Abstract
Neoplastic adrenocortical lesions are common in humans and several species of domestic animals. Although there are unanswered questions about the origin and evolution of adrenocortical neoplasms, analysis of human tumor specimens and animal models indicates that adrenocortical tumorigenesis involves both genetic and epigenetic alterations. Chromosomal changes accumulate during tumor progression, and aberrant telomere function is one of the key mechanisms underlying chromosome instability during this process. Epigenetic changes serve to expand the size of the uncommitted adrenal progenitor population, modulate their phenotypic plasticity (i.e., responsiveness to extracellular signals), and increase the likelihood of subsequent genetic alterations. Analyses of heritable and spontaneous types of human adrenocortical tumors have documented alterations in either cell surface receptors or their downstream effectors that impact neoplastic transformation. Many of the mutations associated with benign human adrenocortical tumors result in dysregulated cyclic AMP signaling, whereas key factors/signaling pathways associated with adrenocortical carcinomas include dysregulated expression of the IGF2 gene cluster, activation of the Wnt/β-catenin pathway, and inactivation of the p53 tumor suppressor. A better understanding of the factors and signaling pathways involved in adrenal tumorigenesis is necessary to develop targeted pharmacologic and genetic therapies.
Keywords: adrenal cortex neoplasms, ferrets, models, animal, ovariectomy, orchiectomy, steroidogenesis
Introduction
Adrenocortical neoplasms are common in humans and domestic animals. Post-mortem studies have shown that ∼5 % of people over the age of 50 years have at least one grossly visible adrenocortical nodule.25 The vast majority of these “incidentalomas” are non-functioning adenomas, but others secrete steroid hormones that cause Cushing syndrome or other complications.11 Adrenocortical carcinoma (ACC) is rare (∼1 case per million people per year) but carries a grim prognosis because of its propensity to metastasize before detection.7,150 The overall frequency of adrenocortical neoplasia in dogs is similar to that in humans, although dogs are more prone to cortisol-secreting adrenal lesions.49,116 The frequency of functional adrenocortical neoplasms is even higher in certain gonadectomized animals, such as goats, ferrets, hamsters, and mice (Table 1).132 These gonadectomy-induced adrenocortical tumors, which are more often benign than malignant, may produce ectopic sex steroids that cause significant morbidity.
Table 1.
Frequency of adrenocortical neoplasia in different species.
The factors accounting for the frequent occurrence of benign adrenocortical neoplasms and the low rate of ACC have been the subject of intense investigation over the past decade.15,54,150 Recent advances in our understanding of the molecular pathogenesis of benign and malignant adrenocortical tumors have come from genetic analysis of sporadic and familial human adrenocortical tumors and from studies of naturally-occurring and genetically-engineered animal models.7,18,72,118,145 In this paper we review the genetic and epigenetic events involved in adrenocortical tumorigenesis in humans and domestic animals, and we discuss the relevant animal models.
Development of the adrenal cortex
The adrenal cortex and gonads are major sites of steroid production. Steroidogenic cells in the adrenal cortex and gonads appear to arise from a common pool of mesodermal progenitors in the urogenital ridge.79 During mammalian embryogenesis, progenitors fated to become adrenocortical cells associate with neural crest derivatives that will give rise to the adrenal medulla; cells destined to become gonadal stroma associate with primordial germ cells. Mutations in steroidogenic factor-1 (Sf1) and Wilms tumor-1 (Wt1), two transcription factor genes expressed in the urogenital ridge, disrupt development of both adrenal and gonadal steroidogenic cells, underscoring the close relationship between these lineages.79,144
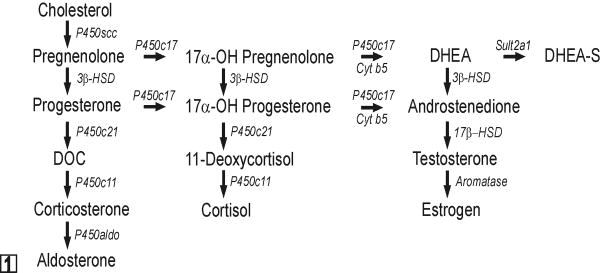
In early human gestation, the fetal adrenal cortex consists of a large inner layer, termed the fetal zone, and a thin outer rim of more immature cells, known as the definitive zone.42 The principal function of the fetal zone is to produce the adrenal androgens, dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEA-S), which are metabolized by the placenta into estrogens that serve to maintain pregnancy.124 Accordingly, the fetal zone expresses enzymes and allosteric regulators required for androgen production, including cytochrome P450 side chain cleavage (P450scc), cytochrome P450 17α-hydroxylase 17,20-lyase (P450c17), cytochrome b5 (cyt b5), and the steroid sulfotransferase SULT2A1 (Fig 1).124 3β-hydroxysteroid dehydrogenase type 2 (HSD3β2), an enzyme required for synthesis of glucocorticoids and mineralocorticoids, is transiently expressed in the fetal zone from weeks 7-12 of gestation and serves to safeguard against virilization of the female genital anlage.58 After birth, the fetal zone regresses, and the production of DHEA and DHEA-S ceases.8 The definitive zone of the human adrenal cortex begins to partition into anatomically and functionally distinct compartments: the zona glomerulosa (zG), zona fasciculata (zF), and zona reticularis (zR).42 Cells in the zG express HSD3β2 but lack P450c17 activity and consequently produce mineralocorticoids. zF cells express HSD3β2 and possess the P450c17 17α-hydroxylase activity but not P450c17 17,20-lyase activity and therefore produce cortisol. This absence of 17,20-lyase activity in the zF has been attributed in part to a lack of expression of its allosteric regulator, cyt b5.5,153 Adrenal production of DHEA and DHEA-S resumes at adrenarche (ages 6-8 years) and localizes to the zR.5
Fig. 1.
Steroid hormone biosynthetic pathways. All steroidogenic cells share the capacity to mobilize and cleave cholesterol. The repertoire of enzymes distal to P450scc determines the steroidogenic capacity of a given cell. Note that P450c17 has both 17α-hydroxylase and 17,20-lyase activities. Cyt b5 selectively enhances the 17,20-lyase activity of P450c17 through allosteric effects. Abbreviations: 3β-HSD, 3β-hydroxysteroid dehydrogenase; 17β-HSD, 17β-hydroxysteroid dehydrogenase; cyt b5, cytochrome b5; DOC, deoxycorticosterone; DHEA, dehydroepiandrosterone; P450aldo, aldosterone synthase; P450c11, cytochrome P450 11β-hydroxylase; P450c17, cytochrome P450 17α-hydroxylase/17,20-lyase; P450c21, cytochrome P450 21-hydroxylase.
The adrenal cortex of most domestic animals resembles that of humans. Two less prominent layers, the zona intermedia and zona juxtamedullaris, are also found in the adrenal cortex of the ferret and other carnivores; the functional significance of these layers is unclear.70 Cortisol is the principal glucocorticoid secreted by the adrenal cortex of most domestic animals, although the adrenal glands of some species produce significant amounts of corticosterone.55,158
In contrast to humans, zonation of the adrenal cortex in the mouse is completed by birth.79 The zG and zF are well-defined in the mouse adrenal, but there is no discernable zR. The adrenal cortex of the young mouse contains an additional layer, termed the X-zone, which is adjacent to the medulla and regresses at puberty in males and during first pregnancy in females.79 The function and steroidogenic potential of the X-zone are unknown, although recent evidence indicates that it may be involved in progesterone catabolism.67 Transgenic expression of LacZ driven by a specific Sf1 fetal enhancer element suggests that the X-zone is a remnant of the adrenal primordia that forms before the definitive adrenal cortex.165 Unlike most other mammals, adrenocortical cells in the adult mouse lack P450c17; consequently, corticosterone is the principal glucocorticoid secreted by the mouse adrenal cortex, and under physiological conditions androgenic steroids are not produced in this tissue.81
Growth and differentiation of stem/progenitor cells in the adrenal cortex
In all mammals the definitive adrenal cortex is a dynamic organ in which steroidogenic cells undergo constant turnover. Cells in each zone of the adult adrenal cortex are theorized to be derived from a common set of stem/progenitor cells in the subcapsular region.18,84,128 These progenitors divide and give rise to daughter cells that differentiate, migrate centripetally, gain zone-specific characteristics, and replenish senescing cells.84 As a result, the adrenal cortex is arranged in radial cords of clonal cells that extend from the zG to the zR.113 This cell migration model is supported by experimental evidence although alternative theories, such as the existence of undifferentiated stem cells exist within each zone, have been proposed to account for the constant renewal and zonal specification of the adrenal cortex.82
The differentiation, growth, and survival of adrenocortical stem cells and their progeny are influenced by a diverse array of hormones and autocrine factors, including adrenocorticotropic hormone (ACTH), angiotensin-II, and members of the insulin-related growth factor (IGF) family.82,127 Endocrine hormones and paracrine factors traditionally associated with the function of gonadal steroidogenic cells, such as luteinizing hormone (LH) and members of the transforming growth factor-β (TGFβ) superfamily (e.g., TGFβ, activin, inhibin) also affect the differentiation, proliferation, and function of cells in the adrenal cortex, both in physiological12 and pathophysiological13 states.
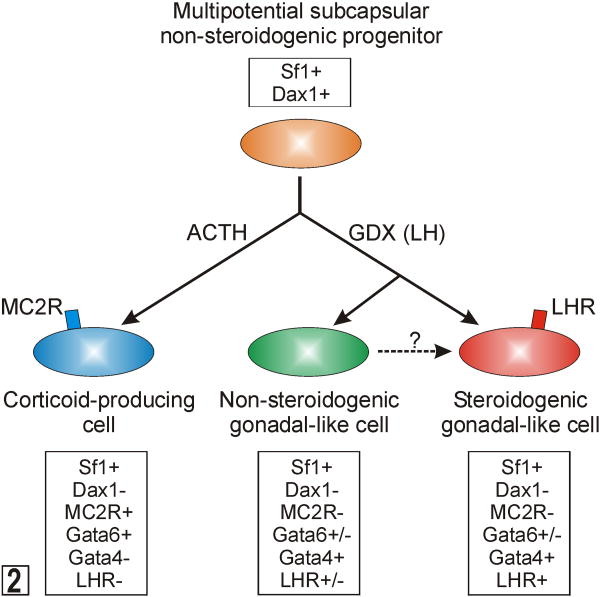
The molecular characteristics of quiescent subcapsular stem cells are unknown. However, differentiation markers characteristic of their descendants have been identified (Fig 2).18,84 Proliferating early progenitors express SF1, a transcription factor that promotes cell growth, limits apoptosis, and activates a wide array of genes involved in both adrenal and gonadal steroidogenesis.39,50,120 Sf1-/- mouse embryos exhibit aberrant adrenal and gonadal development,164 and SF1 haploinsufficiency in mice impairs the compensatory adrenocortical cell growth that follows unilateral adrenalectomy.14 In humans, heterozygous SF1 mutations cause adrenal insufficiency and male-to-female sex reversal.1 Subcapsular progenitors expressing Sf1 have limited steroidogenic capacity due to the SF1-dependent expression of Dax1, an X-linked gene that encodes a repressor of steroidogenic gene expression.78,95,162 In response to ACTH, subcapsular progenitors down-regulate Dax1, and cells differentiate into corticoid-producing cells that express GATA6, a transcription factor that acts in synergy with SF1 and other factors to enhance the expression of genes involved in corticoid biosynthesis.149 DAX1 deficiency in humans and mice leads to excessive differentiation of subcapsular progenitors and eventual depletion of the stem/progenitor cell compartment.1 Consequently, DAX1 deficient males may exhibit transient hypercorticism when young followed by progressive and irreversible hypocorticism.1 Histologically, the adrenals of older DAX1 deficient individuals are characterized by a disorganized steroidogenic cortex that contain cytomegalic cells.61
Fig. 2.
Differentiation of multipotential subcapsular adrenocortical progenitors into corticoid-producing or gonadal-like cells. See the accompanying text for details. Abbreviations: ACTH, adrenocorticotropic hormone; GDX, gonadectomy; LH, luteinizing hormone; LHR, LH receptor; MC2R, melanocortin-2 receptor (ACTH receptor); Sf1, steroidogenic factor 1.
