2006 | Gender (female), lower Glasgow coma score, nonsedation treatment |
| Sandhu and colleagues [26] | 2000 | Duration of intubation (>3 days) | |
| Daley and colleagues [9] | 1996 | Tracheostomy, time to reintubation | |
| Ho and colleagues [7] | 1996 | Gender (female) | |
| Jaber and colleagues [30] | 2003 | High SAPS II, medical patients, difficult intubation, history of self-extubation, prolonged intubation, high cuff pressure | |
| Kriner and colleagues [8] | 2005 | Gender (female), duration of intubation (>6 days), ratio tube size to laryngeal size >45% | |
| Wang and colleagues [14] | 2007 | Gender (female) | |
| Maury and colleagues [25] | 2004 | Gender (female) | |
| Erginel and colleagues [15] | 2005 | Duration of ventilation (>5 days), body mass index (>26.5) | |
| Reintubation | Daley and colleagues [9] | 1996 | Tracheostomy, post-extubation stridor |
| Jaber and colleagues [30] | 2003 | Post-extubation stridor | |
| Epstein and colleagues [1] | 1997 | APACHE II score, age, cardiopulmonary cause for reintubation | |
| Sandhu and colleagues [26] | 2000 | Duration of previous intubation (>3 days) |
APACHE, Acute Physiology and Chronic Health Evaluation; SAPS, Simplified Acute Physiology Score.
Controversy remains in the literature about the duration of intubation and the subsequent risk of developing complications. For example, the duration of intubation is identified as a risk factor for laryngeal injury by Kastanos and colleagues and by Esteller and colleagues [17,19], while Colice and colleagues and Stauffer and colleagues failed to show this relation [2,35].
Diagnosis
Early recognition of laryngeal edema is essential since these patients have the highest risk of evolving to respiratory distress and extubation failure. Even before extubation, signs indicative of laryngeal edema may be present.
The search for a test that adequately identifies patients at risk for extubation failure is ongoing. Recently, the cuff leak test has gained interest. The test is non-invasive, relatively easy to perform and is thought to give an indication of the patency of the upper airway. When the ventilated patient is allowed to exhale with a deflated cuff, expired air normally escapes from the otherwise closed circuit. The volume of leaked air can be measured by spirometry functions of the ventilator. In a case of significant laryngeal edema, the lumen of the larynx is narrowed - this results in a smaller measured air leak, and the cuff leak test will then be classified as positive (Table 3).
Table 3.
Measurement of the cuff leak volume in mechanically ventilated patients
| Before performing the cuff leak test, first suction endotracheal and oral secretions and set the ventilator in the assist control mode. |
| With the cuff inflated, record displayed inspiratory and expiratory tidal volumes to see whether these are similar. |
| Deflate the cuff. |
| Directly record the expiratory tidal volume over the next six breathing cycles as the expiratory tidal volume will reach a plateau value after a few cycles. |
| Average the three lowest values. |
| The difference between the inspiratory tidal volume (measured before the cuff was deflated) and the averaged expiratory tidal volume is the cuff leak volume. |
Edited from Miller and Cole [27].
Miller and Cole made the first attempts to make the cuff leak test quantitative, by measuring the amount of air leak and correlating the cuff leak volume to the likelihood of developing laryngeal edema and PES. They calculated the cut-off value with the highest sensitivity and specificity [27]. Almost none of the patients with cuff leak volume >110 ml developed PES: the specificity of this cut-off value was 99% and the negative predictive value for absence of PES was 98%. If a leak <110 ml was present, only two-thirds of patients developed PES - making the sensitivity 67% [27].
Different cut-off values have been reported in the literature, but all nevertheless result in high specificity and negative predicting values (Table 4). The results show that the quantitative cuff leak test is an indicator of risk for the development of PES and reintubation, rather than an instrument to preclude the extubation attempt [36,37].
Table 4.
