SUMMARY
Copper is a cofactor for many cellular enzymes and transporters1. To load onto secreted and endomembrane cuproproteins, copper is translocated from the cytosol into membrane-bound organelles by ATP7A or ATP7B transporters, the genes for which are mutated in the copper imbalance syndromes, Menkes and Wilson disease, respectively2. Endomembrane cuproproteins are thought to stably incorporate copper upon transit through the trans Golgi network (TGN), within which ATP7A3 accumulates by dynamic cycling through early endocytic compartments4. Here we show that the pigment cell-specific cuproenzyme tyrosinase acquires copper only transiently and inefficiently within the TGN of melanocytes. To catalyze melanin synthesis, tyrosinase is subsequently reloaded with copper within specialized organelles called melanosomes. Copper is supplied to melanosomes by ATP7A, a cohort of which localizes to melanosomes in a Biogenesis of Lysosome-related Organelles Complex-1 (BLOC-1)-dependent manner. These results indicate that cell type-specific localization of a metal transporter is required to sustain metallation of an endomembrane cuproenzyme, providing a mechanism for exquisite spatial control of metalloenzyme activity. Moreover, as BLOC-1 subunits are mutated in subtypes of the genetic disease, Hermansky-Pudlak syndrome (HPS), these results also show that defects in copper transporter localization contribute to hypopigmentation, and hence perhaps other systemic defects, in HPS.
Copper is essential for all cells, but is particularly important for vertebrate pigmentation as a cofactor for tyrosinase5. Tyrosinase is expressed in epidermal melanocytes and ocular pigment cells, and catalyzes the initial steps of melanin biosynthesis within melanosomes6. Mutations in either of two copper binding sites in tyrosinase ablate enzymatic activity and result in oculocutaneous albinism7. Melanin synthesis is limited to melanosomes, but tyrosinase is thought to acquire copper within the TGN as it traverses the secretory pathway toward melanosomes, since copper-dependent tyrosinase enzyme activity is first detected in the TGN8 and in nearby clathrin-coated vesicles9. Copper is supplied to tyrosinase by ATP7A10; accordingly ATP7A-deficient mice are severely hypopigmented11,12.
Hypopigmentation is one feature of HPS13, a multisystem disorder of subcellular organelle formation. HPS and corresponding mouse models result from mutations in any of 15 genes, most of which encode subunits of cytoplasmic complexes that regulate membrane trafficking. Among them, mutations in components of the eight-subunit BLOC-1 cause the most severe hypopigmentation14 and a nearly complete absence of pigment in melanocytes15. Several melanosomal proteins, including tyrosinase-related protein-1 (Tyrp1), are dramatically missorted and excluded from melanosome precursors in BLOC-1-deficient (BLOC-1−) cells15, but tyrosinase is only partially missorted such that a significant cohort properly localizes to non-pigmented melanosome precursors15. We hypothesized that these organelles lack pigment because the tyrosinase within them is inactive, perhaps due to lack of its essential cofactor, copper. We thus assessed copper transporter localization in melanocytes and its dependence on BLOC-1.
