Abstract
Colon cancer is second leading cause of cancer-related deaths in Western countries. Diet and smoking, which contain aromatic and heterocyclic amines, are major risk factors for colon cancer. Colorectal cancers have a natural history of long latency and therefore provide ample opportunities for effective chemoprevention. 3,2’-Dimethyl-4-aminobiphenyl (DMABP) is an experimental aromatic amine that causes cancer in rat colon and serves as an experimental model for arylamine and heterocyclic amine mutagens derived from diet and smoking. In this study, we investigated the effects of celecoxib, a selective cyclooxygenase-2 (COX-2) inhibitor on DMABP-induced DNA adduct formation in rat liver and colon. Male F-344 rats (5-weeks old) were provided free access to modified AIN-76A rat chow containing 0 (control), 500, 1000, or 1500 ppm celecoxib. Two weeks later, the rats received a subcutaneous injection of 100 mg/kg DMABP in peanut oil. Two days after DMABP treatment, the rats were killed and DMABP-derived adducts were analyzed in colon and liver DNA by butanol extraction-mediated 32P-postlabeling. Two major DNA adducts, identified as dG-C8-DMABP and dG-N2-DMABP, were detected in liver and colon of rats treated with DMABP. These DNA adducts were diminished approximately 35–40% with 500 ppm and 65–70% with 1,000 ppm celecoxib. In the colon, no further decline in DNA adducts was observed at 1500 ppm. The same DMABP-DNA adducts also were detected in the liver and were also diminished by celecoxib treatment. The reduction in DMABP-DNA adduct levels in celecoxib-treated animals provides further support for celecoxib as a chemopreventive agent for colorectal cancer.
Keywords: 3,2’-Dimethyl-4-aminobiphenyl; DNA adducts; celecoxib; colon cancer; 32P-postlabeling
1. Introduction
Colorectal cancer is one of the leading causes of cancer-related deaths in the Western world [1]. Family history, diet and genetic pre-disposition are major risk factors for this cancer [2]. However, environmental carcinogens have also been implicated [3]. Several aromatic amines present in the environment, such as tobacco smoke, and heterocyclic amines derived from well-done meat have been considered carcinogenic to humans [4]. 3,2’-dimethyl-4-aminobiphenyl (DMABP) is an experimental aromatic amine carcinogen used for studying the induction of colon cancer in rodents [5] that has close structural similarity with mutagens isolated from well-done meat. DMABP undergoes N-hydroxylation by cytochrome P450 1A2 [6], followed by O-acetylation, which upon hydrolysis reacts with DNA [7,8] to form adducts that are mutagenic and carcinogenic [9].
Celecoxib is a COX-2 specific inhibitor which has less toxicity than traditional COX inhibitors. The two isoforms of cyclooxygenase, COX-1 and COX-2, catalyze the synthesis of prostaglandins from arachidonic acid. While COX-1 is expressed constitutively in most tissues and control normal physiological functions, COX-2 is not detected in most normal tissues and is induced by mitogenic and inflammatory stimuli [10], resulting in the increased synthesis of prostaglandins. Several studies have demonstrated overexpression of COX-2 in cancers [11–14]. Studies that knocked out COX-2 expression demonstrated reduced incidence of skin papillomas and intestinal tumors [15,16]. COX-2 overexpression in colon cancers has been demonstrated by several studies [17–19]. Thus, several lines of evidence suggest that COX-2 is a potential target for prevention and treatment of colon cancer. Celecoxib has shown inhibition of tumorigenesis in several animal models such as azoxymethane-induced colon tumors in F344 rats [20], 9,10-dimethyl-1,2-benzanthracene-induced breast tumors in Sprague-Dawley rats [21], and N-butyl-N-(4-hydroxybutyl)-nitrosamine-induced urinary bladder carcinogenesis [22]. This drug has been approved for the treatment of rheumatoid arthritis and osteoarthritis and has also been approved for the treatment of familial adenomatous polyposis, following a clinical trial that showed positive effect with 400 mg celecoxib twice a day for 6 months [23]. A recent report on significant reduction of colorectal adenomas with 400 mg celecoxib once daily for three years after polypectomy was published [24], although a nearly 2 fold-increased cardiovascular risk was observed [25]. However, there are no studies that investigated the role of celecoxib in inhibiting aromatic amine-induced tumorigenesis. In this study, we have investigated the effect of celecoxib on DMABP-derived DNA adducts, a biomarker of aromatic-amine-induced tumorigenesis.
2. Materials and methods
2.1. Chemicals
DMABP was purchased from Toronto Research Chemicals, Inc. (Toronto, Canada). dG-C8-DMABP and dG-N2-DMABP standards, derived by reacting N-OH-DMABP with calf thymus DNA were kindly provided by Dr. Tom Flamming, National Center for Toxicological Research (Jefferson, AR). Celecoxib was kindly donated by Pfizer Inc. (Groton, CT).
