Abstract
Trans-cellular migration, the movement of one cell directly through another, seems an unlikely, counterintuitive, and even bizarre process. Trans-cellular migration has been reported for nearly half a century in leukocyte transendothelial migration in vivo, but is not well enough accepted to widely feature in textbook accounts of diapedesis. Recently, the first in vitro and additional in vivo observations of trans-cellular diapedesis have been reported. Mechanisms by which this occurs are just beginning to be elucidated and point to podosome-like protrusive activities in leukocytes and specific fusogenic functions in endothelial cells. Emerging evidence for a quantitatively significant contribution of trans-cellular migration to leukocyte trafficking in increasingly diverse settings suggests that this phenomenon represents an important and physiologic cell biological process.
Introduction
Compared to most cells of the adult body, blood leukocytes are exceptionally migratory. The majority of tissue cells exhibit relatively stable interactions with neighboring cells and matrix in order to define tissue/organ architecture and boundaries. By contrast, in order to conduct their immune functions in patrolling and eliminating pathogens, leukocytes must continually traffic throughout all compartments of the body [1]. This requires an ability to efficiently cross tissue barriers such as endothelial and epithelial cell layers. Thus, leukocytes are truly ‘invasive’ cells, which, in fact, are far more adept at border crossing than the metastatic tumor cells for which we usually reserve this term. A central question, which relates to, but nonetheless is distinct from issues of adhesion, locomotion, and chemotaxis per se, is precisely how leukocytes negotiate and ultimately cross tissue barriers.
The vascular endothelium lining the circulatory system, though significant heterogeneity exists [2,3], is principally a monolayer of endothelial cells growing on an abluminal matrix (i.e. basement membrane). Junctions between endothelial cells are elaborate and complex, with different adherens, tight and gap junction zones, each formed from distinct molecular components [4] (Figure 1a, b). The endothelium forms the selectively permeable barrier between the blood circulation and the underlying tissues. Furthermore, by controlling leukocyte entry into (i.e. ‘intravasation’) and exit from (i.e. ‘extravasation’) the circulation, the endothelium plays a central role in leukocyte trafficking.
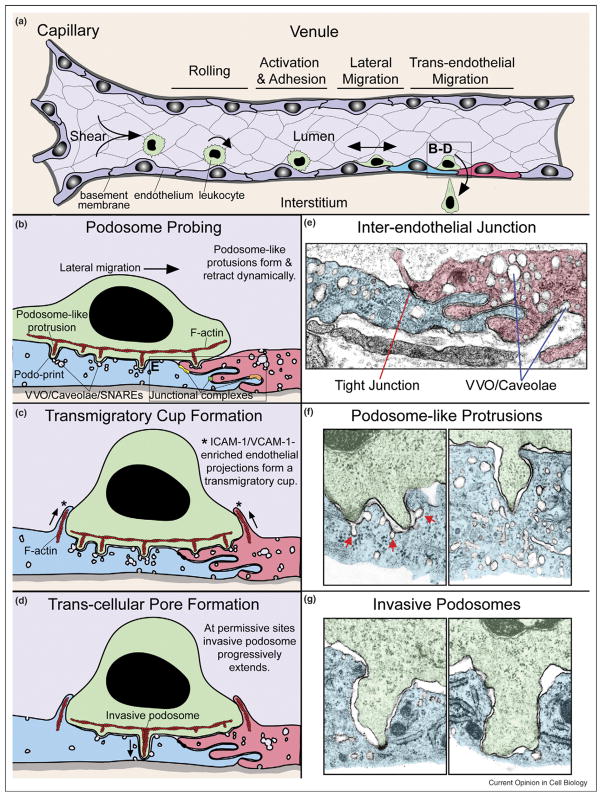
Figure 1.
Mechanisms for trans-cellular diapedesis. (a) Stages of extravasation. Leukocytes (green) entering a postcapillary venule (purple) initially undergo transient rolling type interactions mediated predominantly by selectins. This facilitates chemokine-dependent activation and firm arrest mediated by leukocyte integrins (e.g. LFA-1, Mac1, and VLA-4) binding to endothelial cell adhesion molecules (e.g. ICAM-1/2 and VCAM-1). Subsequently leukocytes migrate laterally over the surface of the endothelium probing for a site to penetrate the endothelium. Finally leukocytes cross the endothelial barrier (i.e. diapedese) and enter the intersitium (tan) by migrating either directly through individual endothelial cells via a trans-cellular pore or between them via a para-cellular gap. Two individual endothelial cells are highlighted (blue and red) and depicted in expanded views during initiation of trans-cellular diapedesis in (b)–(d). (b) Podosome probing. The schematic depicts a ‘snapshot’ of a lymphocyte laterally migrating toward an intact interendothelial junction (see ultrastructure in (e)). Locations where specific junctional adhesion complexes, including tight (yellow), gap (green), and adherens (orange) junctions, form are indicated. During migration dozens of actin (red)-dependent podosome-like protrusions dynamically form and retract, concomitantly forcing endothelial invaginations termed ‘podo-prints’ (see ultrastructure in (f)). Endothelial vesicles, VVO, and caveolae are seen enriched near or directly fused to podo-prints. Fusogenic proteins (i.e. SNAREs) also accumulate at such sites. This dynamic protrusion behavior is thought serve in migratory pathfinding as a means of ‘probing’ or ‘palpating’ the endothelial surface for sites permissive to trans-cellular diapedesis. (c) Transmigratory cup formation. Overlapping temporally with podosome palpation (b), endothelial cells proactively protrude actin/intermediate filament-dependent, ICAM-1/VCAM-1-enrhiched protrusions (*) that embrace adherent leukocytes, forming ‘transmigratory cups’ that are thought to facilitate transition from lateral to transendothelial migration. (d) Trans-cellular pore formation. At permissive sites podosome-like protrusions progressively extend, transitioning to ‘invasive podosomes’, to initiate trans-cellular pore formation for diapedesis (see ultrastructure in (g)). Active SNARE complex-dependent endothelial vesicle fusion at the site of protrusion may facilitate this process. (e) Electron micrograph of a mouse skin postcapillary venule interendothelial junction. Note the highly interdigitated interface. A density toward the top of the interface probably represents a tight junction. Other junctional adhesion complexes cannot be identified unambiguously in this micrograph and are therefore not specifically indicated. Significant amounts of vesicles, VVO, and caveolae are also present. (f) Electron micrographs depicting representative lymphocyte podosome-like protrusions forcing podo-print invaginations into the endothelial surface. Significant levels of endothelial vesicles and caveolea are evident near these protrusions both free in the cytoplasm and fused to the podo-prints (red arrows). (g) Electron micrographs depicting representative lymphocyte invasive podosomes spanning across the majority of the endothelial thickness and forcing endothelial apical and basal membranes into close opposition, as though poised for trans-cellular pore formation. Electron micrographs were provided by Tracey Sciuto and Ann Dvorak.
