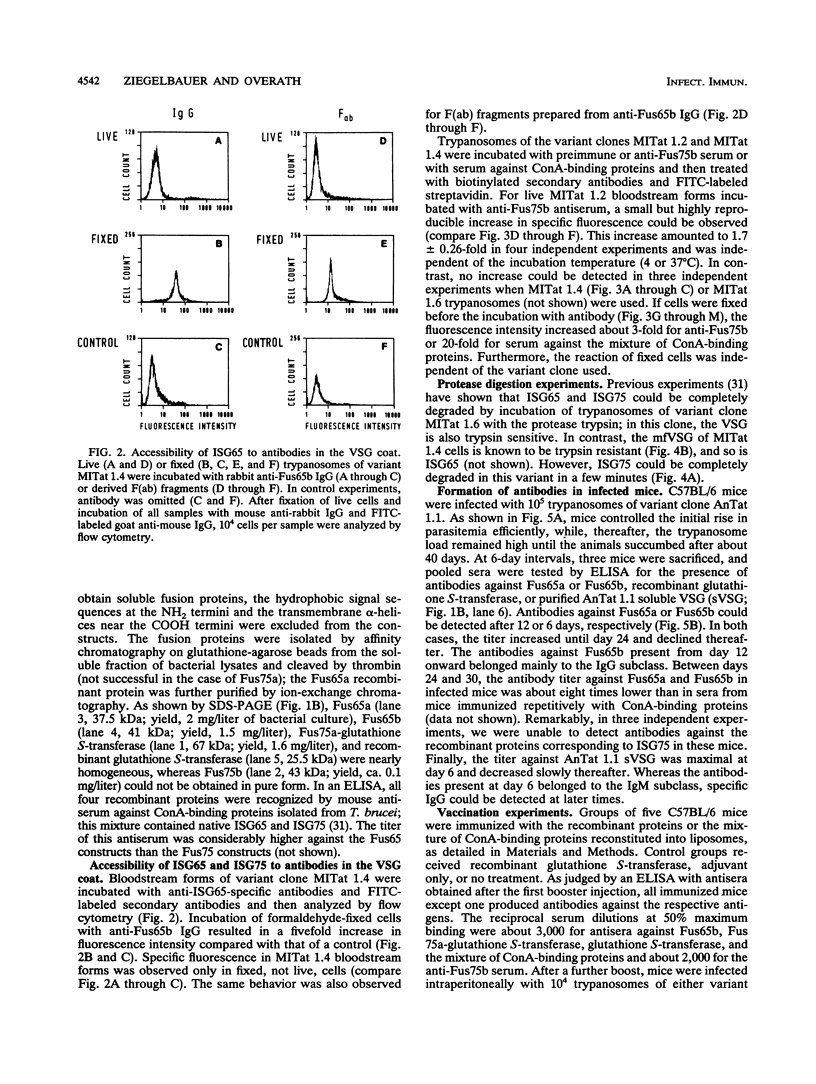
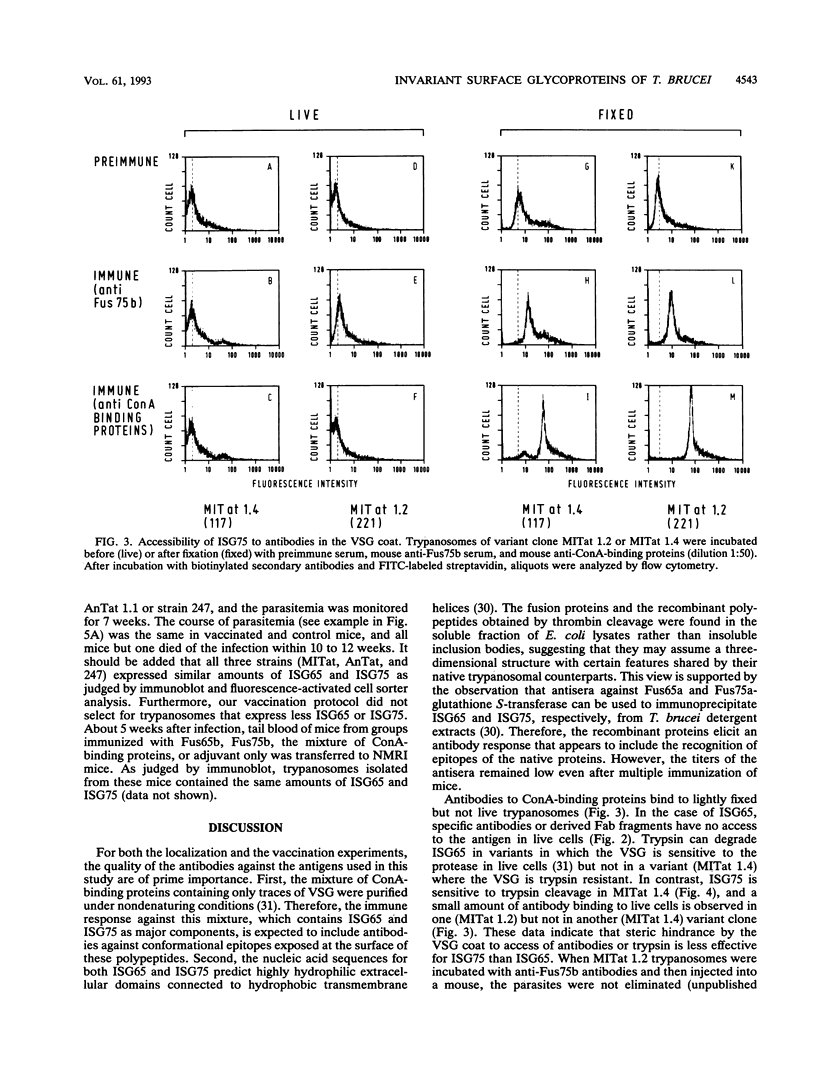
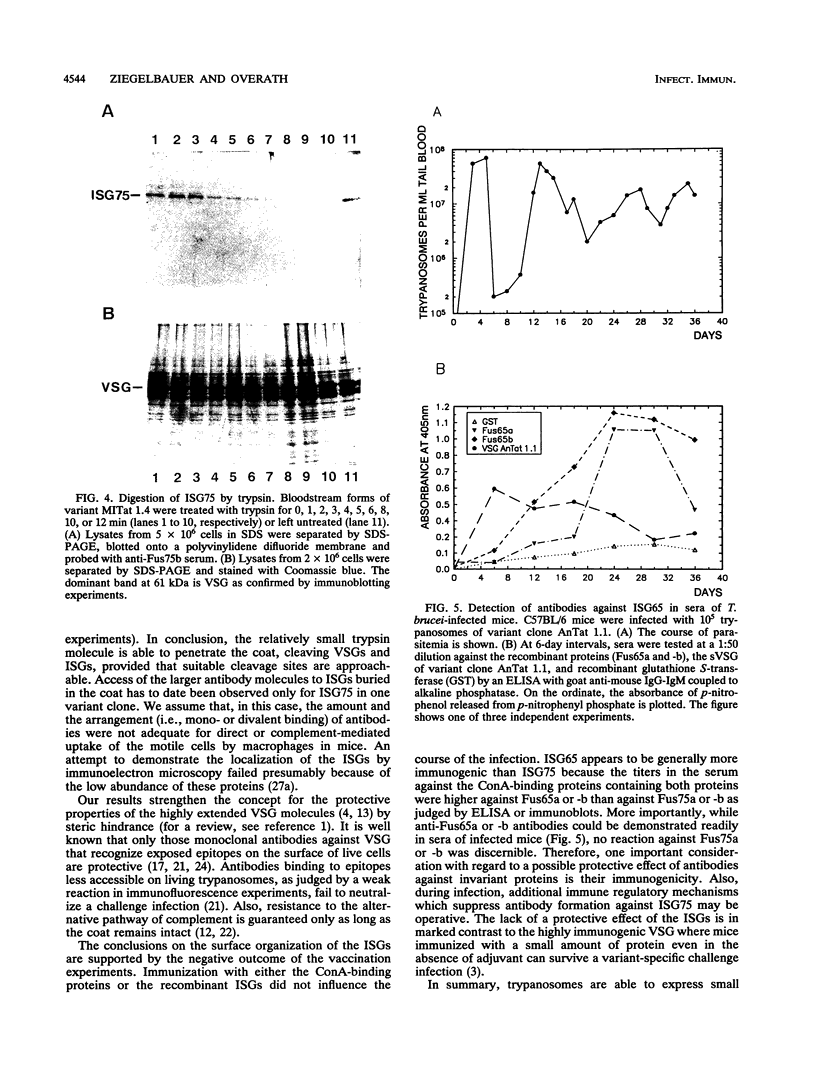
Abstract
The surface coat of Trypanosoma brucei, formed by about 10(7) molecules of the membrane-form variant surface glycoprotein (mfVSG) per cell, is generally considered to constitute a barrier against the access of antibodies directed to invariant surface proteins. The recent characterization of two invariant surface glycoproteins (ISGs) with apparent molecular masses of 65 and 75 kDa (ISG65 and ISG75; 70,000 and 50,000 molecules per cell, respectively), which are both predicted to be composed of large extracellular domains, single transmembrane alpha-helices, and small intracellular domains, enabled a critical test of this hypothesis. Although ISG65 is distributed over the entire surface of the parasites, it is not accessible to antibodies or to the proteinase trypsin in live cells provided the mfVSG is also proteinase resistant. ISG75 is similarly distributed; its accessibility to antibodies depends on the expressed mfVSG, and it is sensitive to trypsin in a variant clone in which the mfVSG is proteinase resistant. Vaccination experiments using recombinant proteins to a mixture of the native ISGs were unsuccessful. ISG65 but not ISG75 elicited an antibody response in chronically infected mice. The results strengthen the view of the protective properties of the variant surface glycoprotein coat by steric hindrance and suggest that additional factors such as low abundance or low immunogenicity of invariant surface proteins may prevent a control of the disease by the humoral immune response.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balber A. E. The pellicle and the membrane of the flagellum, flagellar adhesion zone, and flagellar pocket: functionally discrete surface domains of the bloodstream form of African trypanosomes. Crit Rev Immunol. 1990;10(3):177–201. [PubMed] [Google Scholar]