2.2. Animals and treatment
Weanling F-344 inbred rats were fed AIN-76A diet for a week after acclimation. At 5 weeks, rats were separated into groups and fed AIN-76A diet supplemented with either 0, 500, 1000 or 1500 ppm celecoxib. At 7 weeks, rats were injected subcutaneously with DMABP (100 mg/kg in peanut oil) or vehicle control once. After 2 days, rats were euthanized with CO2, liver and entire colorectum were removed and stored at −80°C until DNA isolation.
2.3. DNA isolation
Colon tissue was cut open, washed with saline to remove residual fecal content and mucosal scrapes were collected in Tris-EDTA buffer, pH 8.0. DNA was isolated from the colon samples by a solvent extraction procedure as described in detail previously [26]. Briefly, crude nuclei were isolated from frozen liver tissues or mucosal scrapes prior to digestion with RNAses and proteinase K, followed by sequential extractions with phenol, phenol:Sevag (chloroform:isoamyl alcohol, 24:1) and Sevag. DNA was recovered by precipitation with ethanol in the presence of sodium chloride and dissolved in HPLC-grade water. The purity and concentration of DNA was determined spectrophotometrically.
2.4. Analysis of DNA adducts by 32P-postlabeling
DNA samples were analyzed by 32P-postlabeling method [26]. Briefly, 7 µg DNA was digested with micrococcal nuclease and spleen phosphodiesterase (enzyme: substrate 1:5). An aliquot was removed and used for evaluation of normal nucleotide levels. Adducts were enriched by butanol extraction in the presence of phase-transfer agent tetrabutylammonium chloride. Adducts were labeled with molar excess of [γ-32P] ATP (6000 Ci/mmol). Adducts were resolved by multidirectional polyethyleneimine (PEI)-cellulose TLC in the following solvents: D1 = 2.3 M sodium phosphate, pH, 6.0; D3 = 2.5 M lithium formate/7 M urea, pH 3.5; D4 = 0.6 M sodium phosphate/7 M urea, pH 8.0; D5 = 1.7 M sodium phosphate, pH 6.0. Development in D2 direction was omitted. Normal nucleotides were resolved in 180 mM sodium phosphate, pH 6.0. Adducts were detected and quantitated using Packard InstantImager. Relative adduct labeling was expressed as adducts/109 nucleotides.
2.5. Co-chromatography of liver and colon DNA from with reference DMABP-derived DNA adducts
DNA adducts from liver and colon were identified by co-chromatography with dG-C8-DMABP and dG-N2-DMABP adducts derived from calf thymus DNA reacted with N-OH-DMABP. Aliquots of labeled DNA digest and reference adducts were chromatographed individually and in combination. Adducts were resolved by multi-directional PEI-cellulose TLC as described above.
2.5. Statistics
Adduct analysis was carried out in all 8 animals within each group. Data are expressed as mean ± SE. Overall dose effect relationship with celecoxib intervention was determined by one-way analysis of variance. All other comparisons between the groups were carried out by negative binomial regression with logarithm as link function. A p value of <0.05 was considered statistically significant.