In a proper hormonal microenvironment, the multipotential stem/progenitor cells in the subcapsular region of the adrenal can differentiate into sex steroidogenic cells that resemble gonadal stroma.18 In response to the hormone changes that accompany gonadectomy (increased LH, decreased inhibin, etc.), SF1-positive subcapsular progenitors down-regulate Dax1 and up-regulate GATA4, a transcription factor that drives expression of P450c17 and other genes involved in sex steroidogenesis (Fig 2).149
Subcapsular stem/progenitor cells are hypothesized to be the principal targets of neoplastic transformation in the adrenal cortex,18,39,84 although it is possible that the adrenal cortex harbors other stem cell populations that undergo malignant transformation. Adrenocortical tumors that arise in young children usually express markers typical for the fetal zone and produce DHEA-S, leading investigators to hypothesize that these tumors arise from a stem cell in this zone.156 Similarly, certain genetically-engineered mouse strains develop adrenocortical tumors that appear to arise from progenitors in the X-zone.18,118 Efforts to purify stem-like cells from one or more of these anatomical sites within the adrenal cortex have met with limited success.104
Conceptual models for the pathogenesis of spontaneous adrenocortical neoplasia
Based on recent genetic and epigenetic data, two conceptual frameworks have been put forward to explain the origin/evolution of adrenocortical tumors: the multistep, or clonal, genetic model and the epigenetic progenitor model. These models are complementary rather than mutually exclusive. As discussed below and summarized in Table 2, most cases of adrenocortical tumorigenesis reflect a combination of clonal genetic changes and epigenetic alterations.
Table 2.
Evidence in support of two conceptual models of adrenocortical neoplasia.
|
The multistep (clonal) genetic model
X-chromosome inactivation studies have shown that hyperplastic adrenal tissue usually consists of a polyclonal population of cells, whereas most adrenocortical adenomas (ACAs) and all carcinomas consist of a monoclonal population of cells.16,20,89 On the basis of these observations it has been proposed that adrenocortical tumorigenesis is a multistep process in which the initial event is the growth of a polyclonal neoplasm in response to activation of paracrine or endocrine signaling pathways.101 Increased cell proliferation enhances the susceptibility of cells to oncogene or tumor suppressor gene mutations, leading to the accumulation of specific genetic changes characteristic of these tumors. The clonal genetic model posits that these changes result in the sequential progression from normal cells to benign lesions and eventually to cancer.34
Comparative genomic hybridization (CGH) studies have shown a positive correlation between adrenocortical tumor size and the number of chromosomal alterations, supporting the premise that chromosomal changes accumulate during tumor progression.102,131 Chromosomal amplifications and/or deletions are present in 28-61% of benign human adrenocortical neoplasias, including cortisol-producing nodular hyperplasias.68,88,131,145 However, the average number of chromosomal aberrations per tumor cell is low in benign lesions.145 The most common chromosomal gain is 9q (14%), and the most common loss is 1p (5%).145 Similar chromosomal abnormalities have been detected in cases of adrenocortical hyperplasia and ACA (e.g., gain of chromosome 17q), suggesting that hyperplastic lesions may evolve into adenomas.145,163
In contrast to the limited chromosomal amplifications/deletions seen in benign adrenocortical tumors, widespread chromosomal alterations are evident in nearly all ACCs.88,131,145 The most common changes in human ACC are gains affecting chromosomes 4, 5, 12, and 19 and losses on chromosomes 1, 2, 11, 17, and 22.74 Aneuploidy often accompanies these widespread chromosomal imbalances.20 In addition to deletions or amplifications, a high percentage of ACC cells exhibit loss of heterozygosity (LOH) at key loci.56,90 Collectively, these findings support a multistep model of adrenocortical tumorigenesis in which genomic instability begins in benign lesions and progresses during malignant transformation. At present, however, there is no definitive evidence to either prove or refute the premise that ACAs evolve into ACC.145
The epigenetic progenitor model
Epigenetic alterations occur in cancer cells as commonly as genetic mutations and can mimic the effects of the latter.46,47 The term epigenetics refers to non-sequence-based modifications of DNA or its associated factors (e.g., histones) that are maintained during cell division. One such epigenetic alteration is global DNA hypomethylation, a hallmark of both benign and malignant tumors.151 The consequences of widespread hypomethylation include increased expression of oncogenes, chromosome instability, and LOH.151 Other epigenetic alterations associated with tumorigenesis include hyper- or hypo-methylation of specific genes, chromatin modification, and loss of imprinting (LOI).46,47 Epigenetic alterations are thought to be central to tumorigenesis in many tissues. Indeed, it has been suggested that tumors would neither arise nor progress without a disruption of normal epigenetic programs.45
Epigenetic alterations could be the consequence of tumor progression, but recent data suggests an alternative explanation; namely, that epigenetic alterations precede tumor formation and increase the probability of cancer when genetic changes arise.46,47,151 According to the epigenetic progenitor model, the first step in tumorigenesis is epigenetic disruption of stem/progenitor cells in a tissue, which alters the normal balance between undifferentiated stem/progenitor cells and their progeny, leading to polyclonal proliferation of “neoplasia-ready” progenitor cells.46,47
A set of “tumor-progenitor” genes is hypothesized to promote epigenetic disruption of stem/progenitor cells.46,47 The normal function of these genes might be to promote “stemness”, the tendency toward pluripotency and replication over the tendency toward differentiation and limited replication.46,47 An example of a tumor-progenitor gene is IGF2. In intestinal epithelium, LOI at the IGF2 locus leads to increased IGF-II expression, which in turn promotes expansion the stem/progenitor cell compartment and increases the probability of neoplasia.77 Of note, IGF2 is the most commonly over-expressed gene in human ACC.57,102 This suggests that in adrenocortical progenitors, as in intestinal epithelial progenitors, dysregulated expression of IGF2 may increase tumor risk by expanding the target cell population and/or modulating the effect of subsequent genetic alterations on these cells.
In addition to expanding the progenitor cell compartment, preexisting epigenetic alterations are thought to impact phenotypic plasticity, the ability of stem/progenitor cells to change their behavior in response to hormones and other environmental cues.46 Epigenetic alteration of adrenal progenitor cells is followed by mutation of tumor suppressor genes (e.g., p53), which enhances genetic instability and increases the likelihood of additional mutations. If progressive, these changes can culminate in a malignant phenotype. In summary, this model proposes that epigenetic changes may contribute to adrenocortical tumorigenesis by modulating size of the stem/progenitor population, altering phenotypic plasticity, and enhancing sensitivity to subsequent mutations.
Gonadectomy-induced adrenocortical neoplasia as a model of epigenetically-influenced tumorigenesis
Approximately 60 years ago researchers first noted that gonadectomy causes cells in the subcapsular region of the mouse adrenal cortex to transform into sex steroid-producing cells that are histologically and functionally similar to gonadal tissue.48 Subsequent experiments established that gonadectomy-induced adrenocortical neoplasia is highly strain dependent.18 The most susceptible strain, CE/J, develops adrenocortical carcinomas, while DBA/2J, C3H, and NU/J mice develop adenomas. Other strains including C57BL/6J and FVB/N are resistant to gonadectomy-induced adrenocortical neoplasia. This process is believed to represent metaplasia of cells in the adrenal cortex, which under the influence of continuous gonadotropin stimulation transform into tissue resembling gonadal stroma. This transformation is accompanied by ectopic expression of GATA4, a sex steroidogenic transcription factor that is normally absent from the adrenal cortex of adult mice.18 Like other classic examples of metaplasia (e.g., Barrett's esophagus), gonadectomy-induced adrenocortical lesions arise in a self-renewing epithelium, are driven by chronic hormonal stimulation or tissue injury, and have the potential to evolve into frank neoplasia.143 Gonadectomy-induced adrenocortical metaplasia/neoplasia is not limited to mice; sex steroid-producing adrenocortical tumors are common in gonadectomized ferrets, hamsters, and goats (Table 1).18
Gonadectomy-induced adrenocortical neoplasia may be viewed as an example of epigenetically-triggered tumorigenesis. Stem/progenitor cells in the adrenal cortex are subject to epigenetic alteration even in the absence of tumor formation, as illustrated by studies of mice harboring a β-galactosidase transgene driven either by a P450c21 promoter113 or a P450scc promoter.73 X-gal staining of the adrenal glands from these mice demonstrates radial columns of cells in the cortex that either express or do not express β-galactosidase, reflecting random epigenetic activation (or silencing) of the transgene in subcapsular stem/progenitor cells. Each of these columns of cells is presumed to be derived from a distinct, epigenetically-defined stem/progenitor cell. In cases of gonadectomy-induced adrenocortical neoplasia, preexisting epigenetic alterations might impact the phenotypic plasticity of stem/progenitor cells, allowing them to respond to the hormonal changes associated with gonadectomy. This may explain why gonadectomy leads to discrete cords of proliferating cells in the adrenal cortex.
The rapidly expanding database of genomic DNA sequences and single nucleotide polymorphisms in various strains of mice afford a means to characterize the alleles and genetic modifiers influencing gonadectomy-induced adrenocortical neoplasia (i.e., the “tumor-progenitor” genes is that promote epigenetic disruption of stem/progenitor cells) Linkage analysis of crosses between a susceptible (DBA/2J) and a non-susceptible (C57BL/6J) strain of mice has shown that post-gonadectomy tumorigenesis in DBA/2J mice is a dominant trait and that a major locus for tumorigenesis resides on chromosome 8. 9 One of the candidate genes in the linkage region is secreted Frizzled-related Protein-1 (sFRP1), a tumor suppressor that inhibits the Wnt/β-catenin signaling pathway (see below).9 Although Sfrp1 is an attractive candidate gene for tumorigenesis, no causal mutations have been identified in the coding or non-coding regions of the gene in DBA/2J mice. Remarkably, a significant proportion of non-affected F2 animals carry the susceptible DBA/2J allele on chromosome 8, and epistasis analysis suggests that multiple interacting genetic loci contribute to the phenotype. More than 30% of tumorigenic F2 animals were homozygous for the non-susceptible C57BL/6J allele on chromosome 8, indicating that other genes were involved in tumorigenesis. Unbiased genetic approaches such as this should yield valuable insights into the pathogenesis of adrenocortical neoplasia.
Role of telomeres in adrenocortical tumorigenesis
Telomeres, the ends of chromosomes, are specialized nucleoprotein structures consisting of tandem arrays of G-rich repetitive DNA sequences bound to accessory proteins (Fig 3).59 Telomere-associated proteins protect the ends of chromosomes and also function to regulate telomerase, the enzyme responsible for addition of the G-rich DNA repeats. Telomerase is a ribonucleoprotein complex comprising a reverse transcriptase (TERT), an RNA template (TERC), and other proteins.32,147 Most somatic cells, including adrenocortical cells, lack telomerase activity, so telomeres shorten by 50–200 base pairs with each cell division due to the end replication problem, the inability of DNA polymerase to fully replicate the lagging DNA strand. Telomere dysfunction, owing to progressive shortening and/or loss of telomere-associated proteins, triggers a tumor suppressor p53-dependent DNA damage response that results in cell cycle arrest, replicative senescence or apoptosis, eventually leading to impaired tissue function.148 Telomere extension by telomerase is essential for normal stem/progenitor cell function; proper telomere maintenance ensures that stem cells can divide an unlimited number of times and avoid replicative senescence.
Besides its well documented role in telomere maintenance, one of the components of the telomerase complex, TERT, appears to directly enhance the entry of quiescent stem cells into the cell cycle.31,53 This non-canonical function of TERT does not require TERC (i.e., it is independent of reverse transcriptase activity).31 TERT binds Brg-1, a chromatin remodeling protein that interacts with and activates TCF/LEF, a key downstream mediator of Wnt/β-catenin signaling (see below).31 Thus, TERT can directly activate tissue stem cells by controlling gene expression of proliferation genes such as MYC through the Wnt/β-catenin pathway.