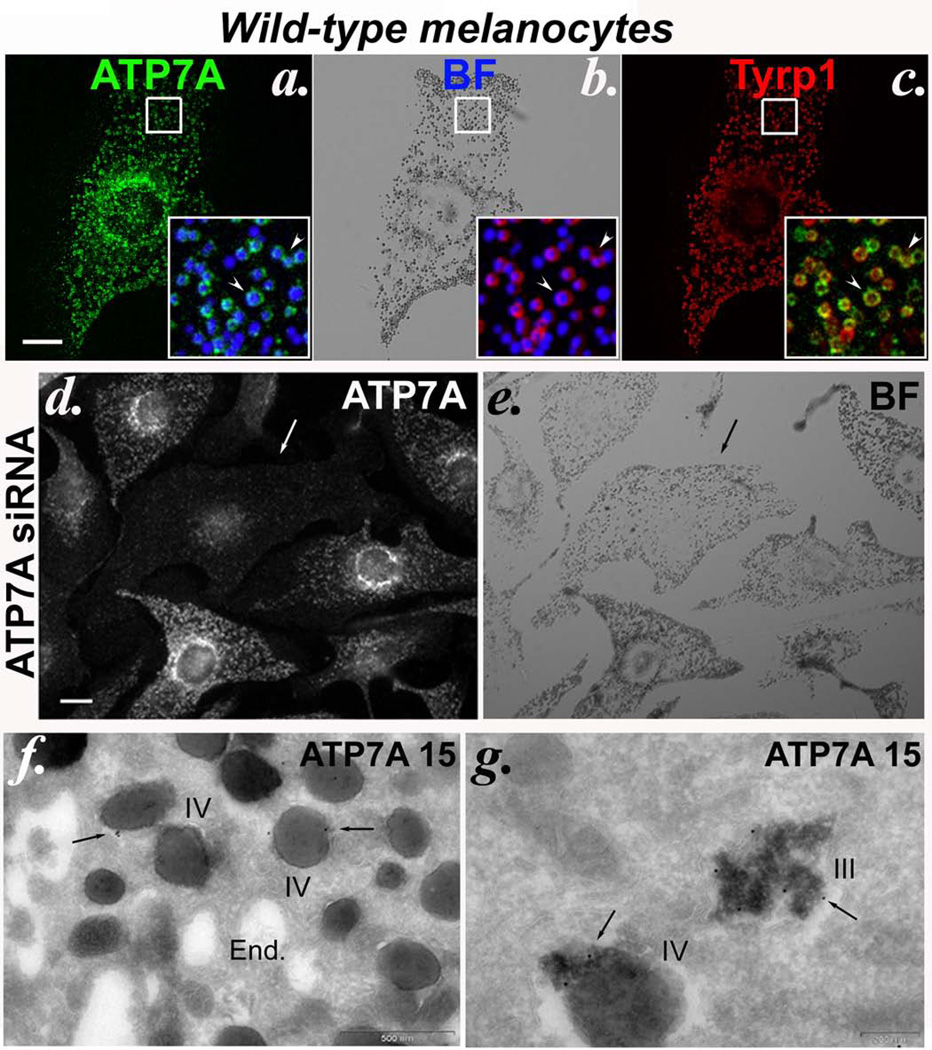
By immunofluorescence microscopy (IFM) analysis of wild-type pigmented mouse melanocyte cell lines, ATP7A was detected not only in the perinuclear/ TGN area and in punctate structures characteristic of endosomes as in most other cell types, but also in ~98% of pigmented cells within mature melanosomes, apparent as “donuts” filled with dark melanin (Figure 1a). These ATP7A-containing structures colocalized with pigment granules detected by bright field microscopy (Figure 1a inset), and with the melanosome membrane protein Tyrp1 (Figure 1c inset). ATP7B was not detected in these cells by IFM (data not shown). The signal obtained with anti-ATP7A antibody was specific, because it was significantly diminished (in an average of 28–39% of cells) upon depletion of endogenous ATP7A following transduction with either of several different ATP7A-specific siRNAs or shRNA expression constructs (Figure 1d and Supplementary Figure S1). Moreover, the signal did not reflect non-specific adherence to melanin, since many of the ATP7A-depleted, antibody-non-reactive cells retained pigment (likely synthesized prior to ATP7A depletion; Figure 1d, e and Supplementary Figure S1). Immunoelectron microscopy (IEM) analysis confirmed that ATP7A localizes not only to Golgi/TGN membranes (see below) but also to the limiting and intralumenal membranes of pigmented melanosomes (Figure 1f, g). This intramelanosomal distribution resembled that of tyrosinase16. ATP7A localization to melanosomes was further verified by subcellular fractionation (see below), consistent with a proteomic analysis of melanosome-enriched subcellular fractions of human MNT-1 melanoma cells17. Together, these results establish that a cohort of ATP7A in wild-type pigmented melanocytes localizes to melanosomes.
Figure 1. Copper transporter ATP7A localizes to melanosomes in wild-type melanocytes.
(a–c) Fixed melan-a (wild-type) cells were analyzed by IFM with antibodies to ATP7A and Tyrp1. The corresponding bright field (BF) image shows pigmented melanosomes. Insets, 3X-magified overlays of the boxed regions comparing ATP7A to melanosomes (pseudocolored blue from an inverted BF image, left), melanosomes to Tyrp1 (middle) and ATP7A to Tyrp1 (right). Arrowheads, colocalized ATP7A, melanosomes and Tyrp1. Bar, 10 µm. (d, e) Wild-type melan-Ink4a melanocytes were transduced with siRNA to ATP7A (ATP7A-3 siRNA) and analyzed 48 h later by IFM for ATP7A and BF microscopy. Arrow, a cell in which labeling for ATP7A on pigment granules is lost. Bar, 10 µm. (f, g) Ultrathin cryosections of wild-type melan-a cells were immunogold labeled with anti-ATP7A antibody and analyzed by electron microscopy. Arrows indicate labeling of stage III and IV melanosomes (III and IV), predominantly on the internal membranes within them. See also Supplementary Figure S4. End., endosomes. Bars, 500 nm (f) and 200 nm (g).