Predictive value of the cuff leak test
| Cuff leak cut off | ||||||||
|---|---|---|---|---|---|---|---|---|
| Author | Year | Volume (ml) | Percentage of tidal volumea | Outcome | Sensitivity | Specificity | PPV | NPV |
| Miller and Cole [27] | 1996 | 110 | PES | 0.67 | 0.99 | 0.80 | 0.98 | |
| Jaber and colleagues[30] | 2003 | 130 | 12 | PES | 0.85 | 0.95 | 0.69 | 0.98 |
| de Bast and colleagues [36] | 2002 | 15.5 | Reintubation | 0.75 | 0.72 | 0.25 | 0.96 | |
| Sandhu and colleagues [26] | 2000 | 10.0 | PES or reintubation | 0.54 | 0.96 | 0.64 | 0.94 | |
| Wang and colleagues [14] | 2007 | 88 | PES | 0.55 | 0.91 | |||
| Maury and colleagues [25] | 2004 | 0 | PES | 1.00 | 0.80 | 0.15 | 1.00 | |
| Chung and colleagues [55] | 2006 | 140 | Laryngeal edema | 0.89 | 0.90 | 0.84 | 0.93 | |
| Engoren [56] | 1999 | 110 | PES | 0.00 | 0.96 | 0.00 | 0.99 | |
| Kriner and colleagues [8] | 2005 | 110 | PES | 0.50 | 0.84 | 0.12 | 0.97 | |
| Cheng and colleagues [3] | 2006 | 18.0 | PES | 0.85 | 0.72 | 0.21 | 0.98 | |
NPV, negative predictive value; PPV, positive predictive value; PES, post-extubation stridor. aCuff leak volume as a percentage of inspiratory or expiratory tidal volume.
Close observation is necessary after extubation, especially in the first hour after extubation. The diagnosis of significant laryngeal edema relies on symptoms. Direct laryngoscopy cannot be performed before extubation, due to the fact that the endotracheal tube blocks sight of the larynx. In addition, laryngoscopy can cause unnecessary complications in patients with respiratory failure if not performed for the purpose of securing the airway [4,6,7]. Naso-endoscopy can be performed to inspect the larynx before and after extubation. Based on the clinical aspect of lesions identified, the severity and expected morbidity can be estimated in experienced hands [38]. The severity of initial laryngeal pathology, however, is not an accurate predictor for the severity of adverse effects. Although every patient with symptoms has some degree of injury, not all injuries present clinically [2,17]. Laryngeal edema therefore remains largely a clinical diagnosis.
Although post-extubation laryngeal edema is described as the development of airway obstruction after extubation, neither a widely accepted definition for laryngeal edema nor a frequently used classification of severity is currently available. In the authors' opinion, the definition and classification by Darmon and colleagues and Francois and colleagues - who use the terms minor and major to classify laryngeal edema - is nevertheless useful (Table 5) [4,6].
Table 5.
Definition for minor and major laryngeal edema
| Minor laryngeal edema: | the presence of stridor (defined as an audible high-pitched inspiratory wheeze) and signs of respiratory distress. Signs of respiratory distress are a prolonged inspiratory phase and recruitment of accessory respiratory muscles as seen by subcostal, suprasternal or intercostal retraction. |
| Major laryngeal edema: | respiratory distress needing tracheal intubation secondary to upper airway obstruction confirmed by direct or video laryngoscopy. |
Prevention
Prevention of laryngeal edema, and thereby decreasing the incidence of extubation failure, is obviously desirable. The strategies for laryngeal edema prevention will now be discussed.
First, considering tube size as a risk factor, intubation with a 7 or 7.5 mm tube in males and a 6.5 mm tube in females would be desirable. A reduced endotracheal tube diameter, however, may delay weaning, potentially interfere with bronchoscopic procedures and will increase ventilatory resistance, making the use of smaller tube sizes not feasible.
Also, several studies have reported the effect of corticosteroids in preventing post-extubation laryngeal edema. Early animal studies showed that administration of steroids reduces laryngeal edema and can prevent post-extubation laryngeal edema [39,40]. Corticosteroid administration before extubation is part of the extubation protocol in some centers [4,7,41]. Steroid use for 24 hours is considered safe, and no major adverse effects related to its use have been reported in a number of studies [3,6,13,16,32,42,43].
Results of recent randomized controlled trials in humans, however, have been contradictory. These differences may be explained by use of different types of steroids and different administration regimens. Moreover, some protocols only use steroids in patients with a positive cuff leak test, thereby attempting to select patients at high risk for the development of laryngeal edema.