The process of transendothelial migration, especially during extravasation, has been the subject of intense investigation. Extravasation begins with the accumulation of circulating leukocytes on the luminal surface of the endothelium through a well-characterized sequence of rolling, activation, and firm adhesion events [5] (Figure 1a). Subsequent integrin-dependent lateral migration is an important next step that seems to enable leukocytes to search out sites permissive for endothelial barrier penetration [6,7••]. However, the final and crucial steps for identifying such sites and then formally breaching the endothelium remain only partially characterized and have long been subjects of controversy.
In 1873, Arnold posited ‘Leukocytes pass through specialized openings between endothelial cells’ (via ‘para-cellular’ migration) [8]. Shortly thereafter Adami, unfettered by modern conceptions of cell biology, intuitively challenged that ‘leukocytes might pass directly through [individual] endothelial cells as one soap bubble may pass through another’ (via a ‘trans-cellular’ route) [9]. Indeed, the first studies to directly address mechanisms for trans-endothelial migration in vivo (using electron microscopy (EM)) demonstrated evidence for both trans-cellular [10–12] and para-cellular [13,14] pathways. Importantly, among the many subsequent studies to demonstrate trans-cellular diapedesis in vivo (recently reviewed elsewhere [15]) was a subset that (through use of serial-section and scanning EM) provided conclusive proof for the existence of this route [16–24]. Ironically, however, the lack of clear evidence for trans-cellular diapedesis in initial studies using cultured in vitro endothelial models [25–27] seemed, for the most part, to supplant the previous in vivo observations and the concept of trans-cellular migration became largely abandoned.
Recently, the first in vitro observations of trans-cellular diapedesis and additional in vivo reports have been made, reviving interest in, and facilitating experimental investigation of, this process. In this review we will summarize these findings, discuss putative mechanisms, and finally attempt to place this phenomenon in an appropriate perspective as a physiologic cell biological process. Mechanisms for para-cellular migration have been extensively reviewed elsewhere [25–27] and will be discussed here only in the context of specific comparisons to, and in contrast with, trans-cellular migration.
A renaissance for trans-cellular migration
Taking advantage of ever-improving imaging techniques, starting in 2004, a continually expanding group of investigators have begun making the first unambiguous in vitro observations of trans-cellular diapedesis [28••,29•,30•, 31•,32,33••,34••,35]. These observations have been made with a range of leukocytes (neutrophils, monocytes, and naïve, memory and effector lymphocytes), endothelial cells [human umbilical vein endothelial cells (HUVEC), human coronary artery endothelial cells (HCAEC), human dermal (HDMVEC) and lung (HLMVECs) microvascular endothelial cells, and human lymphatic endothelial cells (HLyECs)], and migratory/inflammatory stimuli (TNF-α, IL1-β, IL-8, SDF-1α, fMLP, and PAF), both under both static and physiologic shear flow conditions. The quantitative contribution of the trans-cellular mechanism to overall diapedesis (i.e. including both trans-cellular and para-cellular) in these studies has ranged from 5 to 60%. Importantly, when HUVEC models were used the percentage of trans-cellular migration was typically low (~5–10%) [28••,29•,30•,33••], whereas studies using HDMVECs, HLMVECs, HCAECs, and HLyECs tended to show much higher use of trans-cellular routes (~30–40%) [31•,33••,34••]. These results demonstrate that heterogeneity exists in the propensity of distinct endothelial cell types to support trans-cellular diapedesis. In addition, this suggests one potential reason for the difficulty in documenting trans-cellular diapedesis in the early in vitro studies, which were done predominantly with HUVECs.