3. Results
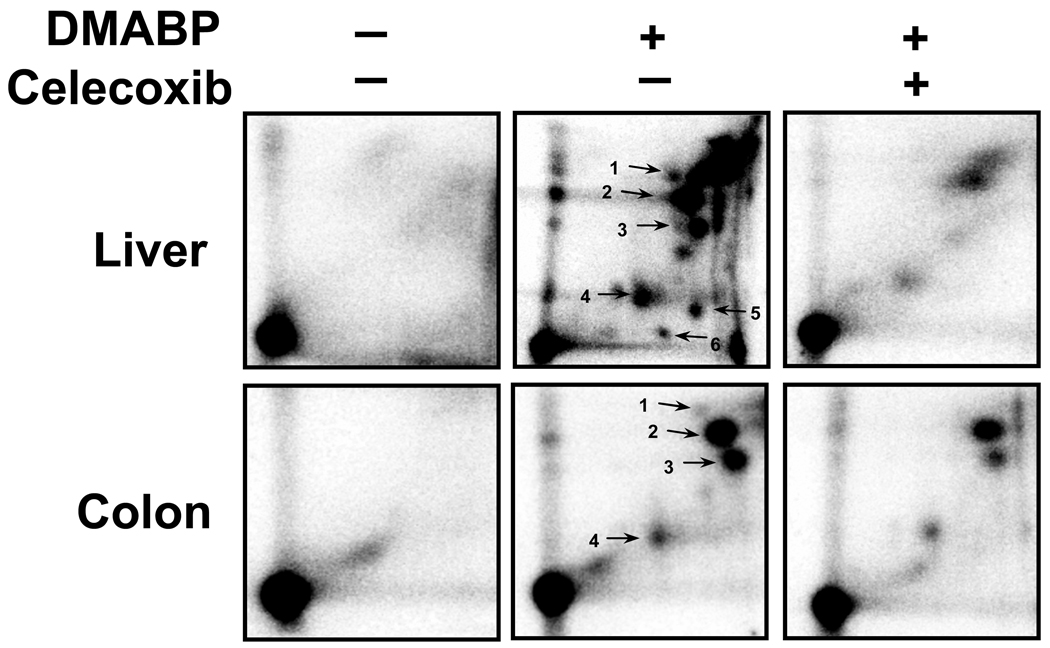
32P-Postlabeling analysis of DNA adducts following treatment with DMABP revealed 3 major adducts both in the liver and colon samples analyzed – adducts 2 (dG-C8-DMABP), 3 (unidentified) and 4 (dG-N2-DMABP) (Fig 1). No adducts were detected in vehicle-treated rats. No qualitative differences were found in the adduct pattern following intervention with celecoxib (Fig 1). However, a dose-dependent decrease was observed upon intervention with celecoxib for all the adducts detected in both liver (Table 1) and colon (Table 2).
Figure 1.
Representative 32P-postlabeling DNA adduct maps from liver and colon of rats exposed to vehicle, DMABP and celecoxib (1000 ppm). Labeled adducts were resolved using TLC conditions described in text. Adduct 2 – dG-C8-DMABP; adduct 4 – dG-N2-DMABP; adducts 1, 3, 4–6 – unidentified.
Table 1.
Effect of celecoxib on DMABP-derived DNA adducts in liver. Values represented are mean ± SEM from 8 animals
| Treatment | Adducts/109 nucleotides | ||||||
|---|---|---|---|---|---|---|---|
| 1 | C8-DMABP | 3 | N2-DMABP | 5 | 6 | Total | |
| Control | 454 ± 66 | 3,208 ± 567 | 2,398 ± 573 | 3,215 ± 647 | 239 ± 99 | 506 ± 138 | 12,571 ± 1,370 |
| 500 ppm celecoxib % reduction |
374 ± 66 17.6 |
1,077 ± 81 66.4 |
1,154 ± 133 51.9 |
2,008 ± 308 37.5 |
149 ± 29 37.6 |
399 ± 61 21.1 |
7,403 ± 330 41.1 |
| 1000 ppm celecoxib % reduction |
194 ± 77 57.2 |
674 ± 118 79.0 |
532 ± 80 77.8 |
787 ± 70 75.5 |
99 ± 13 58.5 |
156 ± 40 69.2 |
3,873 ± 532 69.2 |
| 1500 ppm celecoxib % reduction p value |
128 ± 63 71.8 0.0006 |
534 ± 136 83.3 < 0.0001 |
436 ± 70 81.8 0.0004 |
441 ± 78 86.3 < 0.0001 |
50 ± 13 79.1 0.0997 |
115 ± 24 77.3 0.0053 |
2,328 ± 265 81.5 < 0.0001 |
Table 2.
Effect of celecoxib on DMABP-derived DNA adducts in colon. Values represented are mean ± SEM from 8 animals
| Treatment | Adducts/109 nucleotides | |||
|---|---|---|---|---|
| C8-DMABP | 3 | N2-DMABP | Total | |
| Control | 3,062 ± 335 | 1,489 ± 172 | 1,171 ± 228 | 5,723 ± 465 |
| 500 ppm celecoxib % reduction |
1,815 ± 319 40.7 |
848 ± 106 43.1 |
753 ± 66 35.7 |
3,417 ± 343 40.3 |
| 1000 ppm celecoxib % reduction |
914 ± 119 70.1 |
341 ± 73 77.1 |
392 ± 37 66.5 |
1,646 ± 184 71.2 |
| 1500 ppm celecoxib % reduction p value |
973 ± 202 68.2 <0.0001 |
332 ± 90 77.7 <0.0001 |
277 ± 27 76.3 0.0002 |
1,582 ± 272 72.4 <0.0001 |
In the liver, there was a 66%, 79% and 83% reduction of dG-C8-DMABP (p<0.0001) and 38%, 78% and 86% reduction of dG-N2-DMABP (p<0.0001) at 500, 1000 and 1500 ppm, respectively. The other adducts detected showed significant reduction at doses 1000 and 1500 ppm (Table 1).