Three studies, including two well-described randomized controlled trials, used a single-dose regimen with steroids being administered within 1 hour before extubation. All of these studies failed to show a significant effect on laryngeal edema, PES and reintubation rates [4,7,41]. In contrast, a single dose of 40 mg methylprednisolone 24 hours before extubation was effective in lowering the incidence of PES and the reintubation rate in a randomized controlled trial by Cheng and colleagues [3]. A significant rise in the cuff leak volume was measured 7 hours after the first steroid injection. The authors suggest that steroids may have a time window before initiating an effect on laryngeal edema and should therefore be given at least 7 hours before extubation [3]. Indeed, studies that use regimens with multiple doses of steroids, starting at least 6 to 12 hours before extubation, seem to indicate the best chance of showing a preventive effect [3,6,13,16]. The regimen used by Francois and colleagues is currently the most effective regimen known. The regimen consists of 20 mg methylprednisolone 12 hours before planned extubation, which is repeated every 4 hours until extubation [6].
The effect of a multidose steroid regimen was confirmed in a recent meta-analysis. Fan and colleagues calculated a risk reduction of 0.19 (-0.24 to -0.15; number needed to treat, 5) on the occurrence of laryngeal edema and of 0.04 (-0.07 to -0.02; number needed to treat, 25) on the rate of reintubation [16]. On the contrary, the effect of a single-dose regimen used in older studies was not statistically significant in two meta-analyses [16,44]. The benefit from steroids will be greater in patients at risk for laryngeal edema, who could be identified with a positive cuff leak test [42]. One should bear in mind, however, that the positive predictive value of the cuff leak test is low. This may lead to an overtreatment of patients with a false positive cuff leak test.
Finally, early tracheostomy might be beneficial in patients at risk for extubation failure due to laryngeal edema, but its exact role so far remains unclear. Early tracheostomy, however, has been shown to lead to less laryngeal damage compared with prolonged translaryngeal intubation [45]. Nevertheless, it is impossible to predict morbidity based on the severity of laryngeal damage [17]. There are no data at hand that support the use of tracheostomy as a preventive procedure in patients at risk of extubation failure due to laryngeal edema, but the procedure could be considered in selected cases.
Therapy
Maintaining the airway, adequate oxygenation and relieving distress associated with obstruction are primary treatment goals. Several treatment modalities, including reintubation, are available and will be discussed below.
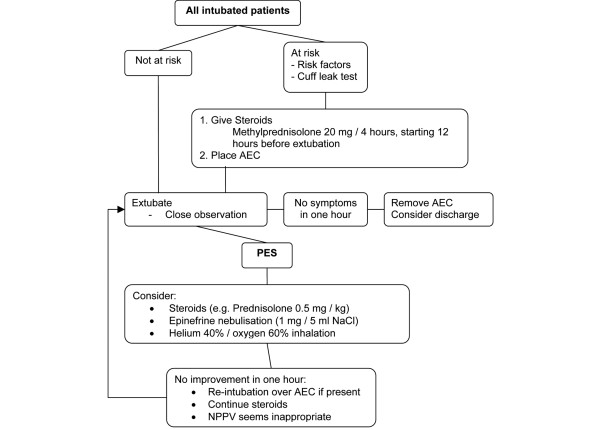
Obviously, intubation in the presence of edema obstructing the airway is life-saving, but may also prove difficult because of impediment of vision [46]. The American Society of Anaesthesiologists nowadays encourages the use of an airway exchange catheter in patients at risk for extubation failure, although evidence mainly consists of case reports and observational studies [47,48]. The airway exchange catheter can be used in patients at high risk for post-extubation laryngeal edema. If necessary, oxygen can be given through the catheter. The catheter is inserted before extubation and remains in situ in the post-extubation period. This facilitates guided (over-the-catheter) intubation. A disadvantage of this method is some degree of patient discomfort. In our experience this disadvantage is outweighed by far by its advantages. Since symptoms of laryngeal edema develop early after extubation, the catheter can be removed if no symptoms develop within approximately 1 hour after extubation (Figure 3).
Figure 3.