In addition to triggering senescence, telomere dysfunction leads to end-to-end chromosome fusions followed by DNA breakage/fusion/bridge cycles, resulting in chromosomal duplications and deletions that are characteristic of cancers.6,43,69 In the setting of an impaired p53 checkpoint, the genomic instability caused by dysfunctional telomeres can initiate malignant transformation, particularly in self-renewing tissues such as the adrenal cortex.4,117 Telomere-based crisis provides a mechanism of chromosomal instability, leading to regional amplifications and deletions that drive tumorigenesis.
Most cancer cells avoid replicative senescence associated with telomere dysfunction and chromosomal imbalances by activating mechanisms of telomere maintenance, either through ectopic expression of TERT or recombination-based mechanism known as alternative lengthening of telomeres (ALT).63 Over 90% of ACC samples display signs of active telomerase maintenance mechanisms, whereas benign adrenocortical tumors do not.41 The importance of telomere maintenance of the malignant phenotype is underscored by a series of xeno-transplantation studies with telomerase-negative bovine adrenocortical cells rendered tumorigenic by transfection with Ha-Ras (G12V) and SV40 large T antigen. These cells do not require exogenous TERT for initial tumor formation;27 however, the cells exhibit a progressive loss of malignant behavior, which can be restored by transfection with TERT.137 Transfection of tumorigenic bovine adrenocortical cells with TERT triggers a progenitor-like state, characterized by increased cell proliferation regulated by the IGF2 pathway.119
Tumor suppressor genes involved in adrenocortical tumorigenesis
p53
The p53 tumor suppressor pathway functions as a feedback loop that integrates with other signal transduction networks, including the IGF-AKT pathway (see below).99 p53 itself acts as a transcriptional regulator of the cell cycle, inducing cell cycle arrest (G1 or G2), senescence, or apoptosis in response to DNA-damage and other stressors.135,161 DNA damage triggers a series of post-translational modifications of p53, including its phosphorylation by the kinases ATM (ataxia telangiectasia-mutated) and CHK2 (checkpoint kinase 2). These modifications render p53 resistant to inactivation by MDM2 (mouse double minute 2), an E3-ubiquitin ligase that binds p53 and targets it for degradation in the proteosome. For efficient degradation of p53, MDM2 needs to be phosphorylated, which can be accomplished by the ATK protein kinase.
There is strong selective pressure for emerging tumors to disrupt the p53 signaling pathway, either through mutation of the p53 gene or overexpression of MDM2 or other negative regulators of p53 function.135,161 Somatic inactivating mutations of TP53, the gene encoding p53, are observed in one third of human ACCs. LOH at the TP53 locus on 17p13 is found in only 14% of cases of human ACA134 but is associated with a worse prognosis in these tumors.56,103 LOH at 17p13 is evident in approximately 80% in ACC.103,134 Fine mapping of 17q13 LOH in ACC suggests that additional tumor suppressor genes besides TP53 may reside in this locus and contribute to tumorigenesis.134
Individuals with Li-Fraumeni Syndrome (LFS), an autosomal dominant tumor predisposition syndrome generally caused by germline mutations in TP53, develop ACC, albeit rarely. An analysis of 91 families with LFS revealed that breast cancer, brain cancer, and sarcomas were common, but ACC was evident in only 1% of the patients.91 Of interest, a specific germline missense mutation in TP53 (R337H) has been observed in a group of children with adrenocortical tumors in southern Brazil.51,125 In contrast to most somatic mutations that affect the DNA binding region of P53, the TP53 R337H mutation affects the oligmerization motif in P53, leading to pH-dependent instability. Carriers of the TP53 R337H mutation have a 20,000-fold increased risk of ACC, but they are not predisposed to other tumor types typical of LFS. Penetrance is incomplete; only 10% of carriers develop ACC. The loss of chromosome 17 harboring the normal TP53 allele is crucial for ACC development in patients with the R337H mutation.121 Other low penetrance constitutional TP53 mutations associated with childhood ACC have also been identified.51,125
MEN1
A heterozygous inactivating germline mutation in MEN1, a tumor suppressor gene located at 11q13, is found in about 90% of patients with multiple endocrine neoplasia type 1, an autosomal dominant syndrome characterized by predisposition to parathyroid, endocrine pancreas, pituitary, and adrenocortical tumors.98 These are typically nonfunctional ACAs; ACC is uncommon. Somatic mutation of the MEN1 gene is very rare in sporadic adrenocortical tumors,66,129 but LOH at 11q13 is present in more than 20% of ACA and 90% of ACC.66,90,129 Mice with a germline mutation in the Men1 gene are predisposed to benign tumors in the adrenal cortex and other tissues.106 Tumors in all sites showed LOH at the Men1 locus.106 Menin, the protein encoded by the MEN1 gene, interacts with Smad3 and other binding partners, and menin deficiency elicits widespread effects on transcription, chromatin remodeling, and cell cycle progression.114
Fumarate hydratase
Hereditary leiomyomatosis and renal cell cancer (HLRCC) is an autosomal dominant disorder caused by mutations in the Fumarate Hydratase (FH) gene on chromosome 1q42.3-43. Patients with HLRCC are also predisposed to Massive Macronodular Adrenocortical Disease (MMAD). MMAD is a heterogeneous condition associated with Cushing syndrome and bilateral hyperplasia of the adrenal glands.97,109 Tumor tissue from these patients harbors both a germline FH mutation and allelic loss at the 1q42.3-43 FH locus. The mechanism through which FH, a mitochondrial protein, participates in adrenocortical tumorigenesis, is unknown.
TPP1
Tpp1, one of the accessory proteins associated with the telomere cap, has been directly linked to adrenocortical stem cell maintenance and tumorigenesis. Mice homozygous for a hypomorphic allele of Tpp1 exhibit a complex phenotype that includes adrenocortical dysplasia and hypofunction.69,80 This allele, termed Tpp1acd (for the adrenocortical dysplasia strain), results in telomeres that are dysfunctional, leading to an increase in senescence-associated markers in adrenocortical cells. When telomere dysfunction-associated senescence is prevented by introducing mutant p53 alleles into Tpp1acd/adc mice, there is a partial normalization of adrenal weight and morphology.30 However, p53 deficiency rescues the adverse effects of telomere dysfunction at the cost of accelerating carcinogenesis.4 Recently it has been shown that p53-deficient mice harboring Tpp1acd mutations are predisposed to a spectrum of tumors, including ACC. Collectively these findings suggest that Tpp1 is essential for proper function of progenitor cells in the adrenal cortex and functions as a tumor suppressor.
Signaling pathways involved in adrenocortical tumorigenesis
In the adrenal cortex, as in other tissues, oncology recapitulates ontogeny. Signaling pathways involved in embryonic/fetal development and in stem cell maintenance/activation are often deranged in adrenocortical tumors.
Wnt/β-catenin
Wnt signaling pathway regulates a variety of developmental processes such as cell proliferation, adhesion, and cell fate determination.122 Members of the Wnt family of signaling molecules bind to Frizzled receptors on the cell surface and initiate a cytoplasmic signaling cascade that results in disruption of a β-catenin degradation complex composed of glycogen synthase kinase 3β (GSK3β), the adenomatous polyposis coli (APC) tumor suppressor, and auxin. The net effect of disruption of this complex is stabilization of β-catenin.122 Once translocated to the nucleus, β-catenin can enhance gene expression through interactions with various transcription factors or co-activators, including TCF/LEF family members, Smad factors, and SF1 in steroidogenic cells.60 Among the targets of activation by the TCF/β-catenin complex are genes that drive cell proliferation, such as c-Myc. 64,141
Wnt2b is expressed in subcapsular cells and might regulate progenitor fate,105 while Wnt4 localizes to the zG and appears to regulate zonal identity.65 Introduction of Wnt4 into primary cultures of adrenocortical cells has been shown to alter steroidogenesis.28 Wnt4 null mice have decreased expression of P450aldo in the adrenal and ectopic expression of the adrenal-specific marker P450c21 in gonadal tissue.65,75 Humans homozygous for WNT4 loss-of-function mutations exhibit adrenal dysgenesis and female-to-male sex reversal.108
Tissue-specific knockout of β-catenin in adrenocortical progenitors (using an Sf1 promoter-Cre transgene) produces mice with adrenal glands that function normally at birth but fail by 30 weeks of age.85 Like in Dax mice, it is theorized that impairment of the Wnt/β-catenin signaling pathway leads to depletion of adrenocortical stem/progenitor cells. Thus, Wnt/β-catenin signaling pathway appears to function as a gatekeeper that serves to maintain the undifferentiated stem cell pool.
Overexpression of genes related to the Wnt/β-catenin signaling pathway has been reported in forms of adrenocortical hyperplasia.23 Abnormal cytoplasmic or nuclear accumulation of β-catenin is seen in 38% of human ACAs and in 85% of adrenocortical carcinomas.142 Somatic activating mutations of the β-catenin gene are present in approximately 30% of human ACAs and carcinomas139,142 and have been linked to bilateral adrenal hyperplasia in humans.140 β-catenin immunoreactivity has also been observed in the nuclei of neoplastic cells that accumulate in the adrenal cortex of gonadectomized inbred mice, suggesting that the Wnt signaling pathway may participate in gonadectomy-induced adrenocortical neoplasia.17 The TCF/β-catenin antagonist PKF115-584 inhibits proliferation of adrenocortical carcinoma cells in vitro, suggesting that inhibitors of the Wnt signaling pathway might be useful in the treatment of adrenocortical tumors.38
Individuals with familial adenomatous polyposis (FAP) have constitutive activation of the Wnt/β-catenin signaling pathway due to germline loss-of-function mutations of the APC gene. These individuals are predisposed to both colonic and extra-colonic tumors, including adrenocortical tumors.19 Most of the adrenocortical neoplasms in patients with FAP are benign.136
IGF1R-AKT
Two members of the IGF family, IGF-I and IGF-II, act as adrenocortical mitogens by promoting progression through the G1/S phase of the cell cycle. IGF-2 expression is normally limited to the embryonic and fetal stages of adrenal development. Both IGF-I and IGF-II bind the tyrosine kinase receptor IGF1R, which in turn phosphorylates the insulin receptor substrates IRS-1 and IRS-2. Phosphorylated IRS recruits phosphatidylinositol 3-kinase (PI3K), resulting in activation of the AKT protein kinase, which then phosphorylates a wide range of target proteins, including GSK3β and MDM2. GSK3β phosphorylation leads to activation of the Wnt/β-catenin pathway. Mice with null mutations in Igf1 or Igf2 appear to have normal adrenocortical function, but mice over-expressing IGF-II develop early adrenal hyperplasia, suggesting that that this growth factor can drive adrenocortical cell growth in vivo.26
The IGF2 locus (11p15) is parentally imprinted, and normally only the paternal allele is expressed.37 Two other imprinted genes in this region, H19 and p57KIP2, are expressed only from the maternal allele. The H19 mRNA is not translated and serves to inhibit IGF2 expression. The p57KIP2 gene encodes a cyclin-dependent kinase inhibitor involved in the G1/S phase of the cell cycle, and p57KIP2 is found primarily in differentiated cells. Mutation and/or aberrant expression of imprinted genes in the IGF2 locus cause Beckwith-Wiedemann syndrome (BWS), an overgrowth syndrome associated with adrenal hyperplasia and ACC.36 BWS resembles Late Offspring Syndrome (LOS), an overgrowth syndrome affecting ruminants.133,159,160 Interestingly, both BWS and LOS are complications of assisted reproduction technology. In vitro culture of embryos during assisted reproduction is believed to cause abnormal genetic imprinting at the IGF2 gene cluster, leading to overgrowth syndromes in children, ruminants, and probably other mammals.100,126
Overexpression of IGF2, caused by genetic or epigenetic alterations at chromosome 11p15, is evident approximately 90% of cases of human ACC.57,102 Transcription analysis has shown that IGF2 is the most over-expressed gene in human ACC compared with ACAs or normal tissue,2,20,156 and downstream AKT phosphorylation is more pronounced in ACC than ACAs.44 On the basis of these observations it has been suggested that activation of IGF2 is essential for ACC growth. The clonal genetic model of tumorigenesis argues that genetic changes at the IGF2 locus are a late event in the evolution of ACAs into carcinomas, whereas the epigenetic progenitor model discussed below posits that LOI at the IGF2 locus is an early event in tumorigenesis.