During the same time, new investigations demonstrating trans-cellular diapedesis in vivo have been reported. Through serial-section EM 100% of lymphocytes crossing the blood–brain barrier endothelium in a murine model of multiple sclerosis (i.e. experimental autoimmune encephalomyelitis) were found to migrate trans-cellularly [36••]. Using a similar approach, lymphocyte extravasation through Peyer’s patch high endothelial venules in both normal homing and inflammation was seen to occur exclusively through trans-cellular pores adjacent to intact junctions [37]. Finally, using the relatively lower resolution imaging technique of whole mount confocal microscopy, ~15% of neutrophils could be unambiguously observed to migrate trans-cellularly across inflamed post-capillary venules [7••]. The remainder of the migration events occurred near junctions (i.e. peri-junctionally) and was inferred to be para-cellular.
Mechanisms for trans-cellular migration
Getting in position
The first step for adherent leukocytes in formally crossing the endothelium is to actively seek out sites permissive to penetration. It has been demonstrated both in vitro [6] and in vivo [7••] that efficient diapedesis requires integrin-dependent lateral migration over the endothelium. This has been generally interpreted as allowing leukocytes to move to junctions for para-cellular diapedesis [6,7••]. However, it is clear that not all endothelial locations are equally permissive to trans-cellular diapedesis. For example, regions directly over the rigid nuclear lamina never serve as sites for trans-cellular migration [34••], whereas relatively attenuated peripheral/peri-junctional regions seem to be used preferentially [16,18,20, 24,36••,37]. Thus, the lateral migration observed to precede trans-cellular diapedesis [29•,30•,33••,34••] probably serves to position leukocytes where trans-cellular pore formation can occur most efficiently. This begs the question, in the absence of a discrete preexisting locus (e.g. the junctions for para-cellular diapedesis), how can such sites be identified?
Probing for a trans-cellular migration locus
Current studies suggest a role for podosome-like protrusions in migratory pathfinding [34••]. Podosomes (i.e. ‘foot-protrusions’) are relatively newly discovered [38] actin-dependent protrusive organelles (~500 nm in both diameter and depth) that form preferentially on the ventral aspect of highly migratory cells including leukocytes [39]. Shortly after their discovery, podosomes were shown to form in leukocytes (NK cells) adhering to endothelium and were speculated to function in extravasation [40]. It was recently demonstrated, through fluorescence and EM, that lymphocytes and monocytes dynamically protrude and retract (with half-lives ~20 s) dozens of podosome-like protrusions during their lateral migration over the endothelium [34••] (Figure 1b, f). These protrusions force cognate invaginations (termed ‘podo-prints’) into the surface of the endothelium causing local displacement of cytoplasm, cytoskeleton, and other organelles [34••]. Though not characterized in detail, remarkably similar lymphocyte-induced endothelial invaginations were also observed using TIRF microscopy [33••]. Importantly, this dynamic protrusive behavior always preceded, and was functionally required for, efficient trans-cellular diapedesis [34••].
In vitro and in vivo ultrastructure analysis revealed a continuum of protrusion depths ranging from ~100 to ~2000 nm [34••]. The longer (>1000 nm) structures (termed ‘invasive podosomes’) bear similarity to invadopodia (protrusions that confer invasive properties to metastatic tumor cells [39,41]) and often span nearly the entire endothelial cell thickness, placing the apical and basal membranes in close opposition (Figure 1g). Thus, it is proposed that dynamic podosomes-like protrusions serve to stochastically ‘probe’ or ‘palpate’ the endothelial surface as a means to efficiently identify locations of relatively low endothelial resistance, at which, protusions are able to progressively extend thereby driving trans-cellular pore formation [34••]. Ultrastructurally defined protrusions remarkably similar to podosomes and invasive podosomes have also previously been observed to be formed by lymphocytes [23,24,36••,42–47], monocytes [19,48,49], and neutrophils [29•,48] in a wide range of in vivo and in vitro settings during trans-cellular diapedesis. Thus, a leukocyte ‘palpation’ mechanism may have broad physiologic relevance.
Endothelial membrane fusion
The endothelium appears to contribute proactively to the process of trans-cellular pore formation through regulated membrane fusion. Recent in vitro studies show enrichment of the caveolae marker caveolin-1, vesicles, vesiculo-vacuolar organelle (VVO), and fusogenic proteins (i.e. the SNAREs VAMP2 and 3) in endothelium at sites of podosome-like protrusion and trans-cellular pore formation [33••,34••] (Figure 1b–d, f). Interestingly, several studies also demonstrated local enrichment and fusion of endothelial vesicles at sites of leukocyte protrusion in vivo [19,34••,47,48,50]. Functional perturbation of the NSF/SNAP/SNARE fusogenic machinery or siRNA knockdown of caveolin-1 in endothelium significantly reduced the efficiency of trans-cellular diapedesis [33••,34••]. Thus, in response to leukocyte adhesion and/or protrusion, endothelial cells trigger SNARE complex mediated recruitment and fusion of vesicles. Vesicle fusion may serve to locally augment the plasma membrane surface area, thereby lowering surface tension and allowing leukocyte invasive podosomes to probe progressively deeper (Figure 1d). Additionally, these vesicles may also deliver adhesion receptors [33••,51] and chemokines [52] to further enhance trans-cellular migration efficiency.