Post-extubation laryngeal edema therapy flow chart. AEC, airway exchange catheter; NaCl, 0.9% saline; NPPV, non-invasive positive pressure ventilation; PES, post-extubation stridor.
Medical therapeutic strategies include systemic administration of steroids and nebulization of epinephrine. Corticosteroids downregulate the inflammatory response by inhibiting the recruitment and action of inflammatory cells [49]. Together with a decrease in capillary vessel dilatation and permeability, this inhibition reduces edema [39]. The most effective dose has not yet been determined. We suggest a dose of 0.5 mg/kg prednisolone intravenously per day. The effectiveness of glucocorticoids in post-extubation laryngeal edema has not been confirmed in randomized controlled trials. In our experience, however, the potential benefit outweighs the risk of adverse events. Moreover, most adverse events are pharmacological effects of corticosteroids that are likely to disappear after the treatment period [50].
Furthermore, epinephrine nebulization is another potentially effective therapy. Epinephrine acts through local stimulation of α-adrenergic receptors on vascular smooth muscle cells, thereby causing vasoconstriction and decreased blood flow, which diminishes edema formation. Randomized controlled trials that prove efficacy of epinephrine in post-extubation laryngeal edema in adults are again lacking. Likewise, there is no consensus about the potentially effective dosage of epinephrine nebulization. A dose of 1 mg epinephrine in 5 ml normal saline has proved successful in some cases of upper airway obstruction in adults [51]. Rebound edema is known to occur and close observation is essential [52]. Side effects can occur, especially in patients with coronary artery disease [52].
Theoretically, non-invasive positive pressure ventilation can help prevent reintubation due to respiratory insufficiency in general [3,8]. Evidence for benefit of non-invasive positive pressure ventilation in laryngeal edema, however, is lacking. In a general intensive care population of patients with respiratory failure after extubation, Esteban and colleagues found an increased mortality in the non-invasive positive pressure ventilation group (25% vs. 14%, P < 0.048), which can possibly be explained by the increased time from onset of respiratory failure to reintubation [53]. The use of non-invasive positive pressure ventilation in patients with laryngeal edema might therefore be harmful, as laryngeal edema progresses and further obstructs the remaining airway - making reintubation more difficult, if not impossible.
Helium administration can also be considered. Because a helium/oxygen mixture has a lower density than oxygen-enriched air, airway resistance is decreased. Again, there is little evidence of its usefulness in adults with laryngeal edema and in adults even with airway obstruction due to asthma, largely consisting of case reports and nonrandomized trials [54]. If a helium/oxygen mixture is used, a minimum of 40% of helium is advised in profound hypoxemia, since this amount of helium reduces airflow resistance most effectively while not compromising oxygenation [52].
Emergency tracheostomy is the ultimate step in severe cases of laryngeal edema in which endotracheal intubation fails. In our hospital, elective tracheostomy has successfully been used in a few patients with several, consecutive episodes of extubation failure, not responding to preventive measures or conservative therapeutic strategies.
A practical flow chart on prevention and therapy of post-extubation laryngeal edema and extubation failure is depicted in Figure 3.
Conclusion
Clinically relevant post-extubation laryngeal edema occurs in up to 30% of extubated patients, and 4% of patients need to be reintubated due to laryngeal edema. Laryngeal edema most often presents as inspiratory stridor and may be associated with respiratory failure due to airway obstruction. Subsequent reintubation leads to increased costs, morbidity and mortality. Female gender, a relatively small tracheal diameter or a large tube size and a long duration of intubation have been identified as risk factors in different studies. A positive cuff leak test with leak volume <110 ml increases the risk for development of PES and subsequent reintubation significantly. Multiple-dose regimens of corticosteroids starting at least 12 hours before extubation can prevent the development of laryngeal edema in patients with these risk factors.
The decision whether or not to treat laryngeal edema depends on the severity of symptoms. Every patient with stridor after extubation should be monitored closely. If an increase in severity of symptoms is noted, it is advised to start with nebulized epinephrine or a helium/oxygen mixture. Corticosteroids might also be helpful, but sound evidence is lacking. The use of non-invasive positive pressure ventilation is not recommended since it may be harmful due to a delay in reintubation. Reintubation should be considered early to secure the airway when possible. The exact role of preventive or therapeutic tracheostomy needs to be established.