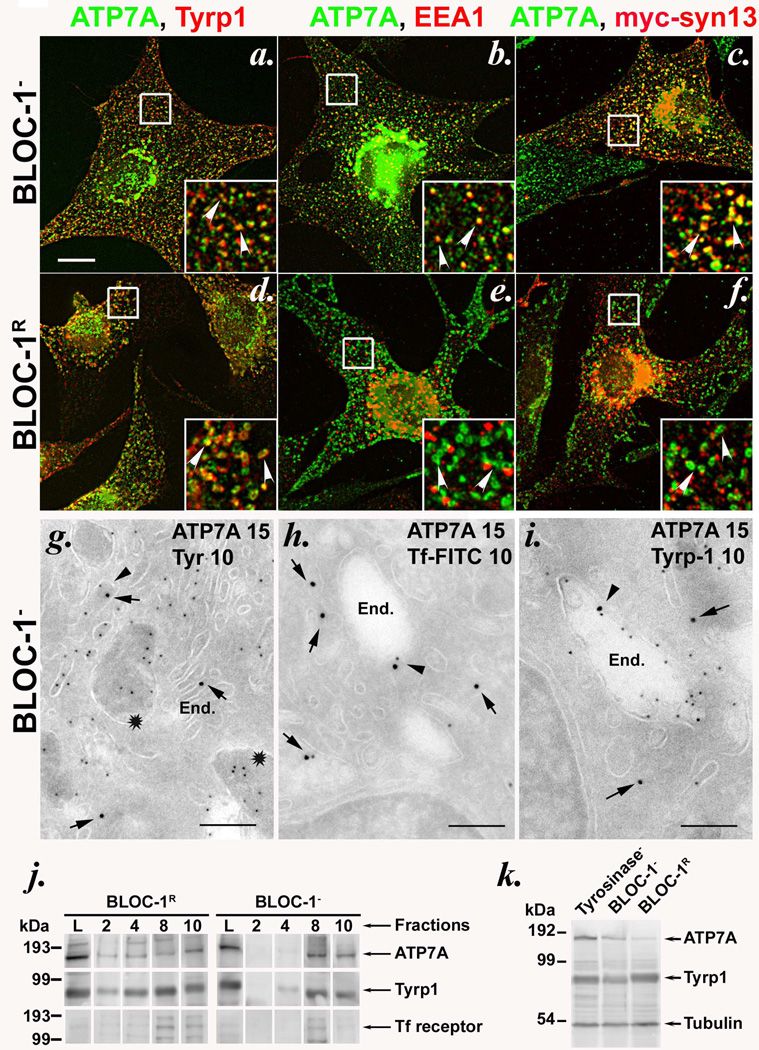
Whereas a cohort of tyrosinase is present in non-pigmented melanosomes in BLOC-1− cells, other melanosome proteins, including Tyrp1, are excluded from melanosomes and accumulate in vacuolar early endosomes15. Like Tyrp1 but not tyrosinase15, ATP7A in these cells was excluded from melanosomes, since it did not colocalize appreciably by IFM with the melanosome precursor marker, Pmel17 (Supplementary Figure S3C) and was not detected in fibrillar melanosomes by IEM (Figure 2g and Supplementary Figure S4a, b). Rather, ATP7A localized predominantly to the perinuclear area and peripheral early endosomes in BLOC-1− melan-mu and melan-rp cells, as shown by extensive colocalization by IFM with the mislocalized Tyrp1 (Figure 2a and Supplementary Figure S2; in melan-mu, 39±11% of peripheral ATP7A puncta contained Tyrp1 and 81±11% of Tyrp1 puncta contained ATP7A) and with the early endosomal markers EEA1 and syntaxin 13 (Figure 2b, c and Supplementary Figure S3A and S3B). IEM analyses confirmed that ATP7A in these cells localized primarily to Golgi membranes and secondarily to tubulovesicular and vacuolar endosomes, which also often contained Tyrp1, internalized transferrin (Tf), and tyrosinase (Fig. 2g–i and Supplementary Figure S4a, b), and to multivesicular late endosomes. BLOC-1 “rescue” by expression of the respective missing Muted or BLOS3 subunit in melan-mu or melan-rp cells, respectively, resulted in reduced ATP7A expression (Figure 2k) but nevertheless restored pigmentation and melanosome localization for both ATP7A and Tyrp1 (25%±7% of peripheral ATP7A localized to Tyrp1-containing rings in BLOC-1- rescued [BLOC-1R] melan-mu:MuHA cells by IFM, and ATP7A was detected in melanosomes, as well as endosomes and Golgi, by IEM; Figure 2d–f and Supplementary Figure S2 and Supplementary Figure S4c–e). IFM and IEM analyses were confirmed by subcellular fractionation on sucrose step gradients, in which a cohort of ATP7A was detected together with Tyrp1 in a high density, pigmented melanosome fraction from BLOC-1R cells, but not from BLOC-1− cells (Figure 2j and Supplementary Figure S4f). In the latter, ATP7A was only detected in low-density fractions and cofractionated with the Tf receptor and mistargeted Tyrp1 (Figure 2j and Supplementary Figure S4f). Finally, results of cross-linking and immunoprecipitation analyses from both BLOC-1− and BLOC-1R cells show that a cohort of ATP7A associates with the BLOC-1-dependent cargo protein Tyrp1, but not BLOC-1-independent cargoes LAMP-1 or Tf receptor (Supplementary Figure S5a–c). Although the interaction with Tyrp1 is not required for ATP7A trafficking to melanosomes (Supplementary Figure S5d), the results nevertheless suggest that ATP7A and Tyrp1 are in close proximity in a transport intermediate prior to BLOC-1 function. Together, the data establish that melanosomal localization of ATP7A requires BLOC-1. By contrast, adaptor protein (AP)-3 is dispensable for ATP7A trafficking to melanosomes, since a large cohort of ATP7A, like Tyrp115, efficiently localized to pigmented melanosomes in AP-3-deficient melan-pe cells derived from HPS type 2 model pearl mice (Supplementary Figure S2m–o).
Figure 2. ATP7A is mislocalized to early endosomes in BLOC-1-deficient melanocytes.