In colon, dG-C8-DMABP adduct was reduced by 40% and 70% at 500 ppm and 1000 ppm doses, but there was no further effect beyond 1000 ppm. dG-N2-DMABP adduct was reduced by 36% and 67% and 76% at 500, 1000 and 1,500 ppm (p=0.0002), respectively. However, total adduct burden was effectively reduced at 1000 ppm but no further reduction was appreciated at 1500 ppm. The other unknown major adduct was reduced similarly (Table 2). The minor adduct was at very low levels or absent. Reduction in total adduct burden was significant from 1000 ppm to 1500 ppm only in the liver (p=0.0298).
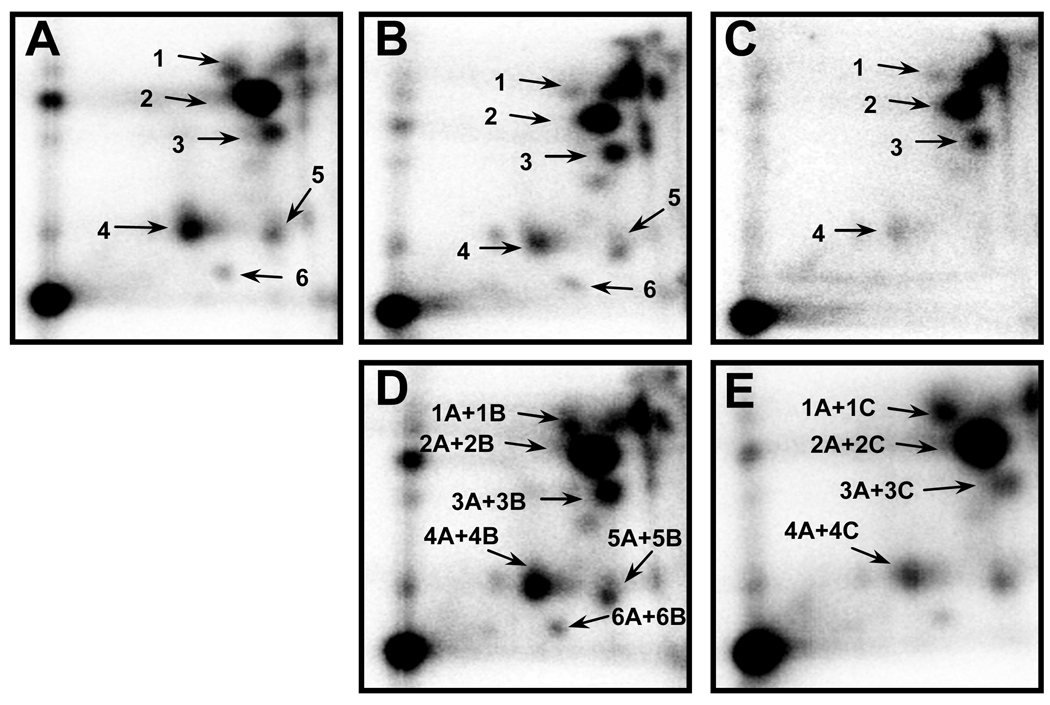
dG-C8-DMABP and dG-N2-DMABP standards, derived by reacting N-OH-DMABP with calf thymus DNA, were run in parallel with the samples to ascertain the identity of the two known major adducts. Identity of the two adduct standards was confirmed by nuclease P1 treatment earlier [27]. Co-chromatography of the liver and colon DNA with reference DMABP-derived adducts confirmed the identity of the tissue DNA adducts detected (Fig 2). Co-chromatography of colon and liver DNA showed that the major and minor adducts were identical (not shown). The third major adduct and minor adducts (#1, #5 and #6) were also detected in calf thymus DNA treated with N-OH-DMABP and were chromatographically identical, and remain unidentified.
Figure 2.
Co-chromatography of liver and colon DNA of rats treated with DMABP with reference DMABP-derived adducts. A–Reference DMABP-derived DNA adducts; B-Liver DNA; C-Colon DNA; D-Liver DNA + reference adducts; E-Colon DNA + reference adducts. 32P-labeled adducts were resolved by multi-directional PEI-cellulose TLC using solvents described in text.