Endothelial cytoskeletal rearrangements
The endothelial cytoskeleton may play both facilitating and modulating roles in trans-cellular migration. Actin/ intermediate filament-dependent, ICAM-1-enriched and VCAM-1-enriched endothelial projections termed ‘transmigratory cups’ or ‘docking structures’ have been demonstrated to partially embrace transmigrating leukocytes both in vitro and in vivo [11,17,28••,32,33••,35,36••,44,45,53–56] (Figure 1c). Perturbation studies suggest that these may serve as guidance/traction structures to facilitate initiation of both trans-cellular and para-cellular diapedesis [28••,32,55]. Other studies demonstrate that the trans-cellular pore itself may be enriched/lined with F-actin [31•,33••]. However, the degree to which this represents laterally displaced [31•] or actively remodeled [33••] F-actin remains unclear. Finally, conditions that activate endothelial actin stress fibers decreased trans-cellular, in favor of para-cellular, migration [31•], whereas toxin-mediated inhibition of stress fibers caused transient micron-scale pores to form in endothelium that facilitated trans-cellular bacterial extravation [57]. These findings suggest a potential role for stress fibers in route modulation.
Adhesion receptors in trans-cellular migration
Adhesion receptors on leukocytes and endothelium may be important for determining the sites for transmigration. In one experimental setting neutrophil trans-cellular migration at sites distant (>5 μm) from interendothelial junctions was strongly favored by high endothelial ICAM-1 expression in a manner largely dependent on the integrins LFA-1 and to a lesser extent Mac-1 [30•]. This is consistent with the known importance of β2 integrin occupancy/signaling in driving podosome formation [39] and with the strong enrichment of LFA-1 and ICAM-1 observed in invasive podosomes and podo-prints, respectively [34••]. Alternatively, in an in vivo model, knockout of Mac-1 integrin was found to be associated with decreased lateral migration, delayed extravasation, and a large increase (~65% compared to ~15% for wild type) in trans-cellular migration events that occurred far from endothelial junctions [7••]. Thus, it was suggested that Mac-1-dependent lateral migration was required for leukocytes to reach ‘optimal’ (i.e. relatively more peripheral) sites for transmigration [7••]. As discussed below, however, it remains unclear whether such sites represent the junctions themselves or peri-junctional trans-cellular migration routes.
Specific adhesion receptors are also important for both traction and barrier maintenance during trans-cellular migration. ICAM-1-enriched and VCAM-1-enriched transmigratory-cups/docking structures provide an adhesion substrate oriented parallel to the direction of diapedesis, which may facilitate leukocyte protrusion against the endothelium during both trans-cellular pore and para-cellular gap formation [11,17,28••,32,33••,35,36••,44,45, 53–56] (Figure 1c). Such structures may also minimize barrier disruption by providing greater leukocyte–endothelial contact area [11,17,28••,32,33••,35,36••,44,45,53–56]. Several studies also show strong enrichment of LFA-1 and ICAM-1, and close cell–cell opposition at the trans-cellular pores themselves [28••,30•,33••,34••]. Additionally, homophilic adhesion molecules, including PECAM-1, JAM-1, and CD99, which are expressed on both endothelium and some leukocytes, are thought to be important for diapedesis [25–27]. Though enriched at endothelial junctions and generally thought to function exclusively in para-cellular migration, PECAM-1 and JAM-1 were also shown to become enriched at, and function in, trans-cellular migration events [34••]. It has been suggested that directed trafficking of intracellular pools of PECAM-1 could facilitate its localization to both para-cellular and trans-cellular migration sites [51].
Putting trans-cellular migration into perspective
Trans-cellular migration as a cell biological process
Although trans-cellular migration has met with widespread skepticism and has been out of fashion for many decades, collective in vitro and in vivo evidence now demonstrates it to be a physiologic cell biological process. Furthermore, the demonstration that it is initiated by podosome protrusion now puts it on a firm mechanistic ‘footing’. It is already well established that membrane fusogenic mechanisms, involving vesicular structures (e.g. caveolae and VVO) and frank trans-cellular pores (i.e. fenestrae), function routinely in endothelial and epithelial cells to facilitate transcytotic passage of fluid and solute [2,3,58]. Although the extent to which molecular mechanisms are actually shared remains to be fully elucidated, trans-cellular movement of leukocytes, compared to solutes, may involve similar endothelial cell events.
Potential advantages of trans-cellular migration
What possible benefits might be gained by using a trans-cellular migration pathway? The problem of crossing cellular barriers might be rephrased as an issue of either disassembling highly organized intercellular adhesion complexes (i.e. a para-cellular route) or circumventing them (i.e. a trans-cellular route). In this light, one can envision both mechanical (see ‘Path of least resistance’) and functional advantages for trans-cellular migration.
Perhaps the most important potential functional benefit may be in barrier preservation. In this regard, let us consider the following analogy: in construction, in order to maximize the overall integrity of a structure, doorways, and windows are never placed where they would interrupt primary beams. The primary feature that defines endothelium as an integrated organ and barrier is the inter-cellular junction [4]. Thus, it might seem intuitive to evolve leukocyte migratory mechanisms that do not excessively stress this central structural element. This could be particularly important in settings of high leukocyte flux, as in homing to lymphoid organs or emigration at sites of acute inflammation. Barrier maintenance also requires efficient closure of the transmigration passage subsequent to diapedesis. Although mechanisms for trans-cellular pore closure remain to be characterized, one could envision relatively enhanced efficiency of a process that relies only on a single endothelial cell compared to one that depends on coordinated activity of two or more endothelial cells, as is the case for para-cellular migration. Potential for such barrier-preserving functions has recently been supported in vivo where it was demonstrated that a predominantly trans-cellular leukocyte extravasation model elicited either similar, or in some conditions significantly less, barrier disruption than a predominantly para-cellular model [54,47].