(a–f) IFM analysis of BLOC-1− (melan-mu, a–c) and BLOC-1R (melan-mu: MuHA, d–f) melanocytes labeled with antibodies to ATP7A and either Tyrp1 (left), EEA1 (middle) or transiently expressed myc epitope-tagged Syntaxin 13 (myc-syn13, right). Insets, boxed regions magnified 3X. Arrowheads, ATP7A colocalized with the indicated marker. Bar, 10 µm. Note that Tyrp1 labels melanosomes in BLOC-1R cells but early endosomes in BLOC-1− cells15; EEA1 and syn13 label early endosomes in both cell types. (g–i) Ultrathin cryosections of BLOC-1− melan-mu cells were immunogold labeled with antibodies to ATP7A (15 nm gold) and tyrosinase (g), internalized Tf (h) or Tyrp1 (i)(10 nm gold) and analyzed by electron microscopy. Arrows indicate labeling of ATP7A and arrowheads indicate ATP7A with Tf or Tyrp1 on endosomes. Stars in (g) indicate striated melanosomes labeled for tyrosinase but not ATP7A. End., endosomes. Bars, 500 nm. See also Supplementary Figure S4. (j) Subcellular fractionation of BLOC-1− (melan-mu) and BLOC-1R (melan-mu:MuHA) cells on sucrose step gradients. Eluted fractions (2, 4, 8 and 10), collected from bottom to top, and lysates (L, input loading control) were probed by immunoblot with antibodies to ATP7A, Tyrp1 and Tf receptor (to label early endosomes). Left, molecular weight markers (in kDa). Arrows, relevant bands. Note the bands for ATP7A and Tyrp1, but not Tf receptor (middle band), in pigment-containing fractions 2 and 4 from melan-mu:MuHA but not melan-mu cells. (k) Whole cell lysates from tyrosinase-deficient (Tyrosinase-, melan-c), BLOC-1− (melan-mu) and BLOC-1R (melan-mu:MuHA) were fractionated by SDS-PAGE and immunoblotted with antibodies to the ATP7A, Tyrp1 and tubulin. Left, molecular weight markers (in kDa). Arrows, relevant bands.
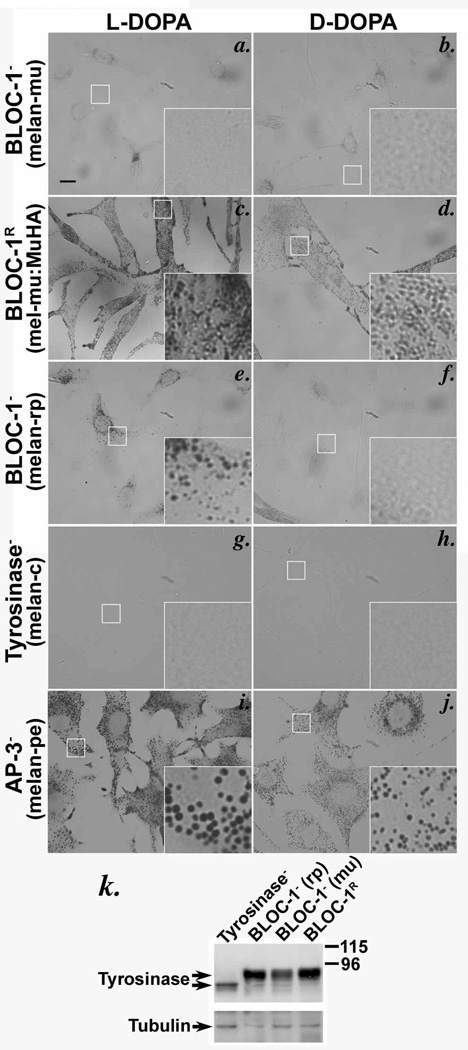
If ATP7A is required to supply copper to tyrosinase in melanosomes, then the cohort of tyrosinase in non-pigmented melanosomes of BLOC-1− cells might be inactive due to a lack of copper resulting from the absence of ATP7A. Indeed, DOPA cytochemistry analyses showed that tyrosinase activity in BLOC-1− melan-mu cells was nearly as low as that of tyrosinase-deficient melan-c cells, as judged by the paucity of melanin deposits formed in the presence of the tyrosinase substrate L-DOPA relative to the negative control D-DOPA (Figure 3a, b, g, h). By contrast, L-DOPA-induced melanin deposits were clearly observed in BLOC-1R melan-mu:MuHA (Figure 3c, d) and AP-3-deficient melan-pe cells (Figure 3i, j). The low tyrosinase activity in BLOC-1− cells did not result from tyrosinase instability, since tyrosinase protein levels by immunoblotting were only modestly lower in BLOC-1− than BLOC-1R cells and much higher than the residual unprocessed tyrosinase in melan-c cells (Figure 3k). Modest tyrosinase activity was detected in melan-rp cells (Figure 3e, f), which lack the BLOC-1 subunit BLOS3; this is consistent with the partial assembly of BLOC-1 in these cells18 and the milder hypopigmentation of reduced pigmentation mice from which melan-rp cells are derived18,19. Together, these results indicate that tyrosinase is largely inactive in BLOC-1− melanocytes.