- Baltz T., Baltz D., Giroud C., Crockett J. Cultivation in a semi-defined medium of animal infective forms of Trypanosoma brucei, T. equiperdum, T. evansi, T. rhodesiense and T. gambiense. EMBO J. 1985 May;4(5):1273–1277. doi: 10.1002/j.1460-2075.1985.tb03772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltz T., Baltz D., Pautrizel R., Richet C., Lamblin G., Degand P. Chemical and immunological characterization of specific glycoproteins from Trypanosoma equiperdum variants. FEBS Lett. 1977 Oct 1;82(1):93–96. doi: 10.1016/0014-5793(77)80893-4. [DOI] [PubMed] [Google Scholar]
- Blum M. L., Down J. A., Gurnett A. M., Carrington M., Turner M. J., Wiley D. C. A structural motif in the variant surface glycoproteins of Trypanosoma brucei. Nature. 1993 Apr 15;362(6421):603–609. doi: 10.1038/362603a0. [DOI] [PubMed] [Google Scholar]
- Borst P. Transferrin receptor, antigenic variation and the prospect of a trypanosome vaccine. Trends Genet. 1991 Oct;7(10):307–309. doi: 10.1016/0168-9525(91)90406-g. [DOI] [PubMed] [Google Scholar]
- Bringaud F., Baltz T. A potential hexose transporter gene expressed predominantly in the bloodstream form of Trypanosoma brucei. Mol Biochem Parasitol. 1992 May;52(1):111–121. doi: 10.1016/0166-6851(92)90040-q. [DOI] [PubMed] [Google Scholar]
- Capbern A., Giroud C., Baltz T., Mattern P. Trypanosoma equiperdum: etude des variations antigéniques au cours de la trypanosomose experimentale du lapin. Exp Parasitol. 1977 Jun;42(1):6–13. doi: 10.1016/0014-4894(77)90055-8. [DOI] [PubMed] [Google Scholar]
- Coppens I., Baudhuin P., Opperdoes F. R., Courtoy P. J. Receptors for the host low density lipoproteins on the hemoflagellate Trypanosoma brucei: purification and involvement in the growth of the parasite. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6753–6757. doi: 10.1073/pnas.85.18.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppens I., Opperdoes F. R., Courtoy P. J., Baudhuin P. Receptor-mediated endocytosis in the bloodstream form of Trypanosoma brucei. J Protozool. 1987 Nov;34(4):465–473. doi: 10.1111/j.1550-7408.1987.tb03216.x. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Cellular and genetic aspects of antigenic variation in trypanosomes. Annu Rev Immunol. 1990;8:83–110. doi: 10.1146/annurev.iy.08.040190.000503. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Identification, purification and properties of clone-specific glycoprotein antigens constituting the surface coat of Trypanosoma brucei. Parasitology. 1975 Dec;71(3):393–417. doi: 10.1017/s003118200004717x. [DOI] [PubMed] [Google Scholar]