Abbreviations
PES: post-extubation stridor.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Bastiaan HJ Wittekamp, Email: icuaawittekampb@zgv.nl.
Walther NKA van Mook, Email: w.van.mook@mumc.nl.
Dave HT Tjan, Email: tjand@zgv.nl.
Jan Harm Zwaveling, Email: jh.zwaveling@mumc.nl.
Dennis CJJ Bergmans, Email: d.bergmans@mumc.nl.
References
- Epstein SK, Ciubotaru RL. Independent effects of etiology of failure and time to reintubation on outcome for patients failing extubation. Am J Respir Crit Care Med. 1998;158:489–493. doi: 10.1164/ajrccm.158.2.9711045. [DOI] [PubMed] [Google Scholar]
- Colice GL, Stukel TA, Dain B. Laryngeal complications of prolonged intubation. Chest. 1989;96:877–884. doi: 10.1378/chest.96.4.877. [DOI] [PubMed] [Google Scholar]
- Cheng KC, Hou CC, Huang HC, Lin SC, Zhang H. Intravenous injection of methylprednisolone reduces the incidence of pos-textubation stridor in intensive care unit patients. Crit Care Med. 2006;34:1345–1350. doi: 10.1097/01.CCM.0000214678.92134.BD. [DOI] [PubMed] [Google Scholar]
- Darmon JY, Rauss A, Dreyfuss D, Bleichner G, Elkharrat D, Schlemmer B, Tenaillon A, Brun Buisson C, Huet Y. Evaluation of risk factors for laryngeal edema after tracheal extubation in adults and its prevention by dexamethasone. A placebo-controlled, double-blind, multicenter study. Anesthesiology. 1992;77:245–251. doi: 10.1097/00000542-199208000-00004. [DOI] [PubMed] [Google Scholar]
- Esteban A, Alia I, Gordo F, Fernandez R, Solsona JF, Vallverdu I, Macias S, Allegue JM, Blanco J, Carriedo D, León M, de la Cal MA, Taboada F, Gonzalez de Velasco J, Palazón E, Carrizosa F, Tomás R, Suarez J, Goldwasser RS. Extubation outcome after spontaneous breathing trials with T-tube or pressure support ventilation. The Spanish Lung Failure Collaborative Group. Am J Respir Crit Care Med. 1997;156:459–465. doi: 10.1164/ajrccm.156.2.9610109. [DOI] [PubMed] [Google Scholar]
- Francois B, Bellissant E, Gissot V, Desachy A, Normand S, Boulain T, Brenet O, Preux PM, Vignon P. 12-h pretreatment with methylprednisolone versus placebo for prevention of postextubation laryngeal oedema: a randomised double-blind trial. Lancet. 2007;369:1083–1089. doi: 10.1016/S0140-6736(07)60526-1. [DOI] [PubMed] [Google Scholar]
- Ho LI, Harn HJ, Lien TC, Hu PY, Wang JH. Postextubation laryngeal edema in adults. Risk factor evaluation and prevention by hydrocortisone. Intensive Care Med. 1996;22:933–936. doi: 10.1007/BF02044118. [DOI] [PubMed] [Google Scholar]
- Kriner EJ, Shafazand S, Colice GL. The endotracheal tube cuff-leak test as a predictor for postextubation stridor. Respir Care. 2005;50:1632–1638. [PubMed] [Google Scholar]
- Daley BJ, Garcia Perez F, Ross SE. Reintubation as an outcome predictor in trauma patients. Chest. 1996;110:1577–1580. doi: 10.1378/chest.110.6.1577. [DOI] [PubMed] [Google Scholar]
- Epstein SK, Ciubotaru RL, Wong JB. Effect of failed extubation on the outcome of mechanical ventilation. Chest. 1997;112:186–192. doi: 10.1378/chest.112.1.186. [DOI] [PubMed] [Google Scholar]
- Gowardman JR, Huntington D, Whiting J. The effect of extubation failure on outcome in a multidisciplinary Australian intensive care unit. Crit Care Resusc. 2006;8:328–333. [PubMed] [Google Scholar]