Supporting the notion of an IGF-II autostimulatory loop, overexpression of the IGF-1 receptor (IGF1R) has been documented in cases of human ACC.52,154,155 The biological effects of IGFs are modulated by a series of IGF binding proteins (IGFBPs). Adrenocortical tumors with IGF-II overproduction have been shown to express large amounts of IGFBP-2, which may regulate the effects of IGF-II in these tumors.22 Interestingly, IGFBP-2 levels correlated with the stage of ACC and are inversely correlated with survival.21 On the other hand, loss of function of a second receptor for IGF-II, IGF2R, is associated with malignant adrenocortical tumors. IGF2R is thought to be a tumor suppressor because of its ability to bind and degrade IGF-II and promote activation of TGFβ.33,96 Recent studies suggest that LOI of at the IGF2 locus increases cancer risk in another way: by increasing the sensitivity of IGF-II signaling pathways.77
Since IGF-II is the highest upregulated transcript in sporadic ACC, this growth factor and its receptors are logical candidates for molecularly targeted drug therapy. IGF1R antagonists cause growth inhibition in vitro in human ACC xenografts in nude mice,62 and early clinical testing in humans is underway.
cAMP signaling
Activation of G-protein coupled receptors on the surface of adrenocortical cells elicits pleiotropic effects on cell growth, differentiation, and steroid production. These effects are mediated in part through the cAMP-protein kinase A signaling pathway, which culminates in the phosphorylation (i.e., activation) of transcription factors and enhanced expression of steroidogenic enzyme genes. The binding of ACTH to its G-protein coupled receptor, MC2R, drives differentiation of adrenocortical progenitors into corticoid-producing cells (Fig 2). Disruption of MC2R leads to adrenocortical atrophy and reduced production of glucocorticoids and aldosterone.29 A constitutively-active, germline mutation of the MC2R gene has been observed in an individual with bilateral adrenal hyperplasia and Cushing's syndrome.138 Genetic experiments with mice and observations on ferrets suggest that the binding of LH to its G-protein coupled receptor, LHR, can redirect differentiation of multipotential stem/progenitor cells in the subcapsular region of the adrenal from corticoid-producing cells fate to a sex steroidogenic cells (Fig 2).18 LHR is expressed in the fetal but not adult mouse adrenal cortex, a developmental expression pattern resembling those of transcription factor GATA4 and P450c17. It is conceivable, albeit unproven, that a subset of adrenocortical stem/progenitor cells express functional LHR and proliferate in response to LH stimulation.
Many of the mutations associated with human adrenocortical hyperplasias and adenomas result in dysregulation of the cAMP-dependent protein kinase signaling pathway. Cases of benign multinodular hyperplasia and ACA in humans have been linked to ectopic expression of certain transmembrane G-protein coupled receptors, including receptors for LH, gastric inhibitory polypeptide, vasopressin, β-adrenergic agonists, and interleukin-1.35,86,92,94,136,152 Enforced expression of LHR or the gastric inhibitory polypeptide receptor in bovine adrenocortical cells is a sufficient genetic event to induce benign adrenocortical tumors in a xeno-transplantation model.110-112
Trimeric G-proteins transduce signals from LHR and other G-coupled transmembrane receptors. Certain benign multinodular adrenocortical hyperplasias have been linked to activating mutations in the gene for Gsα or inactivating mutations in gene for Gi2α, both of which cause excess cAMP signaling. Loss-of-function germline mutations in the genes encoding PDE8 and PDE11, two phosphodiesterases that degrade cAMP, have been linked to bilateral adrenocortical hyperplasia and sporadic ACA.71,136 Inactivating mutations in the protein kinase A regulatory subunit (PRKAR1A) cause Carney Complex, a multiple endocrine neoplasia syndrome associated with Primary Pigmented Adrenocortical disease (PPNAD), abnormal pigmentation of the skin, myxomas, and other neoplasms. Somatic inactivating mutations or allelic losses of the PRKAR1A locus at 17q22-24 are also seen in sporadic cases of ACAs and ACCs.10
TGFβ signaling
Members of this superfamily use a signaling pathway that includes their type I and type II receptor kinases and their downstream mediators, the Smad proteins.130 Activation of the receptors leads to phosphorylation of Smad2 and Smad3. Phosphorylated Smad2 and Smad3, in conjunction with Smad4, are translocated to the nucleus, where they activate transcription of specific genes.
TGFβ1 and TGFβ2 are autocrine growth factors produced by normal adrenocortical cells. TGFβ1 and TGFβ2 have no effect on steroidogenic cell proliferation, but they limit steroidogenesis via downregulation of StAR, P450c17, and other genes.24. Both TGFβ2 and its receptor kinase TGFβ-R1 are over-expressed in human ACC compared to ACA.145
Two other members of the TGFβ superfamily, activin and inhibin, have been shown to impact adrenocortical growth and tumorigenesis. Activin receptors are expressed in the murine adrenal gland, and activins have been shown to inhibit adrenocortical cell growth and steroid production and to enhance apoptosis of X-zone cells.12,93 Inhibins have no direct effect on steroidogenesis or cell survival but can antagonize activin signaling by competing for activin receptors.146 Differentiation of adrenocortical progenitor cells into corticoid- versus sex steroid-producing cells is regulated by inhibin,107 which serves to limit the expansion of LH-primed GATA4-positive progenitors destined for a gonadal fate.84 Inhibins are produced primarily in gonadal somatic cells. Gonadectomy leads to a profound and rapid fall in circulating inhibin levels, which facilitates differentiation of gonadal-like stroma in cases of gonadectomy-induced adrenocortical neoplasia.
Other growth factors
Two members of the fibroblast growth factor (FGF) family, FGF-1 and FGF-2, are expressed in normal adrenal cortex and are potent mitogens in this tissue. FGF-1 and FGF-2 bind to a family of four FGF receptor tyrosine kinases. Two members of this kinase family FGFR1 and FGFR4 are over-expressed in adrenocortical tumors.156 In pediatric patients expression of FGFR4 is much higher in ACC than ACA.145
Epidermal growth factor receptor (EGFR) overexpression has been demonstrated in over 90% of ACCs.40,76,87,123 Although the ligand EGF is not over-expressed in ACC, the receptor is known to bind TGFα, which is often found in adrenocortical tumors.87 Inhibition of the EGFR signaling pathway inhibits proliferation in the ACC cell line NCI-H295.123 The general EGFR tyrosine kinase inhibitor erlotinib is being studied in combination with other chemotherapies for the treatment of advanced ACC, although preliminary results have been disappointing.123
Future directions
Over the past decade research on adrenocortical neoplasia has been dominated by gene expression profiling of tumor specimens and by analysis of human genetic disorders associated with a predisposition to these tumors. Although these studies have identified gene mutations and signaling pathways that are dysregulated in adrenocortical tumors, the molecular events accounting for the frequent occurrence of benign neoplasms and low rate of malignant transformation remain unknown. We are entering a new era in adrenocortical tumor research. The availability of inbred and engineered mouse models is shifting the research focus to genetic and epigenetic events that impact the growth and differentiation of adrenocortical stem/progenitor cells. Once these events are delineated, it may be possible to design rational therapeutic interventions that are specific for benign or malignant adrenocortical neoplasms.
Table 3.
Genetic changes in benign human adrenocortical neoplasms.
|
Table 4.
Genetic changes in malignant human adrenocortical neoplasms.
|
Acknowledgments
This work was supported by NIH grants DK075618 and DK52574 and by the Sigrid Juselius Foundation and the Finnish Cancer Society.
References
- 1.Achermann JC, Meeks JJ, Jameson JL. Phenotypic spectrum of mutations in DAX-1 and SF-1. Mol Cell Endocrinol. 2001;185:17–25. doi: 10.1016/s0303-7207(01)00619-0. [DOI] [PubMed] [Google Scholar]
- 2.Almeida MQ, Latronico AC. The molecular pathogenesis of childhood adrenocortical tumors. Horm Metab Res. 2007;39:461–466. doi: 10.1055/s-2007-981476. [DOI] [PubMed] [Google Scholar]
- 3.Altman NH, Streett CS, Terner JY. Castration and its relationship to tumors of the adrenal gland in the goat. Am J Vet Res. 1969;30:583–589. [PubMed] [Google Scholar]
- 4.Artandi SE, Attardi LD. Pathways connecting telomeres and p53 in senescence, apoptosis, and cancer. Biochem Biophys Res Commun. 2005;331:881–890. doi: 10.1016/j.bbrc.2005.03.211. [DOI] [PubMed] [Google Scholar]
- 5.Auchus RJ. Overview of dehydroepiandrosterone biosynthesis. Semin Reprod Med. 2004;22:281–288. doi: 10.1055/s-2004-861545. [DOI] [PubMed] [Google Scholar]
- 6.Bailey SM, Murnane JP. Telomeres, chromosome instability and cancer. Nucleic Acids Res. 2006;34:2408–2417. doi: 10.1093/nar/gkl303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barlaskar FM, Hammer GD. The molecular genetics of adrenocortical carcinoma. Rev Endocr Metab Disord. 2007;8:343–348. doi: 10.1007/s11154-007-9057-x. [DOI] [PubMed] [Google Scholar]
- 8.Ben-David S, Zuckerman-Levin N, Epelman M, Shen-Orr Z, Levin M, Sujov P, Hochberg Z. Parturition itself is the basis for fetal adrenal involution. J Clin Endocrinol Metab. 2007;92:93–97. doi: 10.1210/jc.2005-2720. [DOI] [PubMed] [Google Scholar]
- 9.Bernichtein S, Petretto E, Jamieson S, Goel A, Aitman TJ, Mangion JM, Huhtaniemi IT. Adrenal gland tumorigenesis after gonadectomy in mice is a complex genetic trait driven by epistatic loci. Endocrinol. 2007;149:651–661. doi: 10.1210/en.2007-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertherat J, Groussin L, Sandrini F, Matyakhina L, Bei T, Stergiopoulos S, Papageorgiou T, Bourdeau I, Kirschner LS, Vincent-Dejean C, Perlemoine K, Gicquel C, Bertagna X, Stratakis CA. Molecular and functional analysis of PRKAR1A and its locus (17q22-24) in sporadic adrenocortical tumors: 17q losses, somatic mutations, and protein kinase A expression and activity. Cancer Res. 2003;63:5308–5319. [PubMed] [Google Scholar]