4. Discussion
The DMABP-derived colon tumorigenesis in F-344 rats has defined stages of premalignant lesions that make it ideal for intervention studies. DMABP has structural similarity to mutagens present in well-done meat. Tumorigenesis in this model was shown to be influenced by dietary fat and fiber [28,29] and therefore has direct relevance to human colon cancers, where Western dietary habits play a major role. This study examined the chemoprotective effect of celecoxib on levels of DMABP-derived adducts in the target organ colon and the non-target liver. Administration of DMABP in F344 rats result in high adduction in both liver and colon DNA and this has been shown in earlier studies by Williams et al [30]. Studies on formation and removal of DNA adducts in target and non-target tissues with DMABP [31,32], with DMBA [33] and from our lab with 2-acetylaminophenanthrene [34] have shown that the adducts levels were as high in non-target liver as in target tissues or higher. However, it has been shown that they do not form tumors in liver [7,33]. These data suggest that DNA adduct formation is essential but additional factors such as cell proliferation are necessary for tissue susceptibility to tumor development. Although the adduct levels were similar in the liver and colon, tumorigenesis occurs only in colon in this model. This could be explained, in part, by the fact that while the lesions are removed in the liver, lesions of the colon, due to high cell turnover, probably get fixed and therefore result in tumors. Westra et al [7] have shown that while the specific activity of the radioactive DMABP-derived adducts remained constant in liver between 1 and 7 days of injection, it decreased in the intestine, which is due to cell turnover and additional adduct formation. The lesions are therefore more likely to be fixed in intestine than liver.
Celecoxib showed a dose-response related reduction in DMABP-derived DNA adducts in both liver and colon. In liver, 1500 ppm showed maximum protective effect, but colon did not show any further effect above 1000 ppm. These results support a protective effect of celecoxib on aromatic amine-induced carcinogenesis. Although celecoxib is shown to have an inhibitory effect on CYP2D6 [35,36], its effect on other phase I enzymes or on conjugation mechanisms are not known. However, NSAIDS have been shown to have several COX-independent mechanisms of protection such as prevention of DNA fragmentation [37], promotion of apoptosis [38,39] and modulation of several other signal transduction molecules [40].
Of the different pathways described for the metabolism of DMABP, the classical N-hydroxylation is the most extensively studied pathway involving cytochrome CYP1A2 and further O-acetylation by N-acetyltransferases to form DNA reactive metabolites [41]. However, an alternate pathway of activation, that involves the enzyme cyclooxygenase (COX) in metabolic activation of the heterocyclic amines has been described, resulting in the formation of metabolites which undergo secondary reactions [42,43]. Studies have shown that the metabolic activation of primary and tertiary aromatic amines by prostaglandin H synthase does not involve the N-hydroxy intermediate step [44]. Studies on aromatic amines such as N-acetylbenzidine (ABZ) have shown that they can be metabolized through alternate pathways by peroxygenase to produce DNA adducts [45]. Comparison of the derived adduct with synthetic standards by HPLC confirmed the formation of dGp-ABZ by this mechanism [46]. Wiese et al [47] have shown that hCOX enzymes have the ability to activate several environmental and dietary carcinogens, including MeIQx, 4-ABP and B[a]P. Alternative mechanisms of metabolism of heterocyclic amines such as IQ and MeIQx yield similar types of adducts as their N-OH analogues. Both N-NO-IQ and N-OH-IQ formed dG-C8-IQ, and co-migration of these adducts was confirmed by 32P-postlabeling and HPLC methods [48]. The N-Nitroso analogues of IQ and MeIQx, activated by conditions that mediate inflammatory responses therefore have the ability to bind to DNA and form dG-C8 adducts, and have genotoxic potential [48,49]. Formation of DMABP-derived adducts by pathways other than N-hydroxylation could partly explain the dose-dependent reduction of these adduct levels by celecoxib. Specific adducts derived by prostaglandin H synthase have been shown to be poorly extracted into butanol [50]. However, no adducts in addition to those described previously could be identified either by butanol extraction or by nuclease P1 in this study (data not shown). Inhibition of prostaglandin H synthase-mediated DNA adduction as a possible mode of action of celecoxib needs more detailed examination.
In summary, this study demonstrates for the first time the role of celecoxib in reducing DMABP-derived DNA adducts. Persistence of these DNA adducts and a dose-dependent reduction suggests that DNA adducts can be used as early biomarkers in colon cancer chemoprevention studies. Elucidating the exact mechanisms by which celecoxib exerts its effects on DMABP metabolism and/or DMABP-derived adducts would prove important in understanding its chemopreventive effects.