- Torres A, Gatell JM, Aznar E, el Ebiary M, Puig de la Bellacasa J, Gonzalez J, Ferrer M, Rodriguez Roisin R. Re-intubation increases the risk of nosocomial pneumonia in patients needing mechanical ventilation. Am J Respir Crit Care Med. 1995;152:137–141. doi: 10.1164/ajrccm.152.1.7599812. [DOI] [PubMed] [Google Scholar]
- Lee CH, Peng MJ, Wu CL. Dexamethasone to prevent postextubation airway obstruction in adults: a prospective, randomized, double-blind, placebo-controlled study. Crit Care. 2007;11:R72. doi: 10.1186/cc5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CL, Tsai YH, Huang CC, Wu YK, Ye MZ, Chou HM, Shu SC, Lin MC. The role of the cuff leak test in predicting the effects of corticosteroid treatment on postextubation stridor. Chang Gung Med J. 2007;30:53–61. [PubMed] [Google Scholar]
- Erginel S, Ucgun I, Yildirim H, Metintas M, Parspour S. High body mass index and long duration of intubation increase post-extubation stridor in patients with mechanical ventilation. Tohoku J Exp Med. 2005;207:125–132. doi: 10.1620/tjem.207.125. [DOI] [PubMed] [Google Scholar]
- Fan T, Wang G, Mao B, Xiong Z, Zhang Y, Liu X, Wang L, Yang S. Prophylactic administration of parenteral steroids for preventing airway complications after extubation in adults: meta-analysis of randomised placebo controlled trials. BMJ. 2008;337:a1841. doi: 10.1136/bmj.a1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastanos N, Estopa Miro R, Marin Perez A, Xaubet Mir A, Agusti Vidal A. Laryngotracheal injury due to endotracheal intubation: incidence, evolution, and predisposing factors. A prospective long-term study. Crit Care Med. 1983;11:362–367. doi: 10.1097/00003246-198305000-00009. [DOI] [PubMed] [Google Scholar]
- Whited RE. Posterior commissure stenosis post long-term intubation. Laryngoscope. 1983;93:1314–1318. doi: 10.1002/lary.1983.93.10.1314. [DOI] [PubMed] [Google Scholar]
- Esteller More E, Ibanez J, Matino E, Adema JM, Nolla M, Quer IM. Prognostic factors in laryngotracheal injury following intubation and/or tracheotomy in ICU patients. Eur Arch Otorhinolaryngol. 2005;262:880–883. doi: 10.1007/s00405-005-0929-y. [DOI] [PubMed] [Google Scholar]
- Thomas R, Kumar EV, Kameswaran M, Shamim A, al Ghamdi S, Mummigatty AP, Okafor BC. Post intubation laryngeal sequelae in an intensive care unit. J Laryngol Otol. 1995;109:313–316. doi: 10.1017/s0022215100130002. [DOI] [PubMed] [Google Scholar]
- Whited RE. A prospective study of laryngotracheal sequelae in long-term intubation. Laryngoscope. 1984;94:367–377. doi: 10.1288/00005537-198403000-00014. [DOI] [PubMed] [Google Scholar]
- Burns HP, Dayal VS, Scott A, van Nostrand AW, Bryce DP. Laryngotracheal trauma: observations on its pathogenesis and its prevention following prolonged orotracheal intubation in the adult. Laryngoscope. 1979;89:1316–1325. doi: 10.1002/lary.1979.89.8.1316. [DOI] [PubMed] [Google Scholar]
- Hartley M, Vaughan RS. Problems associated with tracheal extubation. Br J Anaesth. 1993;71:561–568. doi: 10.1093/bja/71.4.561. [DOI] [PubMed] [Google Scholar]
- Mackle T, Meaney J, Timon C. Tracheoesophageal compression associated with substernal goitre. Correlation of symptoms with cross-sectional imaging findings. J Laryngol Otol. 2007;121:358–361. doi: 10.1017/S0022215106004142. [DOI] [PubMed] [Google Scholar]