- 11.Bertherat J, Mosnier-Pudar H, Bertagna X. Adrenal incidentalomas. Curr Opin Oncol. 2002;14:58–63. doi: 10.1097/00001622-200201000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Beuschlein F, Looyenga BD, Bleasdale SE, Mutch C, Bavers DL, Parlow AF, Nilson JH, Hammer GD. Activin induces x-zone apoptosis that inhibits luteinizing hormone-dependent adrenocortical tumor formation in inhibin-deficient mice. Mol Cell Biol. 2003;23:3951–3964. doi: 10.1128/MCB.23.11.3951-3964.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beuschlein F, Looyenga BD, Reincke M, Hammer GD. Role of the inhibin/activin system and luteinizing hormone in adrenocortical tumorigenesis. Horm Metab Res. 2004;36:392–396. doi: 10.1055/s-2004-814584. [DOI] [PubMed] [Google Scholar]
- 14.Beuschlein F, Mutch C, Bavers DL, Ulrich-Lai YM, Engeland WC, Keegan C, Hammer GD. Steroidogenic factor-1 is essential for compensatory adrenal growth following unilateral adrenalectomy. Endocrinol. 2002;143:3122–3135. doi: 10.1210/endo.143.8.8944. [DOI] [PubMed] [Google Scholar]
- 15.Beuschlein F, Reincke M. Adrenocortical tumorigenesis. Ann N Y Acad Sci. 2006;1088:319–334. doi: 10.1196/annals.1366.001. [DOI] [PubMed] [Google Scholar]
- 16.Beuschlein F, Reincke M, Karl M, Travis WD, Jaursch-Hancke C, Abdelhamid S, Chrousos GP, Allolio B. Clonal composition of human adrenocortical neoplasms. Cancer Res. 1994;54:4927–4932. [PubMed] [Google Scholar]
- 17.Bielinska M, Genova E, Boime I, Parviainen H, Kiiveri S, Leppaluoto J, Rahman N, Heikinheimo M, Wilson DB. Gonadotropin-induced adrenocortical neoplasia in NU/J nude mice. Endocrinol. 2005;146:3975–3984. doi: 10.1210/en.2004-1643. [DOI] [PubMed] [Google Scholar]
- 18.Bielinska M, Kiiveri S, Parviainen H, Mannisto S, Heikinheimo M, Wilson DB. Gonadectomy-induced adrenocortical neoplasia in the domestic ferret (Mustela putorius furo) and laboratory mouse. Vet Pathol. 2006;43:97–117. doi: 10.1354/vp.43-2-97. [DOI] [PubMed] [Google Scholar]
- 19.Blaker H, Sutter C, Kadmon M, Otto HF, Von Knebel-Doeberitz M, Gebert J, Helmke BM. Analysis of somatic APC mutations in rare extracolonic tumors of patients with familial adenomatous polyposis coli. Genes Chromosomes Cancer. 2004;41:93–98. doi: 10.1002/gcc.20071. [DOI] [PubMed] [Google Scholar]
- 20.Blanes A, az-Cano SJ. DNA and kinetic heterogeneity during the clonal evolution of adrenocortical proliferative lesions. Hum Pathol. 2006;37:1295–1303. doi: 10.1016/j.humpath.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 21.Boulle N, Gicquel C, Logie A, Christol R, Feige JJ, Le BY. Fibroblast growth factor-2 inhibits the maturation of pro-insulin-like growth factor-II (Pro-IGF-II) and the expression of insulin-like growth factor binding protein-2 (IGFBP-2) in the human adrenocortical tumor cell line NCI-H295R. Endocrinol. 2000;141:3127–3136. doi: 10.1210/endo.141.9.7632. [DOI] [PubMed] [Google Scholar]
- 22.Boulle N, Logie A, Gicquel C, Perin L, Le BY. Increased levels of insulin-like growth factor II (IGF-II) and IGF-binding protein-2 are associated with malignancy in sporadic adrenocortical tumors. J Clin Endocrinol Metab. 1998;83:1713–1720. doi: 10.1210/jcem.83.5.4816. [DOI] [PubMed] [Google Scholar]
- 23.Bourdeau I, Antonini SR, Lacroix A, Kirschner LS, Matyakhina L, Lorang D, Libutti SK, Stratakis CA. Gene array analysis of macronodular adrenal hyperplasia confirms clinical heterogeneity and identifies several candidate genes as molecular mediators. Oncogene. 2004;23:1575–1585. doi: 10.1038/sj.onc.1207277. [DOI] [PubMed] [Google Scholar]
- 24.Brand C, Cherradi N, Defaye G, Chinn A, Chambaz EM, Feige JJ, Bailly S. Transforming growth factor β1 decreases cholesterol supply to mitochondria via repression of steroidogenic acute regulatory protein expression. J Biol Chem. 1998;273:6410–6416. doi: 10.1074/jbc.273.11.6410. [DOI] [PubMed] [Google Scholar]
- 25.Brunt LM, Moley JF. Adrenal incidentaloma. World J Surg. 2001;25:905–913. doi: 10.1007/s00268-001-0029-0. [DOI] [PubMed] [Google Scholar]
- 26.Caspary T, Cleary MA, Perlman EJ, Zhang P, Elledge SJ, Tilghman SM. Oppositely imprinted genes p57(Kip2) and Igf2 interact in a mouse model for Beckwith-Wiedemann syndrome. Genes Dev. 1999;13:3115–3124. doi: 10.1101/gad.13.23.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen M, Hawks CL, Huang Q, Sun B, Hornsby PJ. Telomerase is not required for experimental tumorigenesis of human and bovine adrenocortical cells. Endocr Res. 2004;30:555–565. doi: 10.1081/erc-200043682. [DOI] [PubMed] [Google Scholar]
- 28.Chen M, Hornsby PJ. Adenovirus-delivered DKK3/WNT4 and Steroidogenesis in Primary Cultures of Adrenocortical Cells. Horm Metab Res. 2006;38:549–555. doi: 10.1055/s-2006-950500. [DOI] [PubMed] [Google Scholar]
- 29.Chida D, Nakagawa S, Nagai S, Sagara H, Katsumata H, Imaki T, Suzuki H, Mitani F, Ogishima T, Shimizu C, Kotaki H, Kakuta S, Sudo K, Koike T, Kubo M, Iwakura Y. Melanocortin 2 receptor is required for adrenal gland development, steroidogenesis, and neonatal gluconeogenesis. Proc Natl Acad Sci U S A. 2007;104:18205–18210. doi: 10.1073/pnas.0706953104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chin L, Artandi SE, Shen Q, Tam A, Lee SL, Gottlieb GJ, Greider CW, DePinho RA. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell. 1999;97:527–538. doi: 10.1016/s0092-8674(00)80762-x. [DOI] [PubMed] [Google Scholar]
- 31.Choi J, Southworth LK, Sarin KY, Venteicher AS, Ma W, Chang W, Cheung P, Jun S, Artandi MK, Shah N, Kim SK, Artandi SE. TERT promotes epithelial proliferation through transcriptional control of a Myc- and Wnt-related developmental program. PLoS Genet. 2008;4:e10. doi: 10.1371/journal.pgen.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen SB, Graham ME, Lovrecz GO, Bache N, Robinson PJ, Reddel RR. Protein composition of catalytically active human telomerase from immortal cells. Science. 2007;315:1850–1853. doi: 10.1126/science.1138596. [DOI] [PubMed] [Google Scholar]
- 33.Coulter CL. Fetal adrenal development: insight gained from adrenal tumors. Trends Endocrinol Metab. 2005;16:235–242. doi: 10.1016/j.tem.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 34.de Fraipont F, El Atifi M, Cherradi N, Le MG, Defaye G, Houlgatte R, Bertherat J, Bertagna X, Plouin PF, Baudin E, Berger F, Gicquel C, Chabre O, Feige JJ. Gene expression profiling of human adrenocortical tumors using complementary deoxyribonucleic acid microarrays identifies several candidate genes as markers of malignancy. J Clin Endocrinol Metab. 2005;90:1819–1829. doi: 10.1210/jc.2004-1075. [DOI] [PubMed] [Google Scholar]
- 35.de Fraipont F, El Atifi M, Gicquel C, Bertagna X, Chambaz EM, Feige JJ. Expression of the angiogenesis markers vascular endothelial growth factor-A, thrombospondin-1, and platelet-derived endothelial cell growth factor in human sporadic adrenocortical tumors: correlation with genotypic alterations. J Clin Endocrinol Metab. 2000;85:4734–4741. doi: 10.1210/jcem.85.12.7012. [DOI] [PubMed] [Google Scholar]
- 36.DeBaun MR, Niemitz EL, McNeil DE, Brandenburg SA, Lee MP, Feinberg AP. Epigenetic alterations of H19 and LIT1 distinguish patients with Beckwith-Wiedemann syndrome with cancer and birth defects. Am J Hum Genet. 2002;70:604–611. doi: 10.1086/338934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeChiara TM, Robertson EJ, Efstratiadis A. Parental imprinting of the mouse insulin-like growth factor II gene. Cell. 1991;64:849–859. doi: 10.1016/0092-8674(91)90513-x. [DOI] [PubMed] [Google Scholar]
- 38.Doghman M, Cazareth J, Lalli E. The Tcf/β-catenin antagonist PKF115-584 inhibits proliferation of adrenocortical carcinoma cells. J Clin Endocrinol Metab. 2008 doi: 10.1210/jc.2008-0247. in press. [DOI] [PubMed] [Google Scholar]
- 39.Doghman M, Karpova T, Rodrigues GA, Arhatte M, De MJ, Cavalli LR, Virolle V, Barbry P, Zambetti GP, Figueiredo BC, Heckert LL, Lalli E. Increased steroidogenic factor-1 dosage triggers adrenocortical cell proliferation and cancer. Mol Endocrinol. 2007;21:2968–2987. doi: 10.1210/me.2007-0120. [DOI] [PubMed] [Google Scholar]
- 40.Edgren M, Eriksson B, Wilander E, Westlin JE, Nilsson S, Oberg K. Biological characteristics of adrenocortical carcinoma: a study of p53, IGF, EGF-r, Ki-67 and PCNA in 17 adrenocortical carcinomas. Anticancer Res. 1997;17:1303–1309. [PubMed] [Google Scholar]
- 41.Else T, Giordano TJ, Hammer GD. Evaluation of telomere length maintenance mechanisms in adrenocortical carcinoma. J Clin Endocrinol Metab. 2008;93:1442–1449. doi: 10.1210/jc.2007-1840. [DOI] [PubMed] [Google Scholar]
- 42.Else T, Hammer GD. Genetic analysis of adrenal absence: agenesis and aplasia. Trends Endocrinol Metab. 2005;16:458–468. doi: 10.1016/j.tem.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Else T, Theisen BK, Wu Y, Hutz JE, Keegan CE, Hammer GD, Ferguson DO. Tpp1/Acd maintains genomic stability through a complex role in telomere protection. Chromosome Res. 2007;15:1001–1013. doi: 10.1007/s10577-007-1175-5. [DOI] [PubMed] [Google Scholar]
- 44.Fassnacht M, Weismann D, Ebert S, Adam P, Zink M, Beuschlein F, Hahner S, Allolio B. AKT is highly phosphorylated in pheochromocytomas but not in benign adrenocortical tumors. J Clin Endocrinol Metab. 2005;90:4366–4370. doi: 10.1210/jc.2004-2198. [DOI] [PubMed] [Google Scholar]
- 45.Feinberg AP. A genetic approach to cancer epigenetics. Cold Spring Harb Symp Quant Biol. 2005;70:335–341. doi: 10.1101/sqb.2005.70.027. [DOI] [PubMed] [Google Scholar]
- 46.Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature. 2007;447:433–440. doi: 10.1038/nature05919. [DOI] [PubMed] [Google Scholar]
- 47.Feinberg AP, Ohlsson R, Henikoff S. The epigenetic progenitor origin of human cancer. Nat Rev Genet. 2006;7:21–33. doi: 10.1038/nrg1748. [DOI] [PubMed] [Google Scholar]