Acknowledgements
This work was supported from USPHS grant CA034627 from NCI and, in part, from James Graham Brown Cancer Center and Agnes Brown Duggan Endowment funds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Bond JH. Colorectal cancer update. Prevention, screening, treatment, and surveillance for high-risk groups. Med Clin North Am. 2000;84:1163–1182. doi: 10.1016/s0025-7125(05)70281-9. viii. [DOI] [PubMed] [Google Scholar]
- 3.Kadlubar FF, Butler MA, Kaderlik KR, Chou HC, Lang NP. Polymorphisms for aromatic amine metabolism in humans: relevance for human carcinogenesis. Environ Health Perspect. 1992;98:69–74. doi: 10.1289/ehp.929869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turesky RJ. Formation and biochemistry of carcinogenic heterocyclic aromatic amines in cooked meats. Toxicol Lett. 2007:219–227. doi: 10.1016/j.toxlet.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Shirai T, Nakamura A, Fukushima S, Yamamoto A, Tada M, Ito N. Different carcinogenic responses in a variety of organs, including the prostate, of five different rat strains given 3,2'-dimethyl-4-aminobiphenyl. Carcinogenesis. 1990;11:793–797. doi: 10.1093/carcin/11.5.793. [DOI] [PubMed] [Google Scholar]
- 6.Nussbaum M, Fiala ES, Kulkarni B, El-Bayoumy K, Weisburger JH. In vivo metabolism of 3,2'-dimethyl-4-aminobiphenyl (DMAB) bearing on its organotropism in the Syrian golden hamster and the F344 rat. Environ Health Perspect. 1983;49:223–231. doi: 10.1289/ehp.8349223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Westra JG, Flammang TJ, Fullerton NF, Beland FA, Weis CC, Kadlubar FF. Formation of DNA adducts in vivo in rat liver and intestinal epithelium after administration of the carcinogen 3,2'-dimethyl-4-aminobiphenyl and its hydroxamic acid. Carcinogenesis. 1985;6:37–44. doi: 10.1093/carcin/6.1.37. [DOI] [PubMed] [Google Scholar]
- 8.Hein DW. Molecular genetics and function of NAT1 and NAT2: role in aromatic amine metabolism and carcinogenesis. Mutat Res. 2002;506–507:65–77. doi: 10.1016/s0027-5107(02)00153-7. [DOI] [PubMed] [Google Scholar]
- 9.Kadlubar FF. DNA adducts of carcinogenic aromatic amines. IARC Sci Publ. 1994:199–216. [PubMed] [Google Scholar]
- 10.Jones DA, Carlton DP, McIntyre TM, Zimmerman GA, Prescott SM. Molecular cloning of human prostaglandin endoperoxide synthase type II and demonstration of expression in response to cytokines. J Biol Chem. 1993;268:9049–9054. [PubMed] [Google Scholar]
- 11.Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 12.Neufang G, Furstenberger G, Heidt M, Marks F, Muller-Decker K. Abnormal differentiation of epidermis in transgenic mice constitutively expressing cyclooxygenase-2 in skin. Proc Natl Acad Sci U S A. 2001;98:7629–7634. doi: 10.1073/pnas.121574098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ristimaki A, Honkanen N, Jankala H, Sipponen P, Harkonen M. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res. 1997;57:1276–1280. [PubMed] [Google Scholar]
- 14.Tucker ON, Dannenberg AJ, Yang EK, Zhang F, Teng L, Daly JM, Soslow RA, Masferrer JL, Woerner BM, Koki AT, Fahey TJ., 3rd Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res. 1999;59:987–990. [PubMed] [Google Scholar]
- 15.Chulada PC, Thompson MB, Mahler JF, Doyle CM, Gaul BW, Lee C, Tiano HF, Morham SG, Smithies O, Langenbach R. Genetic disruption of Ptgs-1, as well as Ptgs-2, reduces intestinal tumorigenesis in Min mice. Cancer Res. 2000;60:4705–4708. [PubMed] [Google Scholar]
- 16.Tiano HF, Loftin CD, Akunda J, Lee CA, Spalding J, Sessoms A, Dunson DB, Rogan EG, Morham SG, Smart RC, Langenbach R. Deficiency of either cyclooxygenase (COX)-1 or COX-2 alters epidermal differentiation and reduces mouse skin tumorigenesis. Cancer Res. 2002;62:3395–3401. [PubMed] [Google Scholar]