- Ferrante A., Allison A. C. Alternative pathway activation of complement by African trypanosomes lacking a glycoprotein coat. Parasite Immunol. 1983 Sep;5(5):491–498. doi: 10.1111/j.1365-3024.1983.tb00763.x. [DOI] [PubMed] [Google Scholar]
- Freymann D., Down J., Carrington M., Roditi I., Turner M., Wiley D. 2.9 A resolution structure of the N-terminal domain of a variant surface glycoprotein from Trypanosoma brucei. J Mol Biol. 1990 Nov 5;216(1):141–160. doi: 10.1016/S0022-2836(05)80066-X. [DOI] [PubMed] [Google Scholar]
- Geigy R., Kauffmann M. Sleeping sickness survey in the Serengeti area (Tanzania) 1971. I. Examination of large mammals for trypanosomes. Acta Trop. 1973;30(1):12–23. [PubMed] [Google Scholar]
- Gruenberg J., Sharma P. R., Deshusses J. D-Glucose transport in Trypanosoma brucei. D-Glucose transport is the rate-limiting step of its metabolism. Eur J Biochem. 1978 Sep 1;89(2):461–469. doi: 10.1111/j.1432-1033.1978.tb12549.x. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991 Feb 1;192(2):262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Hall T., Esser K. Topologic mapping of protective and nonprotective epitopes on the variant surface glycoprotein of the WRATat 1 clone of Trypanosoma brucei rhodesiense. J Immunol. 1984 Apr;132(4):2059–2063. [PubMed] [Google Scholar]
- Herbert W. J., Lumsden W. H. Trypanosoma brucei: a rapid "matching" method for estimating the host's parasitemia. Exp Parasitol. 1976 Dec;40(3):427–431. doi: 10.1016/0014-4894(76)90110-7. [DOI] [PubMed] [Google Scholar]
- Jackson D. G., Windle H. J., Voorheis H. P. The identification, purification, and characterization of two invariant surface glycoproteins located beneath the surface coat barrier of bloodstream forms of Trypanosoma brucei. J Biol Chem. 1993 Apr 15;268(11):8085–8095. [PubMed] [Google Scholar]
- Masterson W. J., Taylor D., Turner M. J. Topologic analysis of the epitopes of a variant surface glycoprotein of Trypanosoma brucei. J Immunol. 1988 May 1;140(9):3194–3199. [PubMed] [Google Scholar]