- 48.Fekete E, Woolley G, Little CC. Histological changes following ovariectomy in mice: I. dba high tumor strain. J Exp Med. 1941;74:1–8. doi: 10.1084/jem.74.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feldman EC, Nelson RW. Comparative aspects of Cushing's syndrome in dogs and cats. Endocrinol Metab Clin North Am. 1994;23:671–691. [PubMed] [Google Scholar]
- 50.Figueiredo BC, Cavalli LR, Pianovski MA, Lalli E, Sandrini R, Ribeiro RC, Zambetti G, DeLacerda L, Rodrigues GA, Haddad BR. Amplification of the steroidogenic factor 1 gene in childhood adrenocortical tumors. J Clin Endocrinol Metab. 2005;90:615–619. doi: 10.1210/jc.2004-0942. [DOI] [PubMed] [Google Scholar]
- 51.Figueiredo BC, Sandrini R, Zambetti GP, Pereira RM, Cheng C, Liu W, Lacerda L, Pianovski MA, Michalkiewicz E, Jenkins J, Rodriguez-Galindo C, Mastellaro MJ, Vianna S, Watanabe F, Sandrini F, Arram SB, Boffetta P, Ribeiro RC. Penetrance of adrenocortical tumours associated with the germline TP53 R337H mutation. J Med Genet. 2006;43:91–96. doi: 10.1136/jmg.2004.030551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Figueiredo BC, Stratakis CA, Sandrini R, DeLacerda L, Pianovsky MA, Giatzakis C, Young HM, Haddad BR. Comparative genomic hybridization analysis of adrenocortical tumors of childhood. J Clin Endocrinol Metab. 1999;84:1116–1121. doi: 10.1210/jcem.84.3.5526. [DOI] [PubMed] [Google Scholar]
- 53.Flores I, Cayuela ML, Blasco MA. Effects of telomerase and telomere length on epidermal stem cell behavior. Science. 2005;309:1253–1256. doi: 10.1126/science.1115025. [DOI] [PubMed] [Google Scholar]
- 54.Fottner C, Hoeflich A, Wolf E, Weber MM. Role of the insulin-like growth factor system in adrenocortical growth control and carcinogenesis. Horm Metab Res. 2004;36:397–405. doi: 10.1055/s-2004-814563. [DOI] [PubMed] [Google Scholar]
- 55.Fox JG, Marini RP. Diseases of the Endocrine System. In: Fox JG, editor. Biology and Diseases of the Ferret. Philadelphia: Lea & Febiger; 1998. pp. 291–305. [Google Scholar]
- 56.Gicquel C, Bertagna X, Gaston V, Coste J, Louvel A, Baudin E, Bertherat J, Chapuis Y, Duclos JM, Schlumberger M, Plouin PF, Luton JP, Le BY. Molecular markers and long-term recurrences in a large cohort of patients with sporadic adrenocortical tumors. Cancer Res. 2001;61:6762–6767. [PubMed] [Google Scholar]
- 57.Gicquel C, Leblond-Francillard M, Bertagna X, Louvel A, Chapuis Y, Luton JP, Girard F, Le BY. Clonal analysis of human adrenocortical carcinomas and secreting adenomas. Clin Endocrinol (Oxf) 1994;40:465–477. doi: 10.1111/j.1365-2265.1994.tb02485.x. [DOI] [PubMed] [Google Scholar]
- 58.Goto M, Piper HK, Marcos J, Wood PJ, Wright S, Postle AD, Cameron IT, Mason JI, Wilson DI, Hanley NA. In humans, early cortisol biosynthesis provides a mechanism to safeguard female sexual development. J Clin Invest. 2006;116:953–960. doi: 10.1172/JCI25091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Greider CW. Telomeres. Curr Opin Cell Biol. 1991;3:444–451. doi: 10.1016/0955-0674(91)90072-7. [DOI] [PubMed] [Google Scholar]
- 60.Gummow BM, Winnay JN, Hammer GD. Convergence of Wnt signaling and steroidogenic factor-1 (SF-1) on transcription of the rat inhibin alpha gene. J Biol Chem. 2003;278:26572–26579. doi: 10.1074/jbc.M212677200. [DOI] [PubMed] [Google Scholar]
- 61.Guo W, Mason JS, Stone CG, Jr, Morgan SA, Madu SI, Baldini A, Lindsay EA, Biesecker LG, Copeland KC, Horlick MN. Diagnosis of X-linked adrenal hypoplasia congenita by mutation analysis of the DAX1 gene. JAMA. 1995;274:324–330. [PubMed] [Google Scholar]
- 62.Haluska P, Shaw HM, Batzel GN, Yin D, Molina JR, Molife LR, Yap TA, Roberts ML, Sharma A, Gualberto A, Adjei AA, de Bono JS. Phase I dose escalation study of the anti insulin-like growth factor-I receptor monoclonal antibody CP-751,871 in patients with refractory solid tumors. Clin Cancer Res. 2007;13:5834–5840. doi: 10.1158/1078-0432.CCR-07-1118. [DOI] [PubMed] [Google Scholar]
- 63.Harrington L, Robinson MO. Telomere dysfunction: multiple paths to the same end. Oncogene. 2002;21:592–597. doi: 10.1038/sj.onc.1205084. [DOI] [PubMed] [Google Scholar]
- 64.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 65.Heikkila M, Peltoketo H, Leppaluoto J, Ilves M, Vuolteenaho O, Vainio S. Wnt-4 deficiency alters mouse adrenal cortex function, reducing aldosterone production. Endocrinol. 2002;143:4358–4365. doi: 10.1210/en.2002-220275. [DOI] [PubMed] [Google Scholar]
- 66.Heppner C, Reincke M, Agarwal SK, Mora P, Allolio B, Burns AL, Spiegel AM, Marx SJ. MEN1 gene analysis in sporadic adrenocortical neoplasms. J Clin Endocrinol Metab. 1999;84:216–219. doi: 10.1210/jcem.84.1.5388. [DOI] [PubMed] [Google Scholar]
- 67.Hershkovitz L, Beuschlein F, Klammer S, Krup M, Weinstein Y. Adrenal 20alpha-hydroxysteroid dehydrogenase in the mouse catabolizes progesterone and 11-deoxycorticosterone and is restricted to the X-zone. Endocrinol. 2007;148:976–988. doi: 10.1210/en.2006-1100. [DOI] [PubMed] [Google Scholar]
- 68.Heyerdahl SL, Boikos S, Horvath A, Giatzakis C, Bossis I, Stratakis CA. Protein Kinase A Subunit Expression is Altered in Bloom Syndrome Fibroblasts and the BLM Protein is Increased in Adrenocortical Hyperplasias: Inverse Findings for BLM and PRKAR1A. Horm Metab Res. 2008 doi: 10.1055/s-2008-1058089. [DOI] [PubMed] [Google Scholar]
- 69.Hockemeyer D, Palm W, Else T, Daniels JP, Takai KK, Ye JZ, Keegan CE, de L T, Hammer GD. Telomere protection by mammalian Pot1 requires interaction with Tpp1. Nat Struct Mol Biol. 2007 doi: 10.1038/nsmb1270. [DOI] [PubMed] [Google Scholar]
- 70.Holmes RL. The adrenal glands of the ferret. J Anat. 1961;95:325–336. [PMC free article] [PubMed] [Google Scholar]
- 71.Horvath A, Giatzakis C, Tsang K, Greene E, Osorio P, Boikos S, Libe R, Patronas Y, Robinson-White A, Remmers E, Bertherat J, Nesterova M, Stratakis CA. A cAMP-specific phosphodiesterase (PDE8B) that is mutated in adrenal hyperplasia is expressed widely in human and mouse tissues: a novel PDE8B isoform in human adrenal cortex. Eur J Hum Genet. 2008 doi: 10.1038/ejhg.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Horvath A, Stratakis CA. Unraveling the molecular basis of micronodular adrenal hyperplasia. Curr Opin Endocrinol Diabetes Obes. 2008;15:227–233. doi: 10.1097/MED.0b013e3282fe7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hu MC, Chou SJ, Huang YY, Hsu NC, Li H, Chung BC. Tissue-specific, hormonal, and developmental regulation of SCC-LacZ expression in transgenic mice leads to adrenocortical zone characterization. Endocrinol. 1999;140:5609–5618. doi: 10.1210/endo.140.12.7177. [DOI] [PubMed] [Google Scholar]
- 74.Igaz P, Wiener Z, Szabó P, Falus A, Gaillard RC, Horányi J, Rácz K, Tulassay Z. Functional genomics approaches for the study of sporadic adrenal tumor pathogenesis: clinical implications. J Steroid Biochem Mol Biol. 2006;101:87–96. doi: 10.1016/j.jsbmb.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 75.Jeays-Ward K, Hoyle C, Brennan J, Dandonneau M, Alldus G, Capel B, Swain A. Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development. 2003;130:3663–3670. doi: 10.1242/dev.00591. [DOI] [PubMed] [Google Scholar]
- 76.Kamio T, Shigematsu K, Sou H, Kawai K, Tsuchiyama H. Immunohistochemical expression of epidermal growth factor receptors in human adrenocortical carcinoma. Hum Pathol. 1990;21:277–282. doi: 10.1016/0046-8177(90)90227-v. [DOI] [PubMed] [Google Scholar]
- 77.Kaneda A, Wang CJ, Cheong R, Timp W, Onyango P, Wen B, Iacobuzio-Donahue CA, Ohlsson R, Andraos R, Pearson MA, Sharov AA, Longo DL, Ko MS, Levchenko A, Feinberg AP. Enhanced sensitivity to IGF-II signaling links loss of imprinting of IGF2 to increased cell proliferation and tumor risk. Proc Natl Acad Sci U S A. 2007;104:20926–20931. doi: 10.1073/pnas.0710359105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kawabe K, Shikayama T, Tsuboi H, Oka S, Oba K, Yanase T, Nawata H, Morohashi K. Dax-1 as one of the target genes of Ad4BP/SF-1. Mol Endocrinol. 1999;13:1267–1284. doi: 10.1210/mend.13.8.0325. [DOI] [PubMed] [Google Scholar]
- 79.Keegan CE, Hammer GD. Recent insights into organogenesis of the adrenal cortex. Trends Endocrinol Metab. 2002;13:200–208. doi: 10.1016/s1043-2760(02)00602-1. [DOI] [PubMed] [Google Scholar]
- 80.Keegan CE, Hutz JE, Else T, Adamska M, Shah SP, Kent AE, Howes JM, Beamer WG, Hammer GD. Urogenital and caudal dysgenesis in adrenocortical dysplasia (acd) mice is caused by a splicing mutation in a novel telomeric regulator. Hum Mol Genet. 2005;14:113–123. doi: 10.1093/hmg/ddi011. [DOI] [PubMed] [Google Scholar]
- 81.Keeney DS, Jenkins CM, Waterman MR. Developmentally regulated expression of adrenal 17α-hydroxylase cytochrome P450 in the mouse embryo. Endocrinol. 1995;136:4872–4879. doi: 10.1210/endo.136.11.7588219. [DOI] [PubMed] [Google Scholar]
- 82.Kempná P, Flück CE. Adrenal gland development and defects. Best Pract Res Clin Endocrinol Metab. 2008;22:77–93. doi: 10.1016/j.beem.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 83.Keyes PH. Adreno-cortical changes in Syrian hamsters following gonadectomy. Endocrinol. 1949;44:274–277. doi: 10.1210/endo-44-3-274. [DOI] [PubMed] [Google Scholar]
- 84.Kim AC, Hammer GD. Adrenocortical cells with stem/progenitor cell properties: recent advances. Mol Cell Endocrinol. 2007;265-266:10–16. doi: 10.1016/j.mce.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim AC, Reuter AL, Zubair M, Else T, Serecky K, Bingham NC, Lavery GG, Parker KL, Hammer GD. Targeted disruption of β-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Development. 2008 doi: 10.1242/dev.021493. in press. [DOI] [PubMed] [Google Scholar]
- 86.Kirschner LS. Signaling pathways in adrenocortical cancer. Ann N Y Acad Sci. 2002;968:222–239. doi: 10.1111/j.1749-6632.2002.tb04338.x. [DOI] [PubMed] [Google Scholar]
- 87.Kirschner LS. Emerging treatment strategies for adrenocortical carcinoma: a new hope. J Clin Endocrinol Metab. 2006;91:14–21. doi: 10.1210/jc.2005-1739. [DOI] [PubMed] [Google Scholar]