- 17.Hull MA, Booth JK, Tisbury A, Scott N, Bonifer C, Markham AF, Coletta PL. Cyclooxygenase 2 is up-regulated and localized to macrophages in the intestine of Min mice. Br J Cancer. 1999;79:1399–1405. doi: 10.1038/sj.bjc.6690224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonoshita M, Takaku K, Oshima M, Sugihara K, Taketo MM. Cyclooxygenase-2 expression in fibroblasts and endothelial cells of intestinal polyps. Cancer Res. 2002;62:6846–6849. [PubMed] [Google Scholar]
- 19.Hasegawa K, Ichikawa W, Fujita T, Ohno R, Okusa T, Yoshinaga K, Sugihara K. Expression of cyclooxygenase-2 (COX-2) mRNA in human colorectal adenomas. Eur J Cancer. 2001;37:1469–1474. doi: 10.1016/s0959-8049(01)00137-x. [DOI] [PubMed] [Google Scholar]
- 20.Kawamori T, Rao CV, Seibert K, Reddy BS. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res. 1998;58:409–412. [PubMed] [Google Scholar]
- 21.Harris RE, Alshafie GA, Abou-Issa H, Seibert K. Chemoprevention of breast cancer in rats by celecoxib, a cyclooxygenase 2 inhibitor. Cancer Res. 2000;60:2101–2103. [PubMed] [Google Scholar]
- 22.Grubbs CJ, Lubet RA, Koki AT, Leahy KM, Masferrer JL, Steele VE, Kelloff GJ, Hill DL, Seibert K. Celecoxib inhibits N-butyl-N-(4-hydroxybutyl)-nitrosamine-induced urinary bladder cancers in male B6D2F1 mice and female Fischer-344 rats. Cancer Res. 2000;60:5599–5602. [PubMed] [Google Scholar]
- 23.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y, Fujimura T, Su LK, Levin B. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 24.Arber N, Eagle CJ, Spicak J, Racz I, Dite P, Hajer J, Zavoral M, Lechuga MJ, Gerletti P, Tang J, Rosenstein RB, Macdonald K, Bhadra P, Fowler R, Wittes J, Zauber AG, Solomon SD, Levin B. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 25.Solomon SD, Pfeffer MA, McMurray JJ, Fowler R, Finn P, Levin B, Eagle C, Hawk E, Lechuga M, Zauber AG, Bertagnolli MM, Arber N, Wittes J. Effect of celecoxib on cardiovascular events and blood pressure in two trials for the prevention of colorectal adenomas. Circulation. 2006;114:1028–1035. doi: 10.1161/CIRCULATIONAHA.106.636746. [DOI] [PubMed] [Google Scholar]
- 26.Gupta RC. 32P-Postlabeling for detection of DNA adducts. In: Pfeifer GP, editor. Technologies for Detection of DNA Damage and Mutations. New York: Plenum Press; 1996. pp. 45–61. [Google Scholar]
- 27.Feng Y, Jiang W, Deitz AC, Hein DW. 3,2'-Dimethyl-4-aminobiphenyl-DNA adduct formation in tumor target and nontarget organs of rapid and slow acetylator Syrian hamsters congenic at the NAT2 locus. Toxicol Appl Pharmacol. 1996;140:315–321. doi: 10.1006/taap.1996.0226. [DOI] [PubMed] [Google Scholar]
- 28.Reddy BS, Mori H. Effect of dietary wheat bran and dehydrated citrus fiber on 3,2'-dimethyl-4-aminobiphenyl-induced intestinal carcinogenesis in F344 rats. Carcinogenesis. 1981;2:21–25. doi: 10.1093/carcin/2.1.21. [DOI] [PubMed] [Google Scholar]
- 29.Reddy BS, Ohmori T. Effect of intestinal microflora and dietary fat on 3,2'-dimethyl-4-aminobiphenyl-induced colon carcinogenesis in F344 rats. Cancer Res. 1981;41:1363–1367. [PubMed] [Google Scholar]
- 30.Williams GM, Iatropoulos MJ, Jeffrey AM, Shirai T. Protective effect of acetaminophen against colon cancer initiation effects of 3,2'-dimethyl-4-aminobiphenyl in rats. Eur J Cancer Prev. 2002;11:39–48. doi: 10.1097/00008469-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Beland FA, Kadlubar FF. Formation and persistence of arylamine DNA adducts in vivo. Environ Health Perspect. 1985;62:19–30. doi: 10.1289/ehp.856219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shirai T, Tada M, Kojima M, Hasegawa R, Masui T, Ito N. DNA adducts in target and nontarget tissues of 3,2'-dimethyl-4-aminobiphenyl in rats. Environ Health Perspect. 1994;102 Suppl 6:167–172. doi: 10.1289/ehp.94102s6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daniel FB, Joyce NJ. 7,12-Dimethylbenz[a]anthracene--DNA adducts in Sprague-Dawley and Long-Evans female rats: the relationship of DNA adducts to mammary cancer. Carcinogenesis. 1984;5:1021–1026. doi: 10.1093/carcin/5.8.1021. [DOI] [PubMed] [Google Scholar]