- Mosser D. M., Roberts J. F. Trypanosoma brucei: recognition in vitro of two developmental forms by murine macrophages. Exp Parasitol. 1982 Dec;54(3):310–316. doi: 10.1016/0014-4894(82)90040-6. [DOI] [PubMed] [Google Scholar]
- Pinder M., van Melick A., Vernet G. Analysis of protective epitopes on the variant surface glycoprotein of a Trypanosoma brucei brucei (DiTat 1.3.) using monoclonal antibodies. Parasite Immunol. 1987 May;9(3):395–400. doi: 10.1111/j.1365-3024.1987.tb00517.x. [DOI] [PubMed] [Google Scholar]
- Schell D., Evers R., Preis D., Ziegelbauer K., Kiefer H., Lottspeich F., Cornelissen A. W., Overath P. A transferrin-binding protein of Trypanosoma brucei is encoded by one of the genes in the variant surface glycoprotein gene expression site. EMBO J. 1991 May;10(5):1061–1066. doi: 10.1002/j.1460-2075.1991.tb08045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfang A., Duszenko M. Functional reconstitution of the Trypanosoma brucei plasma-membrane D-glucose transporter. Eur J Biochem. 1993 Jun 1;214(2):593–597. doi: 10.1111/j.1432-1033.1993.tb17958.x. [DOI] [PubMed] [Google Scholar]
- Van Meirvenne N., Janssens P. G., Magnus E. Antigenic variation in syringe passaged populations of Trypanosoma (Trypanozoon) brucei. 1. Rationalization of the experimental approach. Ann Soc Belg Med Trop. 1975;55(1):1–23. [PubMed] [Google Scholar]
- Van der Ploeg L. H., Bernards A., Rijsewijk F. A., Borst P. Characterization of the DNA duplication-transposition that controls the expression of two genes for variant surface glycoproteins in Trypanosoma brucei. Nucleic Acids Res. 1982 Jan 22;10(2):593–609. doi: 10.1093/nar/10.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegelbauer K., Multhaup G., Overath P. Molecular characterization of two invariant surface glycoproteins specific for the bloodstream stage of Trypanosoma brucei. J Biol Chem. 1992 May 25;267(15):10797–10803. [PubMed] [Google Scholar]
- Ziegelbauer K., Overath P. Identification of invariant surface glycoproteins in the bloodstream stage of Trypanosoma brucei. J Biol Chem. 1992 May 25;267(15):10791–10796. [PubMed] [Google Scholar]