- 88.Kjellman M, Kallioniemi OP, Karhu R, Hoog A, Farnebo LO, Auer G, Larsson C, Backdahl M. Genetic aberrations in adrenocortical tumors detected using comparative genomic hybridization correlate with tumor size and malignancy. Cancer Res. 1996;56:4219–4223. [PubMed] [Google Scholar]
- 89.Kjellman M, Larsson C, Backdahl M. Genetic background of adrenocortical tumor development. World J Surg. 2001;25:948–956. doi: 10.1007/s00268-001-0034-3. [DOI] [PubMed] [Google Scholar]
- 90.Kjellman M, Roshani L, Teh BT, Kallioniemi OP, Hoog A, Gray S, Farnebo LO, Holst M, Backdahl M, Larsson C. Genotyping of adrenocortical tumors: very frequent deletions of the MEN1 locus in 11q13 and of a 1-centimorgan region in 2p16. J Clin Endocrinol Metab. 1999;84:730–735. doi: 10.1210/jcem.84.2.5506. [DOI] [PubMed] [Google Scholar]
- 91.Kleihues P, Schauble B, zur H A, Esteve J, Ohgaki H. Tumors associated with p53 germline mutations: a synopsis of 91 families. Am J Pathol. 1997;150:1–13. [PMC free article] [PubMed] [Google Scholar]
- 92.Koch CA, Pacak K, Chrousos GP. The molecular pathogenesis of hereditary and sporadic adrenocortical and adrenomedullary tumors. J Clin Endocrinol Metab. 2002;87:5367–5384. doi: 10.1210/jc.2002-021069. [DOI] [PubMed] [Google Scholar]
- 93.Kumar TR, Donehower LA, Bradley A, Matzuk MM. Transgenic mouse models for tumour-suppressor genes. J Intern Med. 1995;238:233–238. doi: 10.1111/j.1365-2796.1995.tb00928.x. [DOI] [PubMed] [Google Scholar]
- 94.Lacroix A, Bourdeau I. Bilateral adrenal Cushing's syndrome: macronodular adrenal hyperplasia and primary pigmented nodular adrenocortical disease. Endocrinol Metab Clin North Am. 2005;34:441–58. doi: 10.1016/j.ecl.2005.01.004. x. [DOI] [PubMed] [Google Scholar]
- 95.Lalli E, Melner MH, Stocco DM, Sassone-Corsi P. DAX-1 blocks steroid production at multiple levels. Endocrinol. 1998;139:4237–4243. doi: 10.1210/endo.139.10.6217. [DOI] [PubMed] [Google Scholar]
- 96.Leboulleux S, Gaston V, Boulle N, Le BY, Gicquel C. Loss of heterozygosity at the mannose 6-phosphate/insulin-like growth factor 2 receptor locus: a frequent but late event in adrenocortical tumorigenesis. Eur J Endocrinol. 2001;144:163–168. doi: 10.1530/eje.0.1440163. [DOI] [PubMed] [Google Scholar]
- 97.Lehtonen HJ, Kiuru M, Ylisaukko-Oja SK, Salovaara R, Herva R, Koivisto PA, Vierimaa O, Aittomäki K, Pukkala E, Launonen V, Aaltonen LA. Increased risk of cancer in patients with fumarate hydratase germline mutation. J Med Genet. 2006;43:523–526. doi: 10.1136/jmg.2005.036400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lemos MC, Thakker RV. Multiple endocrine neoplasia type 1 (MEN1): analysis of 1336 mutations reported in the first decade following identification of the gene. Hum Mutat. 2008;29:22–32. doi: 10.1002/humu.20605. [DOI] [PubMed] [Google Scholar]
- 99.Levine AJ, Feng Z, Mak TW, You H, Jin S. Coordination and communication between the p53 and IGF-1-AKT-TOR signal transduction pathways. Genes Dev. 2006;20:267–275. doi: 10.1101/gad.1363206. [DOI] [PubMed] [Google Scholar]
- 100.Li T, Vu TH, Ulaner GA, Littman E, Ling JQ, Chen HL, Hu JF, Behr B, Giudice L, Hoffman AR. IVF results in de novo DNA methylation and histone methylation at an Igf2-H19 imprinting epigenetic switch. Mol Hum Reprod. 2005;11:631–640. doi: 10.1093/molehr/gah230. [DOI] [PubMed] [Google Scholar]
- 101.Libé R, Bertherat J. Molecular genetics of adrenocortical tumours, from familial to sporadic diseases. Eur J Endocrinol. 2005;153:477–487. doi: 10.1530/eje.1.02004. [DOI] [PubMed] [Google Scholar]
- 102.Libé R, Fratticci A, Bertherat J. Adrenocortical cancer: pathophysiology and clinical management. Endocr Relat Cancer. 2007;14:13–28. doi: 10.1677/erc.1.01130. [DOI] [PubMed] [Google Scholar]
- 103.Libé R, Groussin L, Tissier F, Elie C, Rene-Corail F, Fratticci A, Jullian E, Beck-Peccoz P, Bertagna X, Gicquel C, Bertherat J. Somatic TP53 mutations are relatively rare among adrenocortical cancers with the frequent 17p13 loss of heterozygosity. Clin Cancer Res. 2007;13:844–850. doi: 10.1158/1078-0432.CCR-06-2085. [DOI] [PubMed] [Google Scholar]
- 104.Lichtenauer UD, Shapiro I, Geiger K, Quinkler M, Fassnacht M, Nitschke R, Ruckauer KD, Beuschlein F. Side population does not define stem cell-like cancer cells in the adrenocortical carcinoma cell line NCI h295R. Endocrinol. 2008;149:1314–1322. doi: 10.1210/en.2007-1001. [DOI] [PubMed] [Google Scholar]
- 105.Lin Y, Liu A, Zhang S, Ruusunen T, Kreidberg JA, Peltoketo H, Drummond I, Vainio S. Induction of ureter branching as a response to Wnt-2b signaling during early kidney organogenesis. Dev Dyn. 2001;222:26–39. doi: 10.1002/dvdy.1164. [DOI] [PubMed] [Google Scholar]
- 106.Loffler KA, Biondi CA, Gartside M, Waring P, Stark M, Serewko-Auret MM, Muller HK, Hayward NK, Kay GF. Broad tumor spectrum in a mouse model of multiple endocrine neoplasia type 1. Int J Cancer. 2007;120:259–267. doi: 10.1002/ijc.22288. [DOI] [PubMed] [Google Scholar]
- 107.Looyenga BD, Hammer GD. Origin and Identity of Adrenocortical Tumors in Inhibin Knockout Mice: Implications for Cellular Plasticity in the Adrenal Cortex. Mol Endocrinol. 2006 doi: 10.1210/me.2006-0182. [DOI] [PubMed] [Google Scholar]
- 108.Mandel H, Shemer R, Borochowitz ZU, Okopnik M, Knopf C, Indelman M, Drugan A, Tiosano D, Gershoni-Baruch R, Choder M, Sprecher E. SERKAL syndrome: an autosomal-recessive disorder caused by a loss-of-function mutation in WNT4. Am J Hum Genet. 2008;82:39–47. doi: 10.1016/j.ajhg.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Matyakhina L, Freedman RJ, Bourdeau I, Wei MH, Stergiopoulos SG, Chidakel A, Walther M, bu-Asab M, Tsokos M, Keil M, Toro J, Linehan WM, Stratakis CA. Hereditary leiomyomatosis associated with bilateral, massive, macronodular adrenocortical disease and atypical cushing syndrome: a clinical and molecular genetic investigation. J Clin Endocrinol Metab. 2005;90:3773–3779. doi: 10.1210/jc.2004-2377. [DOI] [PubMed] [Google Scholar]
- 110.Mazzuco TL, Chabre O, Feige JJ, Thomas M. Aberrant GPCR expression is a sufficient genetic event to trigger adrenocortical tumorigenesis. Mol Cell Endocrinol. 2007;265-266:23–28. doi: 10.1016/j.mce.2006.12.034. [DOI] [PubMed] [Google Scholar]
- 111.Mazzuco TL, Chabre O, Feige JJ, Thomas M. Aberrant expression of human luteinizing hormone receptor by adrenocortical cells is sufficient to provoke both hyperplasia and Cushing's syndrome features. J Clin Endocrinol Metab. 2006;91:196–203. doi: 10.1210/jc.2005-1975. [DOI] [PubMed] [Google Scholar]
- 112.Mazzuco TL, Chabre O, Sturm N, Feige JJ, Thomas M. Ectopic expression of the gastric inhibitory polypeptide receptor gene is a sufficient genetic event to induce benign adrenocortical tumor in a xenotransplantation model. Endocrinol. 2006;147:782–790. doi: 10.1210/en.2005-0921. [DOI] [PubMed] [Google Scholar]
- 113.Morley SD, Viard I, Chung BC, Ikeda Y, Parker KL, Mullins JJ. Variegated expression of a mouse steroid 21-hydroxylase/β-galactosidase transgene suggests centripetal migration of adrenocortical cells. Mol Endocrinol. 1996;10:585–598. doi: 10.1210/mend.10.5.8732689. [DOI] [PubMed] [Google Scholar]
- 114.Mould AW, Duncan R, Serewko-Auret M, Loffler KA, Biondi C, Gartside M, Kay GF, Hayward NK. Global expression profiling of murine MEN1-associated tumors reveals a regulatory role for menin in transcription, cell cycle and chromatin remodelling. Int J Cancer. 2007;121:776–783. doi: 10.1002/ijc.22734. [DOI] [PubMed] [Google Scholar]
- 115.Murthy AS, Russfield AB. Evidence for three types of benign adrenal tumors in Syrian hamsters. Arch Pathol. 1966;81:140–145. [PubMed] [Google Scholar]
- 116.Myers NC., III Adrenal incidentalomas. Diagnostic workup of the incidentally discovered adrenal mass. Vet Clin North Am Small Anim Pract. 1997;27:381–399. doi: 10.1016/s0195-5616(97)50038-6. [DOI] [PubMed] [Google Scholar]
- 117.O'Hagan RC, Chang S, Maser RS, Mohan R, Artandi SE, Chin L, DePinho RA. Telomere dysfunction provokes regional amplification and deletion in cancer genomes. Cancer Cell. 2002;2:149–155. doi: 10.1016/s1535-6108(02)00094-6. [DOI] [PubMed] [Google Scholar]
- 118.Parviainen H, Kiiveri S, Bielinska M, Rahman N, Huhtaniemi IT, Wilson DB, Heikinheimo M. GATA transcription factors in adrenal development and tumors. Mol Cell Endocrinol. 2007;265-266:17–22. doi: 10.1016/j.mce.2006.12.033. [DOI] [PubMed] [Google Scholar]
- 119.Perrault SD, Hornsby PJ, Betts DH. Global gene expression response to telomerase in bovine adrenocortical cells. Biochem Biophys Res Commun. 2005;335:925–936. doi: 10.1016/j.bbrc.2005.07.156. [DOI] [PubMed] [Google Scholar]
- 120.Pianovski MA, Cavalli LR, Figueiredo BC, Santos SC, Doghman M, Ribeiro RC, Oliveira AG, Michalkiewicz E, Rodrigues GA, Zambetti G, Haddad BR, Lalli E. SF-1 overexpression in childhood adrenocortical tumours. Eur J Cancer. 2006;42:1040–1043. doi: 10.1016/j.ejca.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 121.Pinto EM, Billerbeck AE, Fragoso MC, Mendonca BB, Latronico AC. Deletion mapping of chromosome 17 in benign and malignant adrenocortical tumors associated with the Arg337His mutation of the p53 tumor suppressor protein. J Clin Endocrinol Metab. 2005;90:2976–2981. doi: 10.1210/jc.2004-0963. [DOI] [PubMed] [Google Scholar]
- 122.Prunier C, Hocevar BA, Howe PH. Wnt signaling: physiology and pathology. Growth Fact. 2004;22:141–150. doi: 10.1080/08977190410001720860. [DOI] [PubMed] [Google Scholar]
- 123.Quinkler M, Hahner S, Wortmann S, Johanssen S, Adam P, Ritter C, Strasburger C, Allolio B, Fassnacht M. Treatment of advanced adrenocortical carcinoma with erlotinib plus gemcitabine. J Clin Endocrinol Metab. 2008;93:2057–2062. doi: 10.1210/jc.2007-2564. [DOI] [PubMed] [Google Scholar]
- 124.Rainey WE, Rehman KS, Carr BR. The human fetal adrenal: making adrenal androgens for placental estrogens. Semin Reprod Med. 2004;22:327–336. doi: 10.1055/s-2004-861549. [DOI] [PubMed] [Google Scholar]