Figure 3. Tyrosinase is present but inactive in BLOC-1-deficient melanocytes.
(a–j) Bright field microscopy analysis of BLOC-1− (melan-mu, a, b; melan-rp, e, f), BLOC-1R (melan-mu:MuHA, c, d), AP-3-deficient (melan-pe, i, j) and tyrosinase mutant (melan-c, g, h) mouse melanocytes treated with L- or D-DOPA for 3 h. Tyrosinase activity is indicated by melanin deposition in the presence of L-DOPA but not D-DOPA. Insets, boxed regions magnified 5X. Bars, 10 µm. Note melanin deposits were not observed in melan-mu (a) or control melan-c cells (g), and modest deposits were observed in melan-rp cells in which BLOC-1 is partially assembled (e). (k) Whole cell lysates of mouse melanocytes were fractionated by SDS-PAGE and immunoblotted with anti-tyrosinase antibody. Blots were reprobed with anti-tubulin antibody as a loading control. Right, molecular weight markers (kDa). Arrows, relevant bands. Note that tyrosinase protein levels are only mildly reduced in BLOC-1− melan-mu cells relative to BLOC-1R melan-mu: MuHA cells.
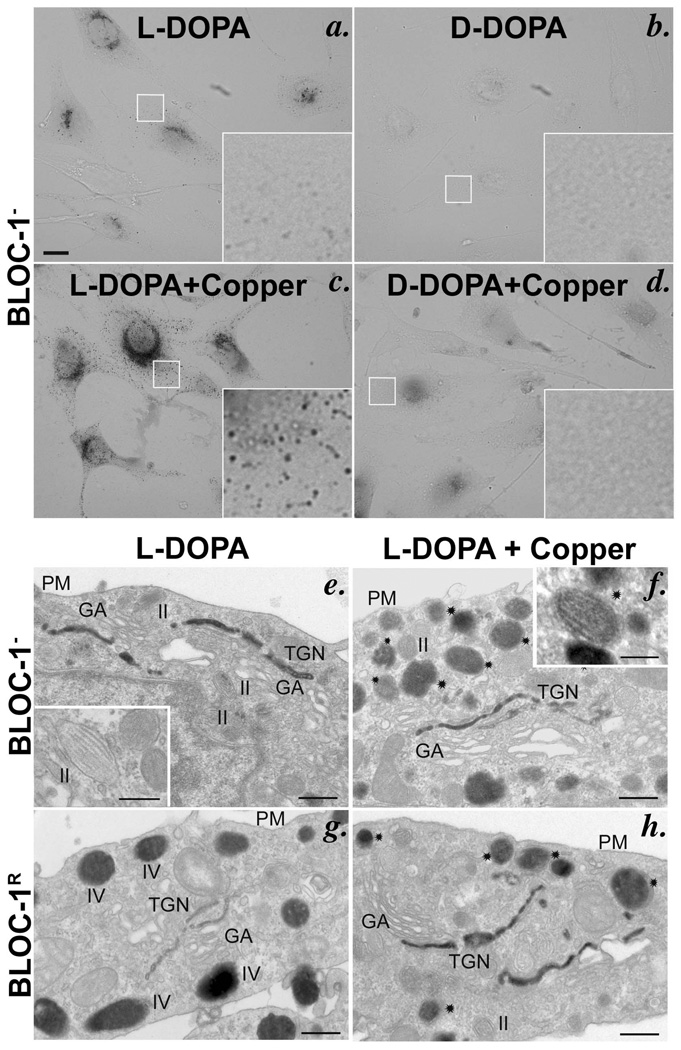
To determine whether tyrosinase in BLOC-1− melanocytes is inactive because it lacks copper, we tested whether the addition of copper to the post-fixation DOPA cytochemistry reaction restored activity. Indeed, L-DOPA-dependent melanin pigmentation was dramatically increased in BLOC-1− melan-mu cells by the addition of 20 µM copper sulfate (Figure 4a–d). Pigmentation of BLOC-1R melan-mu:MuHA cells also increased upon copper addition, albeit not as dramatically (Supplementary Figure S6a–d). To determine the subcellular location of tyrosinase activity in these cells, DOPA cytochemistry reactions were analyzed by EM. As expected, L-DOPA-induced melanin deposits (indicating active tyrosinase) in BLOC-1R melanocytes were observed in mature (stage III and IV) melanosomes (Figure 4g, h) and the TGN in both the absence and presence of copper, although copper consistently increased the signal in the TGN (Figure 4g, h); a cohort (~26–28%; Supplementary Figure S7g) of melanosomes remained unpigmented (Supplementary Figure S7a, b), consistent with the absence of tyrosinase from stage II melanosomes16. By contrast, without added copper, L-DOPA-induced melanin deposits were observed only in the TGN of BLOC-1− melan-mu cells and not in the striated melanosomes (Figure 4e and Supplementary Figure S7c, e, g). Addition of copper to the L-DOPA cytochemistry reaction resulted in additional melanin deposition within the majority of striated melanosomes in BLOC-1− cells (Figure 4f and Supplementary Figure S7d, f); the fraction of melanosomes that contained pigment among these cells was comparable to that of wild-type cells (Supplementary Figure S7g). The pigmented organelles observed in the presence of copper likely correspond to slightly more electron dense fibrillar organelles observed in the absence of copper (Supplementary Figure S7c) and that harbor both Pmel17 and tyrosinase15. Together, these data indicate that tyrosinase is initially activated in the TGN, where ATP7A accumulates in these cells (Supplementary Figure S4a–e), but is inactive within the nonpigmented melanosomes specifically due to a lack of copper. Copper also stimulated tyrosinase activity in BLOC-1− cells within tubular and vacuolar endosomes (Supplemental Figure S7d, f), to which a cohort of tyrosinase is mislocalized in these cells15. Surprisingly, despite the presence of 25–50% of immunodetectable tyrosinase within early endosomes of wild-type melanocytes15,16, very little enzyme activity was detected in endosomes of BLOC-1R cells regardless of the addition of copper (Supplemental Figure S7a, b). Since the TGN and tubular endosomes are intermediates in tyrosinase transport to melanosomes16, the data suggest that tyrosinase inefficiently binds copper ions in the TGN in vivo and loses its bound copper ions within endosomes en route to melanosomes.