- 34.Gupta RC, Earley K, Fullerton NF, Beland FA. Formation and removal of DNA adducts in target and nontarget tissues of rats administered multiple doses of 2-acetylaminophenanthrene. Carcinogenesis. 1989;10:2025–2033. doi: 10.1093/carcin/10.11.2025. [DOI] [PubMed] [Google Scholar]
- 35.Garnett WR. Clinical implications of drug interactions with coxibs. Pharmacotherapy. 2001;21:1223–1232. doi: 10.1592/phco.21.15.1223.33891. [DOI] [PubMed] [Google Scholar]
- 36.Werner U, Werner D, Rau T, Fromm MF, Hinz B, Brune K. Celecoxib inhibits metabolism of cytochrome P450 2D6 substrate metoprolol in humans. Clin Pharmacol Ther. 2003;74:130–137. doi: 10.1016/S0009-9236(03)00120-6. [DOI] [PubMed] [Google Scholar]
- 37.Matthias C, Schuster MT, Zieger S, Harreus U. COX-2 inhibitors celecoxib and rofecoxib prevent oxidative DNA fragmentation. Anticancer Res. 2006;26:2003–2007. [PubMed] [Google Scholar]
- 38.Yamamoto Y, Yin MJ, Lin KM, Gaynor RB. Sulindac inhibits activation of the NF-kappaB pathway. J Biol Chem. 1999;274:27307–27314. doi: 10.1074/jbc.274.38.27307. [DOI] [PubMed] [Google Scholar]
- 39.Hsu AL, Ching TT, Wang DS, Song X, Rangnekar VM, Chen CS. The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J Biol Chem. 2000;275:11397–11403. doi: 10.1074/jbc.275.15.11397. [DOI] [PubMed] [Google Scholar]
- 40.Gupta RA, Tan J, Krause WF, Geraci MW, Willson TM, Dey SK, DuBois RN. Prostacyclin-mediated activation of peroxisome proliferator-activated receptor delta in colorectal cancer. Proc Natl Acad Sci U S A. 2000;97:13275–13280. doi: 10.1073/pnas.97.24.13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flammang TJ, Westra JG, Kadlubar FF, Beland FA. DNA adducts formed from the probable proximate carcinogen, N-hydroxy-3,2' -dimethyl-4-aminobiphenyl, by acid catalysis or S-acetyl coenzyme A-dependent enzymatic esterification. Carcinogenesis. 1985;6:251–258. doi: 10.1093/carcin/6.2.251. [DOI] [PubMed] [Google Scholar]
- 42.Wise RW, Zenser TV, Kadlubar FF, Davis BB. Metabolic activation of carcinogenic aromatic amines by dog bladder and kidney prostaglandin H synthase. Cancer Res. 1984;44:1893–1897. [PubMed] [Google Scholar]
- 43.Wolz E, Wild D, Degen GH. Prostaglandin-H synthase mediated metabolism and mutagenic activation of 2-amino-3-methylimidazo [4,5-f] quinoline (IQ) Arch Toxicol. 1995;69:171–179. doi: 10.1007/s002040050154. [DOI] [PubMed] [Google Scholar]
- 44.Boyd JA, Harvan DJ, Eling TE. The oxidation of 2-aminofluorene by prostaglandin endoperoxide synthetase. Comparison with other peroxidases. J Biol Chem. 1983;258:8246–8254. [PubMed] [Google Scholar]
- 45.Zenser TV, Lakshmi VM, Hsu FF, Davis BB. Peroxygenase metabolism of N-acetylbenzidine by prostaglandin H synthase. Formation of an N-hydroxylamine. J Biol Chem. 1999;274:14850–14856. doi: 10.1074/jbc.274.21.14850. [DOI] [PubMed] [Google Scholar]
- 46.Lakshmi VM, Hsu FF, Davis BB, Zenser TV. N-Acetylbenzidine-DNA adduct formation by phorbol 12-myristate-stimulated human polymorphonuclear neutrophils. Chem Res Toxicol. 2000;13:785–792. doi: 10.1021/tx0000320. [DOI] [PubMed] [Google Scholar]
- 47.Wiese FW, Thompson PA, Kadlubar FF. Carcinogen substrate specificity of human COX-1 and COX-2. Carcinogenesis. 2001;22:5–10. doi: 10.1093/carcin/22.1.5. [DOI] [PubMed] [Google Scholar]
- 48.Lakshmi VM, Schut HA, Zenser TV. 2-Nitrosoamino-3-methylimidazo[4,5-f]quinoline activated by the inflammatory response forms nucleotide adducts. Food Chem Toxicol. 2005;43:1607–1617. doi: 10.1016/j.fct.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Lakshmi VM, Hsu FF, Schut HA, Zenser TV. Stability and reactivity of 2-nitrosoamino-3,8-dimethylimidazo[4,5-f]quinoxaline. Chem Res Toxicol. 2006;19:325–333. doi: 10.1021/tx050305x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krauss RS, Eling TE. Formation of unique arylamine:DNA adducts from 2-aminofluorene activated by prostaglandin H synthase. Cancer Res. 1985;45:1680–1686. [PubMed] [Google Scholar]