- 125.Ribeiro RC, Rodriguez-Galindo C, Figueiredo BC, Mastellaro MJ, West AN, Kriwacki R, Zambetti GP. Germline TP53 R337H mutation is not sufficient to establish Li-Fraumeni or Li-Fraumeni-like syndrome. Cancer Lett. 2007;247:353–355. doi: 10.1016/j.canlet.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 126.Rivera RM, Stein P, Weaver JR, Mager J, Schultz RM, Bartolomei MS. Manipulations of mouse embryos prior to implantation result in aberrant expression of imprinted genes on day 9.5 of development. Hum Mol Genet. 2008;17:1–14. doi: 10.1093/hmg/ddm280. [DOI] [PubMed] [Google Scholar]
- 127.Romero DG, Rilli S, Plonczynski MW, Yanes LL, Zhou MY, Gomez-Sanchez EP, Gomez-Sanchez CE. Adrenal transcription regulatory genes modulated by angiotensin II and their role in steroidogenesis. Physiol Genomics. 2007;30:26–34. doi: 10.1152/physiolgenomics.00187.2006. [DOI] [PubMed] [Google Scholar]
- 128.Schulte DM, Shapiro I, Reincke M, Beuschlein F. Expression and spatio-temporal distribution of differentiation and proliferation markers during mouse adrenal development. Gene Expr Patterns. 2007;7:72–81. doi: 10.1016/j.modgep.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 129.Schulte KM, Mengel M, Heinze M, Simon D, Scheuring S, Kohrer K, Roher HD. Complete sequencing and messenger ribonucleic acid expression analysis of the MEN I gene in adrenal cancer. J Clin Endocrinol Metab. 2000;85:441–448. doi: 10.1210/jcem.85.1.6312. [DOI] [PubMed] [Google Scholar]
- 130.Shi Y, Massague J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 131.Sidhu S, Marsh DJ, Theodosopoulos G, Philips J, Bambach CP, Campbell P, Magarey CJ, Russell CF, Schulte KM, Roher HD, Delbridge L, Robinson BG. Comparative genomic hybridization analysis of adrenocortical tumors. J Clin Endocrinol Metab. 2002;87:3467–3474. doi: 10.1210/jcem.87.7.8697. [DOI] [PubMed] [Google Scholar]
- 132.Simone-Freilicher E. Adrenal gland disease in ferrets. Vet Clin North Am Exot Anim Pract. 2008;11:125–137. doi: 10.1016/j.cvex.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 133.Sinclair KD, Young LE, Wilmut I, McEvoy TG. In-utero overgrowth in ruminants following embryo culture: lessons from mice and a warning to men. Hum Reprod. 2000;15 5:68–86. doi: 10.1093/humrep/15.suppl_5.68. [DOI] [PubMed] [Google Scholar]
- 134.Soon PS, Libe R, Benn DE, Gill A, Shaw J, Sywak MS, Groussin L, Bertagna X, Gicquel C, Bertherat J, McDonald KL, Sidhu SB, Robinson BG. Loss of heterozygosity of 17p13, with possible involvement of ACADVL and ALOX15B, in the pathogenesis of adrenocortical tumors. Ann Surg. 2008;247:157–164. doi: 10.1097/SLA.0b013e318153ff55. [DOI] [PubMed] [Google Scholar]
- 135.Soussi T, Wiman KG. Shaping genetic alterations in human cancer: the p53 mutation paradigm. Cancer Cell. 2007;12:303–312. doi: 10.1016/j.ccr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 136.Stratakis CA. Adrenocortical tumors, primary pigmented adrenocortical disease (PPNAD)/Carney complex, and other bilateral hyperplasias: the NIH studies. Horm Metab Res. 2007;39:467–473. doi: 10.1055/s-2007-981477. [DOI] [PubMed] [Google Scholar]
- 137.Sun B, Huang Q, Liu S, Chen M, Hawks CL, Wang L, Zhang C, Hornsby PJ. Progressive loss of malignant behavior in telomerase-negative tumorigenic adrenocortical cells and restoration of tumorigenicity by human telomerase reverse transcriptase. Cancer Res. 2004;64:6144–6151. doi: 10.1158/0008-5472.CAN-04-1376. [DOI] [PubMed] [Google Scholar]
- 138.Swords FM, Baig A, Malchoff DM, Malchoff CD, Thorner MO, King PJ, Hunyady L, Clark AJ. Impaired desensitization of a mutant adrenocorticotropin receptor associated with apparent constitutive activity. Mol Endocrinol. 2002;16:2746–2753. doi: 10.1210/me.2002-0099. [DOI] [PubMed] [Google Scholar]
- 139.Tadjine M, Lampron A, Ouadi L, Bourdeau I. Frequent mutations of beta-catenin gene in sporadic secreting adrenocortical adenomas. Clin Endocrinol (Oxf) 2008;68:264–270. doi: 10.1111/j.1365-2265.2007.03033.x. [DOI] [PubMed] [Google Scholar]
- 140.Tadjine M, Lampron A, Ouadi L, Horvath A, Stratakis CA, Bourdeau I. Detection of somatic beta-catenin mutations in primary pigmented nodular adrenocortical disease. Clin Endocrinol (Oxf) 2008 doi: 10.1111/j.1365-2265.2008.03273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 142.Tissier F, Cavard C, Groussin L, Perlemoine K, Fumey G, Hagnere AM, Rene-Corail F, Jullian E, Gicquel C, Bertagna X, Vacher-Lavenu MC, Perret C, Bertherat J. Mutations of beta-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res. 2005;65:7622–7627. doi: 10.1158/0008-5472.CAN-05-0593. [DOI] [PubMed] [Google Scholar]
- 143.Tosh D, Slack JM. How cells change their phenotype. Nat Rev Mol Cell Biol. 2002;3:187–194. doi: 10.1038/nrm761. [DOI] [PubMed] [Google Scholar]
- 144.Val P, Martinez-Barbera JP, Swain A. Adrenal development is initiated by Cited2 and Wt1 through modulation of Sf-1 dosage. Development. 2007;134:2349–2358. doi: 10.1242/dev.004390. [DOI] [PubMed] [Google Scholar]
- 145.van Nederveen FH, de Krijger RR. Precursor lesions of the adrenal gland. Pathobiology. 2007;74:285–290. doi: 10.1159/000105811. [DOI] [PubMed] [Google Scholar]
- 146.Vanttinen T, Liu J, Kuulasmaa T, Kivinen P, Voutilainen R. Expression of activin/inhibin signaling components in the human adrenal gland and the effects of activins and inhibins on adrenocortical steroidogenesis and apoptosis. J Endocrinol. 2003;178:479–489. doi: 10.1677/joe.0.1780479. [DOI] [PubMed] [Google Scholar]
- 147.Venteicher AS, Meng Z, Mason PJ, Veenstra TD, Artandi SE. Identification of ATPases pontin and reptin as telomerase components essential for holoenzyme assembly. Cell. 2008;132:945–957. doi: 10.1016/j.cell.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Verdun RE, Karlseder J. Replication and protection of telomeres. Nature. 2007;447:924–931. doi: 10.1038/nature05976. [DOI] [PubMed] [Google Scholar]
- 149.Viger RS, Guittot SM, Anttonen M, Wilson DB, Heikinheimo M. Role of the GATA Family of Transcription Factors in Endocrine Development, Function, and Disease. Mol Endocrinol. 2008;22:781–798. doi: 10.1210/me.2007-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Volante M, Buttigliero C, Greco E, Berruti A, Papotti M. Pathological and molecular features of adrenocortical carcinoma: an update. J Clin Pathol. 2008 doi: 10.1136/jcp.2007.050625. in press. [DOI] [PubMed] [Google Scholar]
- 151.Vucic EA, Brown CJ, Lam WL. Epigenetics of cancer progression. Pharmacogenomics. 2008;9:215–234. doi: 10.2217/14622416.9.2.215. [DOI] [PubMed] [Google Scholar]
- 152.Vuorenoja S, Rivero-Muller A, Kiiveri S, Bielinska M, Heikinheimo M, Wilson DB, Huhtaniemi IT, Rahman NA. Adrenocortical tumorigenesis, luteinizing hormone receptor and transcription factors GATA-4 and GATA-6. Mol Cell Endocrinol. 2007;269:38–45. doi: 10.1016/j.mce.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 153.Wagner S, Kiupel M, Peterson RA, Heikinheimo M, Wilson DB. Cytochrome b5 expression in gonadectomy-induced adrenocortical neoplasms of the domestic ferret (Mustela putorius furo) Vet Pathol. 2008;45:439–442. doi: 10.1354/vp.45-4-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Weber MM, Auernhammer CJ, Kiess W, Engelhardt D. Insulin-like growth factor receptors in normal and tumorous adult human adrenocortical glands. Eur J Endocrinol. 1997;136:296–303. doi: 10.1530/eje.0.1360296. [DOI] [PubMed] [Google Scholar]
- 155.Weber MM, Fottner C, Wolf E. The role of the insulin-like growth factor system in adrenocortical tumourigenesis. Eur J Clin Invest. 2000;30 3:69–75. doi: 10.1046/j.1365-2362.2000.0300s3069.x. [DOI] [PubMed] [Google Scholar]
- 156.West AN, Neale GA, Pounds S, Figueredo BC, Rodriguez GC, Pianovski MA, Oliveira Filho AG, Malkin D, Lalli E, Ribeiro R, Zambetti GP. Gene expression profiling of childhood adrenocortical tumors. Cancer Res. 2007;67:600–608. doi: 10.1158/0008-5472.CAN-06-3767. [DOI] [PubMed] [Google Scholar]
- 157.Wright BJ, Conner GH. Adrenal neoplasms in slaughtered cattle. Cancer Res. 1968;28:251–263. [PubMed] [Google Scholar]
- 158.Young KM, Walker SL, Lanthier C, Waddell WT, Monfort SL, Brown JL. Noninvasive monitoring of adrenocortical activity in carnivores by fecal glucocorticoid analyses. Gen Comp Endocrinol. 2004;137:148–165. doi: 10.1016/j.ygcen.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 159.Young LE, Fernandes K, McEvoy TG, Butterwith SC, Gutierrez CG, Carolan C, Broadbent PJ, Robinson JJ, Wilmut I, Sinclair KD. Epigenetic change in IGF2R is associated with fetal overgrowth after sheep embryo culture. Nat Genet. 2001;27:153–154. doi: 10.1038/84769. [DOI] [PubMed] [Google Scholar]
- 160.Young LE, Sinclair KD, Wilmut I. Large offspring syndrome in cattle and sheep. Rev Reprod. 1998;3:155–163. doi: 10.1530/ror.0.0030155. [DOI] [PubMed] [Google Scholar]
- 161.Zambetti GP. The p53 mutation “gradient effect” and its clinical implications. J Cell Physiol. 2007;213:370–373. doi: 10.1002/jcp.21217. [DOI] [PubMed] [Google Scholar]
- 162.Zazopoulos E, Lalli E, Stocco DM, Sassone-Corsi P. DNA binding and transcriptional repression by DAX-1 blocks steroidogenesis. Nature. 1997;390:311–315. doi: 10.1038/36899. [DOI] [PubMed] [Google Scholar]
- 163.Zhao J, Speel EJ, Muletta-Feurer S, Rutimann K, Saremaslani P, Roth J, Heitz PU, Komminoth P. Analysis of genomic alterations in sporadic adrenocortical lesions. Gain of chromosome 17 is an early event in adrenocortical tumorigenesis. Am J Pathol. 1999;155:1039–1045. doi: 10.1016/S0002-9440(10)65205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhao L, Bakke M, Hanley NA, Majdic G, Stallings NR, Jeyasuria P, Parker KL. Tissue-specific knockouts of steroidogenic factor 1. Mol Cell Endocrinol. 2004;215:89–94. doi: 10.1016/j.mce.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 165.Zubair M, Ishihara S, Oka S, Okumura K, Morohashi K. Two-step regulation of Ad4BP/SF-1 gene transcription during fetal adrenal development: initiation by a Hox-Pbx1-Prep1 complex and maintenance via autoregulation by Ad4BP/SF-1. Mol Cell Biol. 2006;26:4111–4121. doi: 10.1128/MCB.00222-06. [DOI] [PMC free article] [PubMed] [Google Scholar]