Energetics and the ‘path of least resistance’
Lossinsky and Shivers have proposed that extravasation occurs through the ‘path of least resistance’ [43], an idea that was recently supported by in vivo studies [59]. Pre-existence of the intercellular junctions as potential loci for transmigration does not, however, necessarily indicate that a para-cellular route will be the most energetically favorable. Unlike our common schematic representations, interendothelial junctions in vivo are often tortuous and highly interdigitated, involving (by crosssection) many microns of linear cell–cell contact [36••] (Figure 1e). These junctions are stabilized by well-organized molecular adhesion complexes (i.e. adherens, tight, and gap junctions) coupled to cortical actin networks [4]. Mechanisms for junction disassembly during para-cellular migration are thought to include an energy-expensive process of Rho-mediated stress fiber assembly and contraction in endothelium [25,26,60]. By contrast, the total thickness of the endothelium (i.e. the distance to be traversed during trans-cellular diapedesis) is usually only several hundred nanometers (often ≤100 nm at peripheral/ peri-junctional sites [16,18,20,36••,37]) and is, in effect, often further attenuated by the presence of plasma membrane-attached caveolae and VVO [22,58] (Figure 1e). Thus, while it may not be possible to calculate absolute values, it seems reasonable that in many settings a trans-cellular pathway may provide a relatively lower energy barrier and thereby represent the path of least resistance.
Evaluating transmigration: para-cellular or peri-junctional?
Trans-cellular migration may be significantly underestimated because of technical limitations. Among carefully conducted in vivo serial-section EM studies, many demonstrate that trans-cellular migration preferentially occurs in close proximity, often within 100–200 nm, to intact junctions [16,18,20,24,36••,37] (Figure 1d). However, the majority of quantitative analyses have come from light microscopy where the limit of resolution is ~200 nm. Thus, with exception (i.e. high-resolution serial-section confocal microscopy used in conjunction with VE-cadherin staining), most light microscopy applications cannot reliably distinguish true para-cellular migration events from those that occur through peri-junctional trans-cellular pores. Investigators using light microscopy have accordingly been cautious, only scoring those events that are relatively distant (usually at least several microns) from junctions as trans-cellular, whereas peri-junctional events are by default scored as para-cellular [7••,28••,30•,34••]. Thus, light microscopy may underestimate the extent of trans-cellular migration.
Other settings for trans-cellular migration
Significant evidence exists for the dominant use of a trans-cellular pathway during intravasation of both leukocytes and megakaryocytes across bone marrow endothelium (reviewed elsewhere [15]). In addition nonendothelial barriers may be crossed trans-cellularly. For example, it has been demonstrated in vivo that neutrophils are capable of trans-cellular diapedesis through the pericytes that underlie the vascular endothelium [20]. Extensive leukocyte migration also occurs in vivo across epithelial cell layers, such as the mucosal epithelia of the intestine, airway, and urinary tract, where the mechanisms remain either only partially understood [61] or uninvestigated. Interestingly, we recently found that lymphocytes could readily form trans-cellular pores across epithelial cells in an experimental setting in vitro (i.e. CHO-K1 expressing ICAM-1-GFP) [34••]. It is also important to consider cell types other than leukocytes that are highly migratory such as stem and metastatic tumor cells. Although transendothelial migration of tumor cells is relatively poorly characterized, several in vivo studies provide strong support for the use of a trans-cellular mechanism, in at least some settings [50,62,63]. Stem cells are emerging as important cellular therapeutics that clearly undergo extensive trafficking in vivo [64,65]. Mechanisms and routes for transendothelial migration used by these cells currently remain important open questions.
Conclusions
Extensive previous and current in vivo studies, coupled with emerging in vitro work, establish that trans-cellular migration is an important and physiologic solution to the problem of crossing tissue barriers. Many crucial issues remain to be addressed including the molecular basis for trans-cellular pore formation and closure, and the determinants driving preference for trans-cellular versus para-cellular routes. In addition to continued characterization of basic molecular mechanisms in vitro, it will be crucial to more broadly map the specific in vivo settings in which trans-cellular or para-cellular migration pathways are favored. It is anticipated that the leukocyte type, vascular bed, and specific migratory/inflammatory stimulus will collectively contribute to such behavior. Mapping of route usage will provide a better understanding of the general roles for each pathway and will be crucial for the development of effective anti-inflammatory therapeutics that target leukocyte trafficking.
Acknowledgments
We would like to thank Ann Dvorak for her comments and discussion and both Ann Dvorak and Tracey Sciuto for providing electron micrographs for Figure 1. This work was supported by grants from the Arthritis Foundation (CVC) and the NIH (TAS).