Figure 4. Copper restores in vitro tyrosinase activity in melanosomes of BLOC-1-deficient melanocytes.
DOPA cytochemistry of BLOC-1− melan-mu (a–f) or BLOC-1R melan-mu:MuHA (g, h) cells in the absence or presence (+ Copper) of 20 µM copper sulfate in the reaction buffer. (a–d) Bright field microscopy analysis of cells treated for 4 h as indicated. Insets, boxed regions magnified 10X. Bars, 10 µm. Note the increased melanin deposits in BLOC-1− cells in the presence (c) compared to the absence (a) of copper. (e–h) EM analysis of thin sections of cells treated for 2 h. Note melanin deposits in the trans-most Golgi cisternae, but not in striated melanosomes, of BLOC-1− melan-mu cells in the absence of excess copper (e; inset), and the additional deposition of melanin in striated melanosomes in the presence of copper (f, stars; inset). GA, Golgi apparatus; TGN, trans-Golgi network; PM, Plasma membrane; II, III, IV, melanosome stages II and IV. Bars, 500 nm and inset, 200 nm.
Our studies show that tissue-specific localization of the copper transporter ATP7A to a lysosome-related organelle, the melanosome, is required to supply copper to sustain the activity of a resident metalloenzyme, tyrosinase. We confirm that tyrosinase is first loaded with copper in the TGN8, a normal trafficking intermediate en route to melanosomes. However, unlike for ceruloplasmin, which binds copper very stably20, the copper appears to be loaded onto tyrosinase inefficiently and is subsequently stripped, likely within endosomal intermediates that function in TGN to melanosome trafficking16, and must be reloaded within mature melanosomes. This mechanism provides tight spatial control of tyrosinase activity to ensure that melanin is produced only in mature melanosomes and that endosomal transport intermediates and melanosome precursors are protected from toxic melanin intermediates generated by premature tyrosinase activity (the TGN might additionally lack the L-DOPA or tyrosine substrates). Similar spatial regulation of copper integration likely exists for other endomembrane metalloproteins such as peptidylglycine α-amidating monooxygenase21, and preliminary results (unpublished data) suggest that copper release from tyrosinase can be regulated by extracellular cues to provide fine-tuning of cuproenzyme activity. How bound copper is destabilized within endosomes is not clear, but one contributing factor is likely the low pH of these compartments. Tyrosinase is inactive at acidic pH22, perhaps due to protonation of copper-coordinating histidine residues within the copper-binding domain23, and even addition of excess copper in vitro could not restore tyrosinase activity at pH 5 in BLOC-1− melanocytes (Supplementary Figure S8). However, prolonged exposure to low pH does not appear to be sufficient to destabilize bound copper (data not shown), suggesting that additional factors participate. By contrast, melanosomes become more alkaline as they mature24, providing an environment conducive to the reactivation of tyrosinase upon reexposure to copper.
The localization of ATP7A to melanosomes requires BLOC-1, which facilitates tissue-specific transport of selected cargoes to melanosomes15 and other lysosome-related organelles13,25–27. This dependence in part explains the hypopigmentation of BLOC-1-deficient mice and human patients with HPS types 7 and 8. Mislocalization of metal transporters such as ATP7A or B might contribute to other cell type defects in HPS, including the loss of functional dense granules in platelets and of lamellar bodies in type II pneumocytes. Retinal pigment epithelial cells, which also harbor melanosomes and are hypopigmented in HPS, express both ATP7A and ATP7B28; whether either or both transporters localize to melanosomes during the brief period of melanogenesis in these cells29 remains to be tested. Importantly, tyrosinase activity in BLOC-1− melanocytes could be restored by addition of copper in vitro (Supplementary Figure S7i), but not by incubation of live cells in excess copper (data not shown). This suggests that alternative transporters on melanosomes either cannot substitute for ATP7A or are also mislocalized in BLOC-1− cells.