- Maury E, Guglielminotti J, Alzieu M, Qureshi T, Guidet B, Offenstadt G. How to identify patients with no risk for postextubation stridor? J Crit Care. 2004;19:23–28. doi: 10.1016/j.jcrc.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Sandhu RS, Pasquale MD, Miller K, Wasser TE. Measurement of endotracheal tube cuff leak to predict postextubation stridor and need for reintubation. J Am Coll Surg. 2000;190:682–687. doi: 10.1016/S1072-7515(00)00269-6. [DOI] [PubMed] [Google Scholar]
- Miller RL, Cole RP. Association between reduced cuff leak volume and postextubation stridor. Chest. 1996;110:1035–1040. doi: 10.1378/chest.110.4.1035. [DOI] [PubMed] [Google Scholar]
- Ding LW, Wang HC, Wu HD, Chang CJ, Yang PC. Laryngeal ultrasound: a useful method in predicting post-extubation stridor. A pilot study. Eur Respir J. 2006;27:384–389. doi: 10.1183/09031936.06.00029605. [DOI] [PubMed] [Google Scholar]
- Efferen LS, Elsakr A. Post-extubation stridor: risk factors and outcome. J Assoc Acad Minor Phys. 1998;9:65–68. [PubMed] [Google Scholar]
- Jaber S, Chanques G, Matecki S, Ramonatxo M, Vergne C, Souche B, Perrigault PF, Eledjam JJ. Post-extubation stridor in intensive care unit patients. Risk factors evaluation and importance of the cuff-leak test. Intensive Care Med. 2003;29:69–74. doi: 10.1007/s00134-002-1563-4. [DOI] [PubMed] [Google Scholar]
- Rashkin MC, Davis T. Acute complications of endotracheal intubation. Relationship to reintubation, route, urgency, and duration. Chest. 1986;89:165–167. doi: 10.1378/chest.89.2.165. [DOI] [PubMed] [Google Scholar]
- Balestrieri F, Watson CB. Intubation granuloma. Otolaryngol Clin North Am. 1982;15:567–579. [PubMed] [Google Scholar]
- Harrison GA, Tonkin JP. Prolonged (therapeutic) endotracheal intubation. Br J Anaesth. 1968;40:241–249. doi: 10.1093/bja/40.4.241. [DOI] [PubMed] [Google Scholar]
- Donnelly WH. Histopathology of endotracheal intubation. An autopsy study of 99 cases. Arch Pathol. 1969;88:511–520. [PubMed] [Google Scholar]
- Stauffer JL, Olson DE, Petty TL. Complications and consequences of endotracheal intubation and tracheotomy. A prospective study of 150 critically ill adult patients. Am J Med. 1981;70:65–76. doi: 10.1016/0002-9343(81)90413-7. [DOI] [PubMed] [Google Scholar]
- de Bast Y, De Backer D, Moraine JJ, Lemaire M, Vandenborght C, Vincent JL. The cuff leak test to predict failure of tracheal extubation for laryngeal edema. Intensive Care Med. 2002;28:1267–1272. doi: 10.1007/s00134-002-1422-3. [DOI] [PubMed] [Google Scholar]
- Fisher MM, Raper RF. The 'cuff-leak' test for extubation. Anaesthesia. 1992;47:10–12. doi: 10.1111/j.1365-2044.1992.tb01943.x. [DOI] [PubMed] [Google Scholar]
- Benjamin B. Prolonged intubation injuries of the larynx: endoscopic diagnosis, classification, and treatment. Ann Otol Rhinol Laryngol Suppl. 1993;160:1–15. doi: 10.1177/00034894931020s401. [DOI] [PubMed] [Google Scholar]
- Biller HF, Harvey JE, Bone RC, Ogura JH. Laryngeal edema. An experimental study. Ann Otol Rhinol Laryngol. 1970;79:1084–1087. doi: 10.1177/000348947007900609. [DOI] [PubMed] [Google Scholar]
- Kryzer TC Jr, Gonzalez C, Burgess LP. Effects of aerosolized dexamethasone on acute subglottic injury. Ann Otol Rhinol Laryngol. 1992;101:95–99. doi: 10.1177/000348949210100121. [DOI] [PubMed] [Google Scholar]
- Gaussorgues P, Boyer F, Piperno D, Gerard M, Leger P, Robert D. Do corticosteroids prevent postextubation laryngeal edema? Prospective study of 276 adults [letter] Crit Care Med. 1988;16:649. doi: 10.1097/00003246-198806000-00021. [DOI] [PubMed] [Google Scholar]