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 2.Aird WC. Phenotypic heterogeneity of the endothelium. I. Structure, function, and mechanisms. Circ Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 3.Aird WC. Phenotypic heterogeneity of the endothelium. II. Representative vascular beds. Circ Res. 2007;100:174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 4.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 5.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multi-step paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 6.Schenkel AR, Mamdouh Z, Muller WA. Locomotion of monocytes on endothelium is a critical step during extravasation. Nat Immunol. 2004;5:393–400. doi: 10.1038/ni1051. [DOI] [PubMed] [Google Scholar]
- 7••.Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med. 2006;203:2569–2575. doi: 10.1084/jem.20060925. This paper is the first to characterize in situ trans-cellular diapedesis by fluorescence microscopy. In addition, it suggests that specific adhesion receptor–ligand interactions contribute to the localization of diapedesis events. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold J. Ueber diapedesis. Virchows Archiv. 1873;58:203–230. [Google Scholar]
- 9.Adami JG. Inflammation: An Introduction to the Study of Pathology. 4. London: Macmillan; 1909. [Google Scholar]
- 10.Williamson JR, Grisham JW. Leucocytic emigration from inflamed capillaries. Nature. 1960;188:1203. doi: 10.1038/1881203a0. [DOI] [PubMed] [Google Scholar]
- 11.Williamson JR, Grisham JW. Electron microscopy of leukocytic margination and emigration in acute inflammation in dog pancreas. Am J Pathol. 1961;39:239–256. [PMC free article] [PubMed] [Google Scholar]
- 12.Marchesi VT, Gowans JL. The migration of lymphocytes through the endothelium of venules in lymph nodes: an electron microscope study. Proc R Soc Lond B Biol Sci. 1964;159:283–290. doi: 10.1098/rspb.1964.0002. [DOI] [PubMed] [Google Scholar]
- 13.Marchesi VT, Florey HW. Electron micrographic observations on the emigration of leucocytes. Q J Exp Physiol Cogn Med Sci. 1960;45:343–348. doi: 10.1113/expphysiol.1960.sp001489. [DOI] [PubMed] [Google Scholar]
- 14.Marchesi VT. Some electron microscopic observations on interactions between leukocytes, platelets, and endothelial cells in acute inflammation. Ann NY Acad Sci. 1964;116:774–788. doi: 10.1111/j.1749-6632.1964.tb52545.x. [DOI] [PubMed] [Google Scholar]
- 15.Sage PT, Carman CV. Settings and mechanisms for trans-cellular diapedesis. Front Biosci. 2008 doi: 10.2741/3587. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azzali G, Gatti R, Orlandini G. Macrophage migration through the endothelium in the absorbing peripheral lymphatic vessel of the small intestine. J Submicrosc Cytol Pathol. 1990;22:273–280. [PubMed] [Google Scholar]
- 17.Faustmann PM, Dermietzel R. Extravasation of polymorphonuclear leukocytes from the cerebral microvasculature. Inflammatory response induced by alpha-bungarotoxin. Cell Tissue Res. 1985;242:399–407. doi: 10.1007/BF00214554. [DOI] [PubMed] [Google Scholar]
- 18.Cho Y, De Bruyn PP. Internal structure of the postcapillary high-endothelial venules of rodent lymph nodes and Peyer’s patches and the transendothelial lymphocyte passage. Am J Anat. 1986;177:481–490. doi: 10.1002/aja.1001770406. [DOI] [PubMed] [Google Scholar]
- 19.Greenwood J, Howes R, Lightman S. The blood–retinal barrier in experimental autoimmune uveoretinitis. Leukocyte interactions and functional damage. Lab Invest. 1994;70:39–52. [PubMed] [Google Scholar]
- 20.Feng D, Nagy JA, Pyne K, Dvorak HF, Dvorak AM. Neutrophils emigrate from venules by a transendothelial cell pathway in response to FMLP. J Exp Med. 1998;187:903–915. doi: 10.1084/jem.187.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoshi O, Ushiki T. Scanning electron microscopic studies on the route of neutrophil extravasation in the mouse after exposure to the chemotactic peptide N-formyl-methionyl-leucyl-phenylalanine (fMLP) Arch Histol Cytol. 1999;62:253–260. doi: 10.1679/aohc.62.253. [DOI] [PubMed] [Google Scholar]
- 22.Feng D, Nagy JA, Dvorak HF, Dvorak AM. Ultrastructural studies define soluble macromolecular, particulate, and cellular transendothelial cell pathways in venules, lymphatic vessels, and tumor-associated microvessels in man and animals. Microsc Res Tech. 2002;57:289–326. doi: 10.1002/jemt.10087. [DOI] [PubMed] [Google Scholar]
- 23.Lossinsky AS, Badmajew V, Robson JA, Moretz RC, Wisniewski HM. Sites of egress of inflammatory cells and horseradish peroxidase transport across the blood–brain barrier in a murine model of chronic relapsing experimental allergic encephalomyelitis. Acta Neuropathol. 1989;78:359–371. doi: 10.1007/BF00688172. [DOI] [PubMed] [Google Scholar]
- 24.Chamberlain JK, Lichtman MA. Marrow cell egress: specificity of the site of penetration into the sinus. Blood. 1978;52:959–968. [PubMed] [Google Scholar]
- 25.Luscinskas FW, Ma S, Nusrat A, Parkos CA, Shaw SK. Leukocyte transendothelial migration: a junctional affair. Semin Immunol. 2002;14:105–113. doi: 10.1006/smim.2001.0347. [DOI] [PubMed] [Google Scholar]
- 26.Muller WA. Leukocyte–endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:327–334. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 27.Burns AR, Smith CW, Walker DC. Unique structural features that influence neutrophil emigration into the lung. Physiol Rev. 2003;83:309–336. doi: 10.1152/physrev.00023.2002. [DOI] [PubMed] [Google Scholar]