METHODS SUMMARY
For bright field and immunofluorescence microscopy, cells were fixed with 2–4% formaldehyde, labeled with the indicated primary and Alexafluor-conjugated secondary antibodies, and analyzed on a DM IRBE microscope (Leica Microsystems, Wetzlar, Germany) equipped with an Orca digital camera (Hamamatsu, Bridgewater, NJ) using OpenLab software (Improvision, Waltham, MA). Images obtained from consecutive z-planes were processed using subtractive volume deconvoluton with Improvision OpenLab. DOPA cytochemistry was performed as described30 and similarly analyzed but without image deconvolution; all images were obtained at similar camera and illumination settings. Electron microscopy analyses of immunogold labeled ultrathin cryosections or of DOPA-treated cells and immunoblotting of whole cell lysates was performed as described18,19. Melan-Ink4a cells were transfected with siRNAs using Oligofectamine and analyzed either two to three days later (Figure 1) or transfected a second time on day 3 and analyzed two days later (Supplementary Figure S1). Subcellular fractionation of cells disrupted by Dounce homogenization was performed using sedimentation on sucrose step gradients.
Full Methods including cell lines, antibodies and associated references are described in Methods.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Jonathan Gitlin, Michael Petris, Betty Eipper, Svetlana Lutsenko, and Andrew Peden for reagents, Andrew Dancis, Leslie King and Christopher Burd for comments, and Dawn Harper for technical assistance. This work was supported by National Institute of Health grants R01 EY015625 and R21 GM078474 (to MSM), CNRS, Institut Curie and Fondation pour la Recherche Médicale (to GR), Wellcome Trust program grant # 064583 (to EVS and DCB), and postdoctoral fellowship 0625437U from the American Heart Association (to SRGS).
Footnotes
SUPPLEMENTARY INFORMATION
Supplementary figures are described in supplementary information.
AUTHOR CONTRIBUTIONS
SRGS designed and performed most of the experiments described herein, prepared most of the figures, wrote an initial draft of the manuscript, and participated in all stages of manuscript revision. DT performed all of the electron microscopy analyses described herein and provided valuable insights into data interpretation. EVS and DCB provided the cell lines used in all of the experiments, performed confirmatory experiments and guided others, provided additional data not shown in the manuscript, and participated in manuscript revision. GR oversaw the electron microscopy analyses, prepared electron micrographs for the figures, contributed substantially to experimental design, and participated in manuscript revision. MSM oversaw the entire project, designed many of the experiments in collaboration with SRGS, coordinated work among collaborators, and participated in all stages of manuscript revision.
REFERENCES
- 1.Thiele DJ. Integrating trace element metabolism from the cell to the whole organism. J. Nutr. 2003;133:1579S–1580S. doi: 10.1093/jn/133.5.1579S. [DOI] [PubMed] [Google Scholar]
- 2.Mercer JF. The molecular basis of copper-transport diseases. Trends Mol. Med. 2001;7:64–69. doi: 10.1016/s1471-4914(01)01920-7. [DOI] [PubMed] [Google Scholar]
- 3.Yamaguchi Y, Heiny ME, Suzuki M, et al. Biochemical characterization and intracellular localization of the Menkes disease protein. Proc. Natl. Acad. Sci. U.S.A. 1996;93:14030–14035. doi: 10.1073/pnas.93.24.14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris ED. Cellular copper transport and metabolism. Annu. Rev. Nutr. 2000;20:291–310. doi: 10.1146/annurev.nutr.20.1.291. [DOI] [PubMed] [Google Scholar]
- 5.Lutsenko S, Barnes NL, Bartee MY, et al. Function and regulation of human copper-transporting ATPases. Physiol. Rev. 2007;87:1011–1046. doi: 10.1152/physrev.00004.2006. [DOI] [PubMed] [Google Scholar]
- 6.Marks MS, Seabra MC. The melanosome: membrane dynamics in black and white. Nat. Rev. Mol. Cell Biol. 2001;2:738–748. doi: 10.1038/35096009. [DOI] [PubMed] [Google Scholar]
- 7.Oetting WS, Fryer JP, Shriram S, et al. Oculocutaneous albinism type 1: the last 100 years. Pigment Cell Res. 2003;16:307–311. doi: 10.1034/j.1600-0749.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 8.Novikoff AB, Albala A, Biempica L. Ultrastructural and cytochemical observations on B-16 and Harding-Passey mouse melanomas. The origin of premelanosomes and compound melanosomes. J Histochem. Cytochem. 1968;16:299–319. doi: 10.1177/16.5.299. [DOI] [PubMed] [Google Scholar]
- 9.Maul GG, Brumbaugh JA. On the possible function of coated vesicles in melanogenesis of the regenerating fowl feather. J. Cell Biol. 1971;48:41–48. doi: 10.1083/jcb.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petris MJ, Strausak D, Mercer JF. The Menkes copper transporter is required for the activation of tyrosinase. Hum. Mol. Genet. 2000;9:2845–2851. doi: 10.1093/hmg/9.19.2845. [DOI] [PubMed] [Google Scholar]