- Markovitz BP, Randolph AG, Khemani RG. Corticosteroids for the prevention and treatment of post-extubation stridor in neonates, children and adults. Cochrane Database Syst Rev. 2008;Apr 16(2):CD001000. doi: 10.1002/14651858.CD001000.pub2. [DOI] [PubMed] [Google Scholar]
- Hawkins DB, Crockett DM, Shum TK. Corticosteroids in airway management. Otolaryngol Head Neck Surg. 1983;91:593–596. doi: 10.1177/019459988309100601. [DOI] [PubMed] [Google Scholar]
- Roberts RJ, Welch SM, Devlin JW. Corticosteroids for prevention of postextubation laryngeal edema in adults. Ann Pharmacother. 2008;42:686–691. doi: 10.1345/aph.1K655. [DOI] [PubMed] [Google Scholar]
- Rumbak MJ, Newton M, Truncale T, Schwartz SW, Adams JW, Hazard PB. A prospective, randomized, study comparing early percutaneous dilational tracheotomy to prolonged translaryngeal intubation (delayed tracheotomy) in critically ill medical patients. Crit Care Med. 2004;32:1689–1694. doi: 10.1097/01.CCM.0000134835.05161.B6. [DOI] [PubMed] [Google Scholar]
- Dark A, Armstrong T. Severe postoperative laryngeal oedema causing total airway obstruction immediately on extubation. Br J Anaesth. 1999;82:644–646. doi: 10.1093/bja/82.4.644. [DOI] [PubMed] [Google Scholar]
- American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology. 2003;98:1269–1277. doi: 10.1097/00000542-200305000-00032. [DOI] [PubMed] [Google Scholar]
- Mort TC. Continuous airway access for the difficult extubation: the efficacy of the airway exchange catheter. Anesth Analg. 2007;105:1357–1362. doi: 10.1213/01.ane.0000282826.68646.a1. table of contents. [DOI] [PubMed] [Google Scholar]
- Salmela K, Roberts PJ, Lautenschlager I, Ahonen J. The effect of local methylprednisolone on granulation tissue formation. II. Mechanisms of action. Acta Chir Scand. 1980;146:541–544. [PubMed] [Google Scholar]
- Walters JA, Gibson PG, Wood-Baker R, Hannay M, Walters EH. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2009;Jan 21(1):CD001288. doi: 10.1002/14651858.CD001288.pub3. [DOI] [PubMed] [Google Scholar]
- MacDonnell SP, Timmins AC, Watson JD. Adrenaline administered via a nebulizer in adult patients with upper airway obstruction. Anaesthesia. 1995;50:35–36. doi: 10.1111/j.1365-2044.1995.tb04510.x. [DOI] [PubMed] [Google Scholar]
- Irwin RS, Rippe JM. Irwin and Rippe's Intensive Care Medicine. 6. Philadelphia: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- Esteban A, Frutos Vivar F, Ferguson ND, Arabi Y, Apezteguia C, Gonzalez M, Epstein SK, Hill NS, Nava S, Soares MA, D'Empaire G, Alía I, Anzueto A. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med. 2004;350:2452–2460. doi: 10.1056/NEJMoa032736. [DOI] [PubMed] [Google Scholar]
- Rodrigo G, Pollack C, Rodrigo C, Rowe BH. Heliox for nonintubated acute asthma patients. Cochrane Database Syst Rev. 2006;Oct 18(4):CD002884. doi: 10.1002/14651858.CD002884.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YH, Chao TY, Chiu CT, Lin MC. The cuff-leak test is a simple tool to verify severe laryngeal edema in patients undergoing long-term mechanical ventilation. Crit Care Med. 2006;34:409–414. doi: 10.1097/01.CCM.0000198105.65413.85. [DOI] [PubMed] [Google Scholar]
- Engoren M. Evaluation of the cuff-leak test in a cardiac surgery population. Chest. 1999;116:1029–1031. doi: 10.1378/chest.116.4.1029. [DOI] [PubMed] [Google Scholar]