- 28••.Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol. 2004;167:377–388. doi: 10.1083/jcb.200404129. This study provided the first conclusive demonstration of a trans-cellular mode of diapedesis in an in vitro system using effector lymphocytes, monocytes, and neutrophils. Moreover, specific endothelial protrusive structures, termed ‘transmigratory cups’ were shown to facilitate both trans-cellular and para-cellular diapedesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Cinamon G, Shinder V, Shamri R, Alon R. Chemoattractant signals and beta 2 integrin occupancy at apical endothelial contacts combine with shear stress signals to promote transendothelial neutrophil migration. J Immunol. 2004;173:7282–7291. doi: 10.4049/jimmunol.173.12.7282. This paper describes a role for fluid shear flow in promoting neutrophil protrusion and transmigration across endothelium by both para-cellular and trans-cellular routes. [DOI] [PubMed] [Google Scholar]
- 30•.Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood. 2005;106:584–592. doi: 10.1182/blood-2004-12-4942. This paper describes the first in vitro setting in which trans-cellular diapedesis can serve as the quantitatively dominant route and demonstrates an important role of the ICAM-1 in supporting this process. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31•.Ferreira AM, McNeil CJ, Stallaert KM, Rogers KA, Sandig M. Interleukin-1beta reduces transcellular monocyte diapedesis and compromises endothelial adherens junction integrity. Microcirculation. 2005;12:563–579. doi: 10.1080/10739680500253493. This work demonstrates for the first time modulation of route usage in response to a physiologic inflammatory cytokine (IL1-β) [DOI] [PubMed] [Google Scholar]
- 32.Nieminen M, Henttinen T, Merinen M, Marttila-Ichihara F, Eriksson JE, Jalkanen S. Vimentin function in lymphocyte adhesion and transcellular migration. Nat Cell Biol. 2006;8:156–162. doi: 10.1038/ncb1355. [DOI] [PubMed] [Google Scholar]
- 33••.Millan J, Hewlett L, Glyn M, Toomre D, Clark P, Ridley AJ. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat Cell Biol. 2006;8:113–123. doi: 10.1038/ncb1356. This study provides evidence for endothelial vesicular structures (caveolae) contributing to trans-cellular pore formation, and that heterogeneity exists among endothelial cell types in their propensity to support trans-cellular diapedesis. [DOI] [PubMed] [Google Scholar]
- 34••.Carman CV, Sage PT, Sciuto TE, de la Fuente MA, Geha RS, Ochs HD, Dvorak HF, Dvorak AM, Springer TA. Transcellular diapedesis is initiated by invasive podosomes. Immunity. 2007;26:784–797. doi: 10.1016/j.immuni.2007.04.015. This study demonstrated a novel cell migratory behavior in which lymphocytes seem to ‘probe’ the endothelial substrate using dynamic podosome-like protrusions in order to identify sites for trans-cellular migration. In addition vesicle fusion activity in endothelium was implicated in the trans-cellular pore formation processs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riethmuller C, Nasdala I, Vestweber D. Nano-surgery at the leukocyte–endothelial docking site. Pflugers Arch. 2008;456:71–81. doi: 10.1007/s00424-007-0412-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36••.Wolburg H, Wolburg-Buchholz K, Engelhardt B. Diapedesis of mononuclear cells across cerebral venules during experimental autoimmune encephalomyelitis leaves tight junctions intact. Acta Neuropathol. 2005;109:181–190. doi: 10.1007/s00401-004-0928-x. This study elegantly employs ultrathin serial-section electron microscopy to demonstrate that mononuclear cells extend processes (similar to recently characterized podosome-like projections [34]) to ‘probe’ through the blood–brain barrier trans-cellularly, while being embraced by ‘cup-like endothelial structures’. [DOI] [PubMed] [Google Scholar]
- 37.Azzali G, Arcari ML, Caldara GF. The “mode” of lymphocyte extravasation through HEV of Peyer’s patches and its role in normal homing and inflammation. Microvasc Res. 2008;75:227–237. doi: 10.1016/j.mvr.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Tarone G, Cirillo D, Giancotti FG, Comoglio PM, Marchisio PC. Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp Cell Res. 1985;159:141–157. doi: 10.1016/s0014-4827(85)80044-6. [DOI] [PubMed] [Google Scholar]
- 39.Linder S, Aepfelbacher M. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 2003;13:376–385. doi: 10.1016/s0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- 40.Allavena P, Paganin C, Martin-Padura I, Peri G, Gaboli M, Dejana E, Marchisio PC, Mantovani A. Molecules and structures involved in the adhesion of natural killer cells to vascular endothelium. J Exp Med. 1991;173:439–448. doi: 10.1084/jem.173.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Curr Opin Cell Biol. 2005;17:559–564. doi: 10.1016/j.ceb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Astrom KE, Webster HD, Arnason BG. The initial lesion in experimental allergic neuritis. A phase and electron microscopic study. J Exp Med. 1968;128:469–495. doi: 10.1084/jem.128.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lossinsky AS, Shivers RR. Structural pathways for macromolecular and cellular transport across the blood–brain barrier during inflammatory conditions. Review. Histol Histopathol. 2004;19:535–564. doi: 10.14670/HH-19.535. [DOI] [PubMed] [Google Scholar]