- 11.Levinson B, Vulpe C, Elder B, et al. The mottled gene is the mouse homologue of the Menkes disease gene. Nat. Genet. 1994;6:369–373. doi: 10.1038/ng0494-369. [DOI] [PubMed] [Google Scholar]
- 12.Mercer JF, Grimes A, Ambrosini L, et al. Mutations in the murine homologue of the Menkes gene in dappled and blotchy mice. Nat. Genet. 1994;6:374–378. doi: 10.1038/ng0494-374. [DOI] [PubMed] [Google Scholar]
- 13.Wei ML. Hermansky-Pudlak syndrome: a disease of protein trafficking and organelle function. Pigment Cell Res. 2006;19:19–42. doi: 10.1111/j.1600-0749.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- 14.Gautam R, Novak EK, Tan J, et al. Interaction of Hermansky-Pudlak Syndrome genes in the regulation of lysosome-related organelles. Traffic. 2006;7:779–792. doi: 10.1111/j.1600-0854.2006.00431.x. [DOI] [PubMed] [Google Scholar]
- 15.Setty SR, Tenza D, Truschel ST, et al. BLOC-1 is required for cargo-specific sorting from vacuolar early endosomes toward lysosome-related organelles. Mol. Biol. Cell. 2007;18:768–780. doi: 10.1091/mbc.E06-12-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theos AC, Tenza D, Martina JA, et al. Functions of adaptor protein (AP)-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes. Mol. Biol. Cell. 2005;16:5356–5372. doi: 10.1091/mbc.E05-07-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chi A, Valencia JC, Hu ZZ, et al. Proteomic and bioinformatic characterization of the biogenesis and function of melanosomes. J. Proteome Res. 2006;5:3135–3144. doi: 10.1021/pr060363j. [DOI] [PubMed] [Google Scholar]
- 18.Starcevic M, Dell'Angelica EC. Identification of snapin and three novel proteins (BLOS1, BLOS2, and BLOS3/reduced pigmentation) as subunits of biogenesis of lysosome-related organelles complex-1 (BLOC-1) J. Biol. Chem. 2004;279:28393–28401. doi: 10.1074/jbc.M402513200. [DOI] [PubMed] [Google Scholar]
- 19.Gwynn B, Martina JA, Bonifacino JS, et al. Reduced pigmentation (rp), a mouse model of Hermansky-Pudlak syndrome, encodes a novel component of the BLOC-1 complex. Blood. 2004;104:3181–3189. doi: 10.1182/blood-2004-04-1538. [DOI] [PubMed] [Google Scholar]
- 20.Sato M, Gitlin JD. Mechanisms of copper incorporation during the biosynthesis of human ceruloplasmin. J. Biol. Chem. 1991;266:5128–5134. [PubMed] [Google Scholar]
- 21.De M, Ciccotosto GD, Mains RE, et al. Trafficking of a secretory granule membrane protein is sensitive to copper. J. Biol. Chem. 2007;282:23362–23371. doi: 10.1074/jbc.M702891200. [DOI] [PubMed] [Google Scholar]
- 22.Wang N, Hebert DN. Tyrosinase maturation through the mammalian secretory pathway: bringing color to life. Pigment Cell Res. 2006;19:3–18. doi: 10.1111/j.1600-0749.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- 23.Martinez JH, Solano F, Garcia-Borron JC, et al. The involvement of histidine at the active site of Harding-Passey mouse melanoma tyrosinase. Biochem. Int. 1985;11:729–738. [PubMed] [Google Scholar]
- 24.Raposo G, Tenza D, Murphy DM, et al. Distinct protein sorting and localization to premelanosomes, melanosomes, and lysosomes in pigmented melanocytic cells. J. Cell Biol. 2001;152:809–824. doi: 10.1083/jcb.152.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raposo G, Marks MS. Melanosomes--dark organelles enlighten endosomal membrane transport. Nat. Rev. Mol. Cell Biol. 2007;8:786–797. doi: 10.1038/nrm2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raposo G, Marks MS, Cutler DF. Lysosome-related organelles: driving post-Golgi compartments into specialisation. Curr. Opin. Cell Biol. 2007;19:394–401. doi: 10.1016/j.ceb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salazar G, Craige B, Styers ML, et al. BLOC-1 complex deficiency alters the targeting of adaptor protein complex-3 cargoes. Mol. Biol. Cell. 2006;17:4014–4026. doi: 10.1091/mbc.E06-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krajacic P, Qian Y, Hahn P, et al. Retinal localization and copper-dependent relocalization of the Wilson and Menkes disease proteins. Invest. Ophthalmol. Vis. Sci. 2006;47:3129–3134. doi: 10.1167/iovs.05-1601. [DOI] [PubMed] [Google Scholar]
- 29.Lopes VS, Wasmeier C, Seabra MC, et al. Melanosome maturation defect in Rab38-deficient retinal pigment epithelium results in instability of immature melanosomes during transient melanogenesis. Mol. Biol. Cell. 2007;18:3914–3927. doi: 10.1091/mbc.E07-03-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boissy RE, Zhao Y, Gahl WA. Altered protein localization in melanocytes from Hermansky-Pudlak syndrome: support for the role of the HPS gene product in intracellular trafficking. Lab. Invest. 1998;78:1037–1048. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.