- 44.Raine CS, Cannella B, Duijvestijn AM, Cross AH. Homing to central nervous system vasculature by antigen-specific lymphocytes. II. Lymphocyte/endothelial cell adhesion during the initial stages of autoimmune demyelination. Lab Invest. 1990;63:476–489. [PubMed] [Google Scholar]
- 45.Fujita S, Puri RK, Yu ZX, Travis WD, Ferrans VJ. An ultrastructural study of in vivo interactions between lymphocytes and endothelial cells in the pathogenesis of the vascular leak syndrome induced by interleukin-2. Cancer. 1991;68:2169–2174. doi: 10.1002/1097-0142(19911115)68:10<2169::aid-cncr2820681014>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 46.Wolosewick JJ. Distribution of actin in migrating leukocytes in vivo. Cell Tissue Res. 1984;236:517–525. doi: 10.1007/BF00217218. [DOI] [PubMed] [Google Scholar]
- 47.Olah I, Glick B. Lymphocyte migration through the lymphatic sinuses of the chicken’s lymph node. Poult Sci. 1985;64:159–168. doi: 10.3382/ps.0640159. [DOI] [PubMed] [Google Scholar]
- 48.Migliorisi G, Folkes E, Pawlowski N, Cramer EB. In vitro studies of human monocyte migration across endothelium in response to leukotriene B4 and f-Met-Leu-Phe. Am J Pathol. 1987;127:157–167. [PMC free article] [PubMed] [Google Scholar]
- 49.Bamforth SD, Lightman SL, Greenwood J. Ultrastructural analysis of interleukin-1 beta-induced leukocyte recruitment to the rat retina. Invest Ophthalmol Vis Sci. 1997;38:25–35. [PubMed] [Google Scholar]
- 50.De Bruyn PP, Cho Y, Michelson S. Endothelial attachment and plasmalemmal apposition in the transcellular movement of intravascular leukemic cells entering the myeloid parenchyma. Am J Anat. 1989;186:115–126. doi: 10.1002/aja.1001860202. [DOI] [PubMed] [Google Scholar]
- 51.Mamdouh Z, Chen X, Pierini LM, Maxfield FR, Muller WA. Targeted recycling of PECAM from endothelial surface-connected compartments during diapedesis. Nature. 2003;421:748–753. doi: 10.1038/nature01300. [DOI] [PubMed] [Google Scholar]
- 52.Middleton J, Patterson AM, Gardner L, Schmutz C, Ashton BA. Leukocyte extravasation: chemokine transport and presentation by the endothelium. Blood. 2002;100:3853–3860. doi: 10.1182/blood.V100.12.3853. [DOI] [PubMed] [Google Scholar]
- 53.Carman CV, Jun CD, Salas A, Springer TA. Endothelial cells proactively form microvilli-like membrane projections upon intercellular adhesion molecule 1 engagement of leukocyte LFA-1. J Immunol. 2003;171:6135–6144. doi: 10.4049/jimmunol.171.11.6135. [DOI] [PubMed] [Google Scholar]
- 54.Phillipson M, Kaur J, Colarusso P, Ballantyne CM, Kubes P. Endothelial domes encapsulate adherent neutrophils and minimize increases in vascular permeability in paracellular and transcellular emigration. PLoS ONE. 2008;3:e1649. doi: 10.1371/journal.pone.0001649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Buul JD, Allingham MJ, Samson T, Meller J, Boulter E, Garcia-Mata R, Burridge K. RhoG regulates endothelial apical cup assembly downstream from ICAM1 engagement and is involved in leukocyte trans-endothelial migration. J Cell Biol. 2007;178:1279–1293. doi: 10.1083/jcb.200612053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, Furthmayr H, Sanchez-Madrid F. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol. 2002;157:1233–1245. doi: 10.1083/jcb.200112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boyer L, Doye A, Rolando M, Flatau G, Munro P, Gounon P, Clement R, Pulcini C, Popoff MR, Mettouchi A, et al. Induction of transient macroapertures in endothelial cells through RhoA inhibition by Staphylococcus aureus factors. J Cell Biol. 2006;173:809–819. doi: 10.1083/jcb.200509009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tuma PL, Hubbard AL. Transcytosis: crossing cellular barriers. Physiol Rev. 2003;83:871–932. doi: 10.1152/physrev.00001.2003. [DOI] [PubMed] [Google Scholar]
- 59.Wang S, Voisin MB, Larbi KY, Dangerfield J, Scheiermann C, Tran M, Maxwell PH, Sorokin L, Nourshargh S. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J Exp Med. 2006;203:1519–1532. doi: 10.1084/jem.20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wojciak-Stothard B, Ridley AJ. Rho GTPases and the regulation of endothelial permeability. Vasc Pharmacol. 2002;39:187–199. doi: 10.1016/s1537-1891(03)00008-9. [DOI] [PubMed] [Google Scholar]
- 61.Zen K, Parkos CA. Leukocyte–epithelial interactions. Curr Opin Cell Biol. 2003;15:557–564. doi: 10.1016/s0955-0674(03)00103-0. [DOI] [PubMed] [Google Scholar]
- 62.Azzali G. On the transendothelial passage of tumor cell from extravasal matrix into the lumen of absorbing lymphatic vessel. Microvasc Res. 2006;72:74–85. doi: 10.1016/j.mvr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 63.Azzali G. Tumor cell transendothelial passage in the absorbing lymphatic vessel of transgenic adenocarcinoma mouse prostate. Am J Pathol. 2007;170:334–346. doi: 10.2353/ajpath.2007.060447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laird DJ, von Andrian UH, Wagers AJ. Stem cell trafficking in tissue development, growth, and disease. Cell. 2008;132:612–630. doi: 10.1016/j.cell.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 65.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]